Introduction
Thromboembolic events are a significant complication
in patients with β-thalassaemia, particularly in splenectomised
patients. These patients have an increased risk for both arterial
and venous thromboembolic events affecting different organs
(1). A comprehensive
epidemiological study involving 8,860 patients with β-thalassaemia,
the largest clinical investigation to date, revealed that
thromboembolic events occurred in 0.9% of β-thalassaemia major and
3.9% of patients with intermedia β-thalassaemia (2). Endothelial cells (ECs) play a pivotal
role in maintaining vascular homeostasis and haemostasis by
producing several regulatory factors. Endothelial dysfunction in
patients with β-thalassaemia has been reported, as evidenced by
elevated levels of endothelial activation markers, such as ICAM-1,
E-selectin, VCAM-1, von Willebrand factor (vWF) and thrombomodulin,
in serum and/or plasma samples (3-5).
Nitric oxide (NO), an important protective molecule in the
vasculature, is synthesised by endothelial-nitric oxide synthase
(eNOS) in the vascular endothelium, and plays a crucial role in
maintaining vasomotor tone, regulating coagulation, suppressing
platelet activation, cellular adhesion to the endothelium and
reducing inflammation. Impaired NO bioavailability in patients with
β-thalassaemia/haemoglobin E (HbE) has been documented (6).
Microparticles or medium extracellular vesicles
(mEVs), characterized by a size in the 50-300 nm range and isolated
by high-speed centrifugation at 14,000 x g (7), have emerged as potential mediators of
EC dysfunction in β-thalassaemia/HbE disease. Elevated levels of
mEVs have been observed in splenectomised patients with
β-thalassaemia/HbE (8). It has been
previously demonstrated that mEVs obtained from splenectomised
patients with β-thalassaemia/HbE can induce EC activation, leading
to the increased expression of tissue factor, inflammatory
cytokines and adhesion molecules, and promoting leucocyte adhesion
to ECs (9). Moreover, a recent
proteomic analysis performed by the authors revealed significantly
elevated levels of haemoglobin subunits, including α-, γ-, δ- and
β-globin, (HBA, HBG, HBD and HBB, respectively) in mEVs from
splenectomised patients with β-thalassaemia/HbE and an increase of
3-6-fold compared with those in mEVs from healthy donors (7). Haemoglobin can rapidly react with NO
to form a vasodilator-inactive nitrate (10). This suggests that mEVs may play a
role in modulating NO bioavailability and contributing to vascular
dysfunction in patients with β-thalassaemia/HbE.
The present study aimed to investigate the effects
of mEVs isolated from splenectomised patients with
β-thalassaemia/HbE on NO production, NO scavenging, and eNOS
phosphorylation in human pulmonary artery ECs (HPAECs). By
elucidating the mechanisms through which mEVs impact NO
bioavailability, the present study seeks to enhance our
understanding of the pathophysiological mechanisms underlying
thromboembolic complications in β-thalassaemia, with implications
for potential therapeutic interventions.
Materials and methods
Patients and blood samples
The present study was performed in accordance with
The Declaration of Helsinki and was approved (approval nο. MU-CIRB
2014/013.0502) by the Mahidol University Central Institutional
Review Board, (Bangkok, Thailand). Written informed consent was
obtained from all individual participants included in the present
study. A total of 11 splenectomised patients with
β-thalassaemia/HbE and 10 healthy donors were recruited into the
present cohort study. The volunteers were enrolled at Nakhonpathom
Hospital (Nakhon Pathom, Thailand) from January 2020 to December
2021. All patients had a DNA diagnosis of β-thalassaemia/HbE
disease and had undergone splenectomy >5 years ago. All patients
received folic acid (5 mg) daily. Patients treated with aspirin,
antibiotics, anti-depressants, β-blockers and anti-platelets, or
those with severe anaemia (Hb <5 g/dl) were excluded, and none
had been hospitalised or transfused within the preceding 4 weeks.
All healthy donors had a normal complete blood count, normal Hb
typing and were negative for DNA diagnosis of common α-globin gene
deletions in Thailand, including α-thalassaemia-1 both
-SEA (Southeast Asian deletion) and -THAI
(Thai deletion), and α-thalassaemia-2 both -α3.7 (3.7 kb
deletion) and -α4.2 (4.2 kb deletion), using multiplex
gap PCR. All subjects had no evidence of concurrent infection or a
history of atherosclerotic vascular disease. Venous blood samples
were collected by the two-syringe technique at room temperature and
were processed within 1 h (7).
Haematological parameters are summarised in Table I.
 | Table IHaematological parameters of patients
with β-thalassaemia/HbE and normal subjects. |
Table I
Haematological parameters of patients
with β-thalassaemia/HbE and normal subjects.
| Description | Healthy donor | Splenectomised
patients with β-thalassaemia/HbE | Reference
range |
|---|
| Number (Male:
Female) | 10 (7:3) | 11 (6:5) | |
| Age, years | 37±9 | 40±9 | |
| (Range
Min-Max) | (25-47) | (28-55) | |
| Hb typing | A2A | EF | A2A |
| HbA (%) | 96.7±3.6 | - | 85-90 |
| HbA2
(%) | 2.8±0.2 | - | 2-3 |
| HbE (%) | - |
48.3±8.6a | - |
| HbF (%) | 0.2±0.1 |
28.6±11.6a | 0.8-2.0 |
| RBC count
(x106/µl) | 4.8±0.5 |
3.5±0.5a | 4.2-5.4 |
| Hb (g/dl) | 14.2±1.4 |
6.9±0.8a | 12-18 |
| Hct (%) | 42.6±3.8 |
23.7±2.7a | 37-52 |
| MCV (fl) | 89±3 | 70±8a | 80-99 |
| MCH (pg) | 29±1 | 21±2a | 27-31 |
| MCHC (g/dl) | 33±1 | 30±2a | 31-35 |
| RDW (%) | 12.6±0.7 |
33.2±16.4a | 11.5-14.5 |
| NRBCs (cells/100
WBCs) | None |
184±139a | None |
| WBC count
(x103/µl) | 6±2 | 29±23 | 4-11 |
| Platelet count
(x103/µl) | 280±54 |
621±184a | 150-450 |
Isolation of mEVs from peripheral
blood samples
The mEVs were isolated by sequential centrifugation
as described in a previous study by the authors (11). Briefly, fresh whole blood samples
collected in 3.2% trisodium citrate anticoagulant were centrifuged
at 1,500 x g for 15 min at 25˚C to collect platelet-poor plasma and
were re-centrifuged at 14,000 x g for 2 min at 4˚C to obtain
platelet-free plasma (PFP). Pellet mEVs were collected after
centrifugation of PFP at 14,000 x g at 4˚C for another 45 min. The
pellet was washed once with PBS.
Detection and quantification of mEVs
by flow cytometry (Fig. S1)
The flow cytometric analysis of mEVs followed the
guidelines of the International Society for Extracellular Vesicles
(ISEV) and MIFlowCyt-EV (12,13).
The characterisation of β-thalassaemia/HbE mEVs was previously
reported, as required by ISEV, using three different techniques
(nanoparticle tracking analysis, flow cytometry and
cryo-transmission electron microscopy) (7). The blood samples were processed within
1 h, as previously demonstrated that the absolute numbers and
cellular origins of mEVs did not significantly change within 1 h of
blood collection (7). Total numbers
of mEVs were quantitated using flow cytometry as described in a
previous study (9). Briefly,
samples were stained with PE-conjugated Annexin V (BD Biosciences)
and analysed using an Accuri C6 plus flow cytometer (BD
Biosciences). The mEV population was determined by comparison with
nano polystyrene beads of sizes 0.79 and 1.32 µm in diameter
(Spherotech, Inc.) (Fig. S1). The
absolute number of mEVs was calculated using TruCount beads (BD
Biosciences).
HPAECs culture and treatment
The primary HPAECs (cat. no. 302-05a, Cell
Application) were used within 3-8 passages. HPAECs were cultured in
EC growth medium (Cell Applications, Inc.) at 37˚C and 5%
CO2. HPAECs were plated in a 6-well-plate until they
formed an 80% confluent monolayer. The cells were then treated for
10 min with 1x106 mEVs/ml or 10 ng/ml vascular
endothelial growth factor (VEGF) as a positive control (14). After treatment, culture medium was
kept at -80˚C for the measurement of nitrite concentration, and
cells were harvested for analysis of eNOS and eNOS phosphorylation
by western blotting.
Measurement of NO
The NO production of HPAECs was determined by
measuring nitrite, the stable oxidation product of NO, in the
culture medium using the tri-iodide-based chemiluminescence method
with a NO chemiluminescence analyser (Analyzer CLD88; ECO MEDICS
AG) (15). Briefly, samples were
directly injected into an acidified tri-iodide solution in the
purge vessel, while purging with nitrogen connected to a gas-phase
chemiluminescence NO analyser. The area under the curve was
calculated using Origin 7 (OriginLab Corporation) and was converted
into the amount of nitrite by comparing with a 0.005-0.025 nmol
sodium nitrite (MilliporeSigma) standard curve. The change (∆) in
nitrite concentration in treated cells compared with untreated
controls was then calculated.
Analysis of eNOS phosphorylation
A total of 8 µg protein extracted with RIPA lysis
and extraction buffer (cat. no. 89901; Thermo Fisher Scientific,
Inc.) from HPAECs was separated by SDS-PAGE on a 10% gel. The
proteins were then transferred to a PVDF membrane and blocked with
5% skim milk for 1 h at room temperature. The same membrane was
used to probe for total eNOS, phosphorylated-eNOS at Ser1177 and
Thr495, and β-actin. The membrane was cut into sections before
probing with the respective antibodies to β-actin (45 kD) and eNOS
and phosphorylated-eNOS (140 kD). The membrane was incubated
overnight at 4˚C with primary mouse anti-total eNOS type 3 or mouse
anti-phosphorylated-eNOS position Ser1177 or Thr495 antibodies,
followed by incubation with the secondary horseradish peroxidase
(HRP)-conjugated goat anti-mouse polyclonal antibody or
HRP-conjugated mouse anti-β-actin antibody for 1 h at room
temperature, in dark. After probing for total eNOS, the membrane
was stripped and re-probed for phosphorylated-eNOS. List of
antibodies is presented in Table
SI. The bands on immunoblots were detected using the Enhanced
Chemiluminescence Plus system (ECL plus; Bio-Rad Laboratories,
Inc.). Subsequently, the films were scanned and the intensity of
protein bands was measured using ImageJ software version 1.47
(National Institutes of Health). The expression of all proteins was
first normalised to the individual β-actin protein intensity. Then,
the phosphorylated-eNOS expression was normalised again to total
eNOS expression.
Measurement of potential NO
scavenging
NO scavenging by mEVs was directly measured using a
NO chemiluminescence analyser, Eco Medics Analyzer CLD88(15). A total of 2x106 mEVs from
healthy donors and patients with β-thalassaemia/HbE were injected
into the purge vessel that contained 50 µM NO donor, DETA NONOate
(cat. no. ALX-430-014; Enzo Life Science, Inc.). The area under the
curve of the potential NO scavenging peak was calculated by the
Origin 7 program and was converted into amount of nitrite using the
sodium nitrite standard curve.
Analysis of α-globin protein in
mEVs
An equal number of 5x106 mEVs was used
for western blot analysis of α-globin protein content. This
approach was taken because the present study's proteomic analysis
did not identify a protein with equal amounts between mEVs from
healthy donors and splenectomised patients with β-thalassaemia/HbE
that could serve as an internal control (7). The mEVs were lysed with RIPA lysis and
extraction buffer (Thermo Fisher Scientific, Inc.). Total protein
from an equal number of mEVs (5x106 particles) in each
individual sample was loaded into each lane and separated by
SDS-PAGE on a 12% gel, then, it was transferred to a PVDF membrane.
The membrane was then blocked with 5% skim milk for 1 h at room
temperature and incubated with a mouse anti-human α-globin chain
monoclonal antibody (Santa Cruz Biotechnology, Inc.), followed by
incubation with an HRP-conjugated goat anti-mouse polyclonal
antibody (BD Biosciences) (Table
SI). Before signal detection, an ECL blotting substrate
cocktail (Bio-Rad Laboratories, Inc.) was added. The films were
scanned and the intensity of the protein bands was measured using
ImageJ software version 1.47 (National Institutes of Health).
Statistical analysis
All descriptive statistics (mean and SD) were
performed using Statistical Package for the Social Science (SPSS),
version 17.0 (SPSS, Inc.). The distribution of all data was
estimated using the one-sample Kolmogorov-Smirnov test, and the
histograms were verified using P-P and Q-Q plots. All data, except
the fold changes of α-globin expression detected by western blot
analysis, were non-parametric; therefore, the comparisons between
two groups were analysed using a Mann-Whitney U test while multiple
comparisons of more than two groups were analysed using a
Kruskal-Wallis test, followed by the Dunn-Bonferroni. The fold
changes of α-globin expression were normal distribution; therefore,
the comparisons between groups were performed using unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
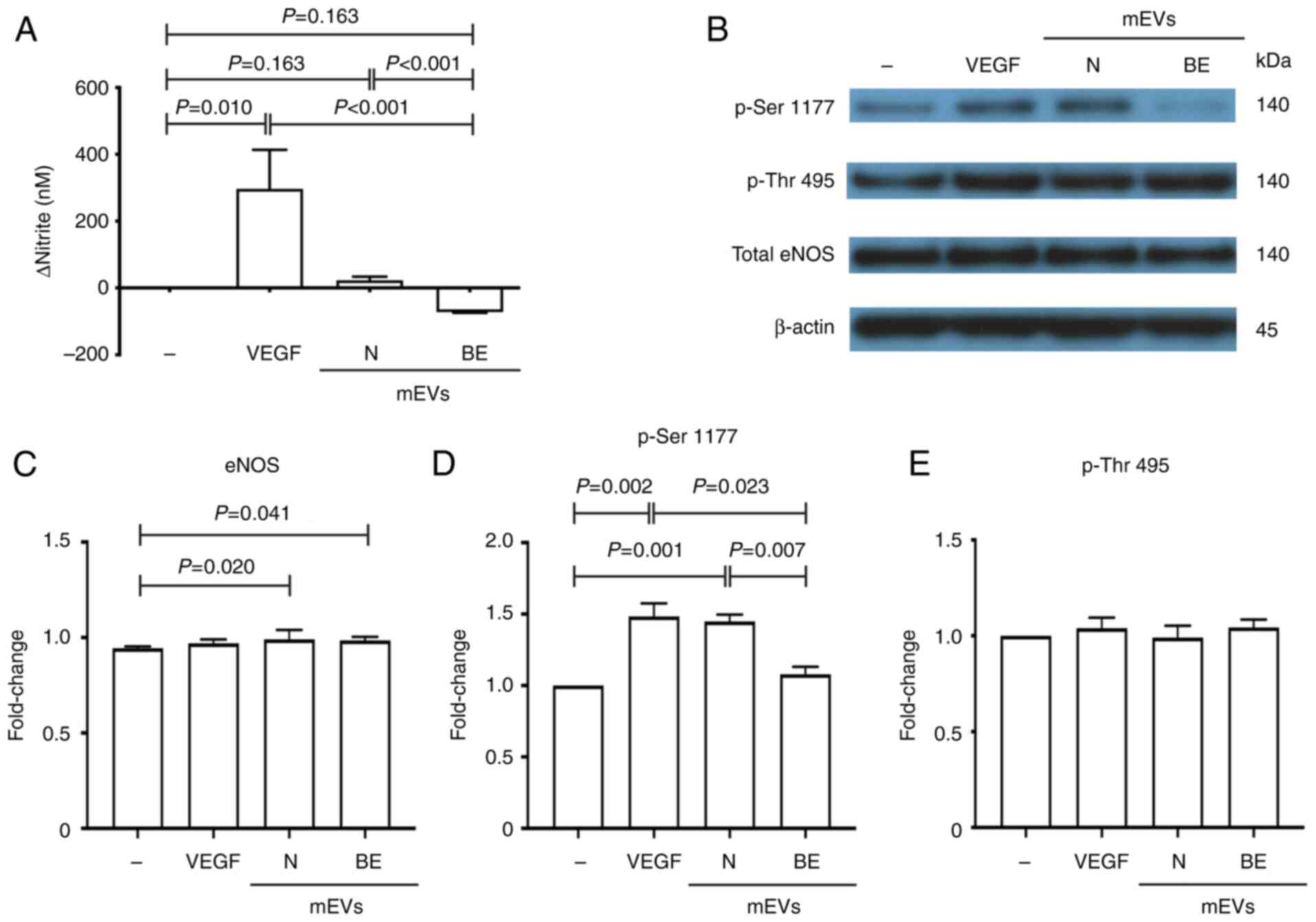
β-Thalassaemia/HbE mEVs decrease NO
production in HPAECs
The difference in nitrite concentrations between
treated and untreated HPAEC samples was determined. HPAECs treated
with healthy donor-mEVs exhibited a significant increase in nitrite
levels in the culture medium (23±10 nM) as compared with untreated
cells. An increase in nitrite was also observed in VEGF-treated
HPAECs (Fig. 1A). Notably, the
nitrite level in HPAECs treated with splenectomised mEVs was
significantly decreased (-72±2 nM) compared with that in the
untreated cells. These results suggested that normal mEVs could
induce NO production, while splenectomised mEVs reduced NO levels
in the culture medium.
β-Thalassaemia/HbE mEVs have no effect
on eNOS expression and activation
The protein levels of total eNOS were not
significantly different among the untreated HPAECs, and those
treated with VEGF, healthy donor-mEVs or splenectomised mEVs
(Fig. 1B and C). This suggested that increased or
decreased NO production by mEVs did not cause changes in the levels
of eNOS expression. eNOS activity is predominantly controlled via
phosphorylation and dephosphorylation. For example, phosphorylation
at Ser1177 activates eNOS, whereas phosphorylation at Thr495
inhibits its function (16).
Consistent with the increased NO production, the level of
phosphorylated eNOS at Ser1177 was significantly increased in
HPAECs treated with VEGF and normal mEVs compared with in untreated
cells (Fig. 1B and D). Notably, the levels of eNOS
phosphorylation at Ser1177 in splenectomised mEVs-treated HPAECs
were not significantly different from those in untreated cells.
Furthermore, eNOS phosphorylation at Thr495 did not exhibit
significant differences among untreated HPAECs, and those treated
with VEGF, healthy donor-mEVs or splenectomised mEVs (Fig. 1B and E). These results suggested that the
reduction of NO in HPAECs treated with splenectomised mEVs was not
caused by phosphorylation at Thr495, a negative regulatory
site.
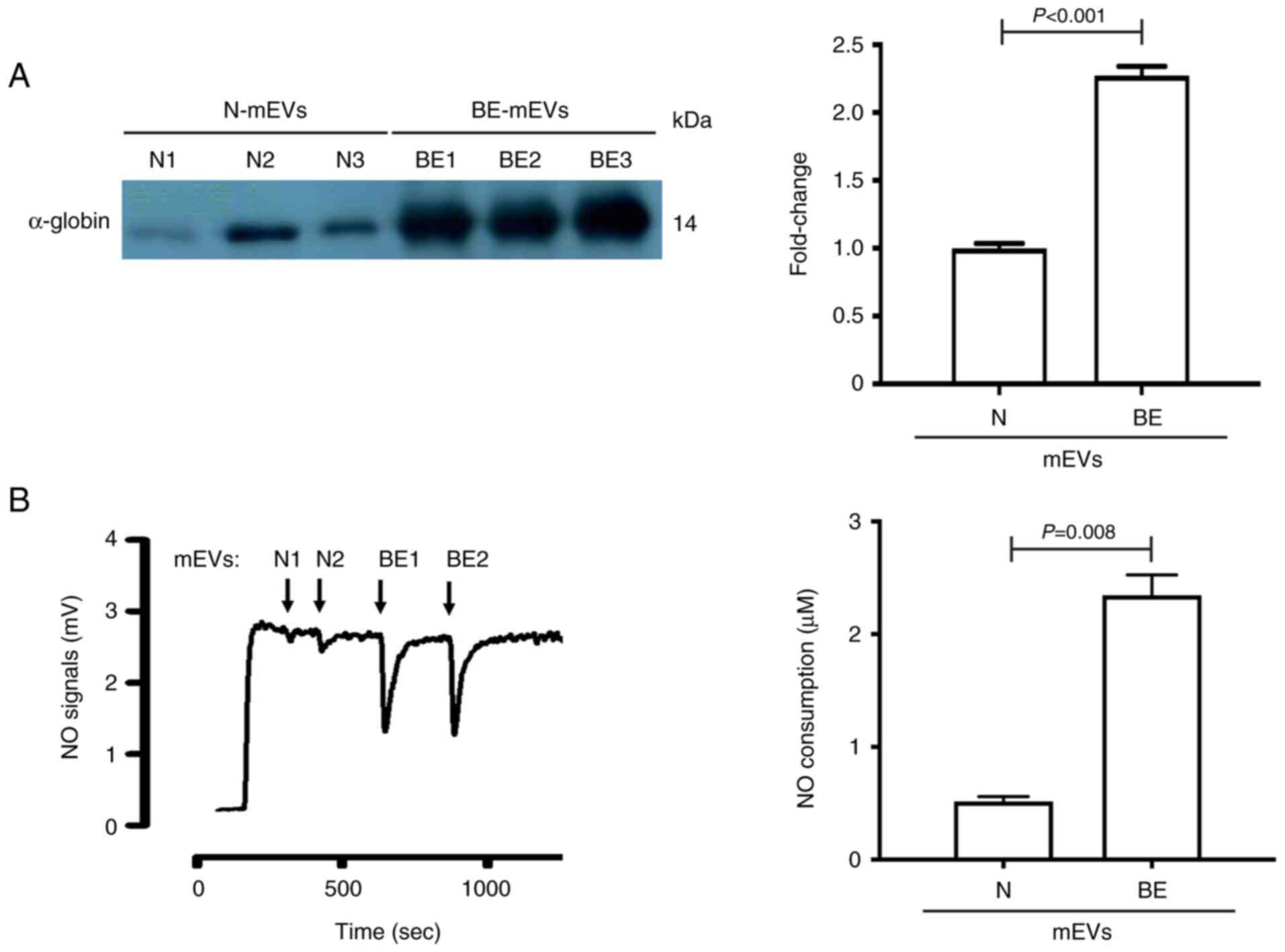
β-Thalassaemia/HbE mEVs can scavenge
NO
Haemoglobin can scavenge NO (17). Additionally, proteomic analysis of
β-thalassaemia/HbE mEVs revealed an increase in haemoglobin in
splenectomised mEVs (7). The
reduction of NO in the culture medium of HPAECs treated with
splenectomised mEVs may be due to NO scavenging. An equal number of
mEVs (5x106 particles) was used for western blot
analysis of α-globin protein content in mEVs, as proteomic analysis
revealed no protein with equal amounts between groups that could be
used as an internal normaliser. The levels of α-globin content in
splenectomised mEVs were significantly higher than those in healthy
donor-mEVs (Fig. 2A), confirming
the previous proteomic analysis performed by the authors. The
increased haemoglobin content in splenectomised mEVs could scavenge
NO. Subsequently, NO scavenging by mEVs was determined by analysing
the NO decay signal (Fig. 2B).
Notably, NO scavenging by splenectomised mEVs (2.27±0.45 µM) was
significantly higher than that by healthy donor-mEVs (0.49±0.08 µM)
(P=0.008).
Discussion
NO is a crucial endothelium-derived molecule that
regulates various vascular functions, including vascular tone,
platelet activation and inflammation. Decreased NO bioavailability,
a marker of vascular dysfunction, is a significant contributing
factor to thrombosis. The present study demonstrated that mEVs
obtained from splenectomised patients with β-thalassaemia/HbE
exhibited elevated haemoglobin content and enhanced NO scavenging,
leading to decreased NO levels.
Clinically, diminished NO production is a hallmark
of endothelial dysfunction, contributing to the development of
atherosclerosis and thrombosis. Individuals with high
altitude-related excessive erythrocytosis (EE) or permanent spinal
cord injury (SCI) are at increased risk of cardiovascular
comorbidities, including endothelial dysfunction, hypertension,
coronary artery disease and thrombosis. NO production has been
significantly reduced in human umbilical vein ECs (HUVECs) treated
with mEVs from individuals with EE or SCI. In both conditions, this
reduction was not due to decreased eNOS expression but was
attributed to impaired eNOS activation, with reduced
phosphorylation at Ser1177 and increased phosphorylation at Thr495
(18,19). By contrast, while mEVs from EE, SCI
and splenectomised β-thalassaemia similarly reduced EC NO
production, the present study revealed that splenectomised mEVs
reduced NO production in HPAECs without affecting eNOS expression
or phosphorylation, suggesting a different mechanism. This
reduction is due to direct NO scavenging by the high haemoglobin
content of splenectomised mEVs, a mechanism distinct from the
impaired eNOS activation observed in EE and SCI, and has important
implications for understanding the unique vascular complications in
patients with β-thalassaemia.
Our proteomic analysis of β-thalassaemia/HbE mEVs
showed an increase in all haemoglobin subunits in splenectomised
mEVs, which was validated in the present study by western blot
analysis of α-globin protein. Haemoglobin in the ferrous redox
state, such as oxyhaemoglobin (HbO2), rapidly and
irreversibly reacts with NO via a dioxygenation reaction, producing
inactive vasodilator nitrate and methaemoglobin (metHb), as
illustrated by the reaction: NO + HbO2 → NO3-
+ metHb. This reaction occurs at a rate ~1,000-fold faster with
cell-free haemoglobin compared with haemoglobin contained within
red blood cells (RBCs). Furthermore, RBC-derived mEVs scavenge NO
in a manner that more closely resembles cell-free haemoglobin, with
a reaction rate only 2.5 to 3-fold slower than that of cell-free
haemoglobin, attributed to their increased surface area compared
with RBCs (17). Blood bank storage
of human RBCs leads to significant haemolysis and the release of
mEVs, resulting in an increased rate of NO scavenging that
increases with the duration of storage. Infusion of plasma from
stored human RBCs into rat circulation can induce notable
vasoconstriction, and this effect is correlated with elevated
levels of haemoglobin-carrying mEVs released during storage
(17).
Plasma NO levels of patients with β-thalassaemia/HbE
are significantly decreased. The flow-mediated dilation, a test
which represents vascular dilation mediated by EC-derived NO
response to the shearing force, has been reported to be
significantly correlated with plasma NO levels in patients with
β-thalassaemia/HbE (6).
Furthermore, decreased local vasodilators, such as NO, and other
factors, including endothelial dysfunction, platelet activation and
a hypercoagulable state, contribute to pulmonary hypertension, a
life-threatening complication in β-thalassaemia. Administration of
inhaled nebulized sodium nitrite can rapidly decrease pulmonary
artery pressure in patients with β-thalassaemia with pulmonary
hypertension, as measured by echocardiography and right heart
catheterization (20).
Additionally, combined treatment of sildenafil and inhaled
nebulized nitrite at 30 mg sodium nitrite twice a day for 12 weeks
resulted in decreased mean pulmonary artery pressure and an
increase in the 6-min walk distance (21). This suggests the importance of NO
bioavailability in patients with β-thalassaemia/HbE. The reduced NO
bioavailability due to increased haemoglobin content in the
splenectomised mEVs could have multiple effects related to
Virchow's triad, endothelial injury, hypercoagulability and stasis
of blood flow. First, reduced NO can contribute to endothelial
dysfunction, as NO plays a crucial role in maintaining endothelial
function by inhibiting platelet activation and inflammation.
Second, low NO levels can exacerbate a hypercoagulable state by
impairing the natural anticoagulant mechanisms and promoting
platelet aggregation. Finally, inadequate NO-induced vasodilation
can lead to stasis of blood flow, further increasing the risk of
clot formation. Collectively, these factors suggest that the
impaired NO bioavailability due to haemoglobin scavenging in
splenectomised mEVs could contribute to thrombosis by influencing
all components of Virchow's triad.
Endothelial dysfunction in patients with
β-thalassaemia is multifactorial, with contributors including iron
overload, oxidative stress, chronic inflammation, reduced NO
bioavailability and increased mEVs. Iron-induced oxidative stress
can damage ECs, reduce NO bioavailability and trigger inflammation.
Increased oxidative stress, as indicated by decreased total
glutathione levels and increased basal production of superoxide
radicals in patients with β-thalassaemia/HbE, has been reported to
be correlated with impaired endothelial function, as demonstrated
by significantly elevated basal forearm blood flow tests (22). Moreover, elevated levels of
pro-inflammatory cytokines, such as TNF-α and IL-6, have been
observed in patients wth β-thalassaemia (23,24).
Circulating mEVs are linked to increased aortic stiffness (25), and promote endothelial expression of
tissue factor, inflammatory cytokines and adhesion molecules
(9).
EVs are associated with the pathology of various
conditions, including genetic diseases, infections, cancer,
metabolic syndrome and traumatic injuries. In type 2 diabetes, EVs
play a crucial role in inter-organ communication, with EVs derived
from organs, such as the liver, adipose tissue and pancreas,
carrying bioactive molecules, including microRNAs and proteins,
that impact insulin signalling pathways and glucose metabolism,
thus contributing to insulin resistance and β-cell dysfunction
(26). EVs also play a dual role in
viral infections by promoting virus dissemination and triggering
immune responses. They facilitate virus-host interactions by
transferring both viral and host-derived proteins and RNAs, a
mechanism observed in several emerging and re-emerging viruses,
including SARS-CoV-2, dengue, Ebola and Zika viral infectious
diseases (27). In traumatic brain
injury, EVs have been shown to induce endothelial dysfunction by
disrupting the endothelial barrier and promoting vascular leakage.
This is mediated by EVs carrying high mobility group box 1, which
triggers a cascade of inflammatory responses. Additionally, vWF on
the surface of EVs facilitates their interaction with ECs, further
amplifying endothelial dysfunction (28). In sickle cell disease, EVs play a
critical role in endothelial activation, promoting RBC adhesion and
contributing to microvascular stasis. RBC-derived EVs have been
shown to activate microvascular ECs, leading to increased vWF
expression and enhanced RBC adhesion under microfluidic conditions
(29).
In splenectomised β-thalassaemia, mEVs play a
significant role in promoting vascular complications. Although no
significant difference has been detected in angiogenesis when
HUVECs are incubated with mEVs from patients with
β-thalassaemia/HbE compared with those from healthy controls
(30), EVs from patients with
β-thalassaemia have been correlated with platelet factor 3-like
activity and prothrombinase complex activity (8). mEVs from splenectomised patients can
induce platelets, leading to platelet activation, platelet
aggregation and platelet-neutrophil aggregation (31). Furthermore, consistent with previous
studies showing that patients with β-thalassaemia/HbE exhibit
endothelial dysfunction, the current study revealed that
splenectomised mEVs induce EC activation, resulting in increased
expression of tissue factor, inflammatory cytokines and adhesion
molecules, promoting leucocyte adhesion to ECs (9). Importantly, the present study further
expands on prior findings by demonstrating that splenectomised mEVs
contribute to EC dysfunction by decreased NO bioavailability in
patients with β-thalassaemia/HbE through direct NO scavenging due
to their high haemoglobin content. This mechanism is similar to
that observed in mEVs from stored human RBCs in blood banks, where
prolonged storage leads to increased hemolysis and a heightened
rate of NO scavenging. However, this mechanism is distinct from the
impaired eNOS activation observed in other conditions, such as
high-altitude-related EE or SCI.
A limitation of the present study was the relatively
small number of patients recruited. However, the variation within
each group was minimal, and statistically significant differences
between the patient and healthy donor groups were observed.
Additionally, the two groups were distinct in their
characteristics. Ethical considerations also guided the decision to
limit the number of participants. Another limitation was the
exclusion of non-splenectomised patients from the study.
Non-splenectomised patients typically exhibit a lower incidence of
thrombotic events, and mEVs isolated from these patients tend to
induce EC activation to a degree between that observed in normal
controls and splenectomised patients. To avoid intermediate data
and to provide a clearer comparison, the current study focused on
the two ends of the spectrum of EC activation, specifically using
mEVs from healthy donors and splenectomised patients. While the
present study demonstrated reduced NO bioavailability, a limitation
was the absence of functional assays, such as endothelial-dependent
vasodilation, endothelial cell migration, or tube formation assays,
to directly assess the physiological impact of these changes.
Although previous studies have shown that mEVs from patients with
β-thalassaemia/HbE do not promote angiogenesis in HUVECs compared
with healthy controls (30) and
that NO donors improve pulmonary hypertension in patients with
β-thalassaemia (20,21), functional assays would have provided
a more comprehensive understanding of the observed NO reduction by
mEVs. The findings in the present study were based on in
vitro models, and further studies using animal models or ex
vivo blood vessels will be necessary to validate these
results.
Future research focusing on elucidating the detailed
molecular mechanisms by which mEVs from splenectomised patients
with β-thalassaemia/HbE induce endothelial dysfunction, and the
downstream effects. This may provide insights into vascular
complications and could shed light on novel alternative therapies.
Furthermore, given the proven benefits of NO donors for patients
with β-thalassaemia with pulmonary hypertension, investigating the
impact of circulating mEV levels on improvements from NO donor
treatment could provide valuable insights into therapeutic
strategies. Exploring the correlation between pulmonary
hypertension improvement and mEV levels following NO donor
treatment is warranted. Additionally, studies targeting mEV
production or uptake could provide new avenues for preventing
vascular complications in β-thalassaemia. Longitudinal studies in
patients would also be beneficial to determine how mEV levels
correlate with disease progression and the risk of thrombotic
events, ultimately aiding in the development of predictive
biomarkers.
In summary, vascular complications, such as
cardiovascular alterations, pulmonary arterial hypertension and
thromboembolic events, are notable complications in splenectomised
patients with β-thalassaemia. It has been previously shown that
splenectomised mEVs are one of the factors that contribute to
vascular complications via several mechanisms. The present study
demonstrated that splenectomised mEVs could decrease NO
bioavailability in the patients. These findings highlight the
significant implications of splenectomised mEVs in the pathogenesis
of thromboembolism in β-thalassaemia disease. The present study
emphasizes the association between splenectomy and thrombotic
complications, reinforcing the need for careful consideration
before performing a splenectomy in these patients.
Supplementary Material
Characterization of mEVs using flow
cytometry. A whole blood sample obtained from a splenectomised
β-thalassaemia/HbE patient was analysed for mEVs using flow
cytometry. (A) The sample was mixed with nano polystyrene beads
(0.79 μm beads in R1 region and 1.32 μm beads in R2
region) to identify the mEV population in the R3 region, the
platelet population in the R4 region, and the RBC population in the
R5 region. (B) The sample was mixed with TruCount beads (R6 region)
to calculate the absolute number of mEVs. (C) Annexin V positive
mEVs were identified in the R7 region. MEVs, medium extracellular
vesicles.
Representative X-ray film of western
blot analysis. (A) Phosphorylated eNOS at Ser1177 (p-Ser1177),
phosphorylated eNOS at Thr495 (p-Thr-495), total eNOS and b-actin
in untreated HPAECs and HPAECs treated with either VEGF, mEVs from
healthy subject (N), or from β-thalassaemia/HbE patient (BE),
respectively. A single membrane was used to probe for eNOS,
phosphorylated eNOS (at p-Ser1177 and p-Thr-495), and b-actin. The
membrane was cut to separately analyse eNOS (140 kD) and β-actin
(45 kD). After probing for total eNOS, the eNOS section of the
membrane was stripped and sequentially re-probed for p-Ser1177,
followed by stripping and re-probing for p-Thr-495. The β-actin
section of the membrane was similarly stripped and re-probed each
time after probing for total eNOS and each phosphorylated eNOS
form. Both sections were then detected together in the final
analysis. (B) α-globin in mEVs from healthy subjects (n=3) and from
β-thalassaemia/HbE patients (n=3). ENOS, endothelial nitric oxide
synthase; HPAECs, human pulmonary artery ECs; mEVs, medium
extracellular vesicles; N, healthy subjects; BE, β-thalassaemia/HbE
patient.
List of antibodies.
Acknowledgements
The authors give special thanks to Professor Duncan
R. Smith (Molecular Pathology Laboratory, Institute of Molecular
Biosciences, Mahidol University, Nakhon Pathom, Thailand) for his
valuable comments and proof reading.
Funding
Funding: The present study was supported by Mahidol University
(grant no. MRC-MGR 01/2565); the National Research Council of
Thailand (NRCT) and Mahidol University (grant no. N42A650349); the
NRCT: High-Potential Research Team Grant Program (grant no.
N42A650870); the NRCT (grant no. N42A670732); the Office of the
Permanent Secretary, Ministry of Higher Education, Science,
Research and Innovation, Thailand Science Research and Innovation
and Mahidol University (grant no. RGNS 64-166); and the Royal
Golden Jubilee Ph.D. Research Scholarship, the NRCT and the
Thailand Research Fund (TRF) (grant no. PHD/0006/2559; Code no.
4.U.MU/59/C.1.O.XX); and KPh was supported by the Royal Golden
Jubilee Ph.D. Research Scholarship, the NRCT and the TRF (grant no.
PHD/0006/2559; Code no. 4.U.MU/59/C.1.O.XX).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KPh performed the experiments, the analysis and
interpretation of the data and drafting the manuscript. WK and NP
performed the isolation of mEVs, flow cytometry and analysis of the
data. TS performed the NO analysis. KPai and SF contributed to
specimen collection. KPat and NS contributed to the concept of the
present study and interpretation of the data. PC contributed to the
concept of the present study, design of the experiments, analysis
and interpretation of the data, and drafting the manuscript. SS was
the principal investigator and takes primary responsibility for the
concept and design of the project, the analysis and interpretation
of the data, drafting and editing the manuscript. All authors read
and approved the final version of the manuscript. SS and PC confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Helsinki Declaration and was approved (approval no.
2014/013.0502) by the Mahidol University Central Institutional
Review Board (Bangkok, Thailand). Written informed consent was
obtained from all individual participants included in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taher AT, Otrock ZK, Uthman I and
Cappellini MD: Thalassemia and hypercoagulability. Blood Rev.
22:283–292. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Taher A, Isma'eel H, Mehio G, Bignamini D,
Kattamis A, Rachmilewitz EA and Cappellini MD: Prevalence of
thromboembolic events among 8,860 patients with thalassaemia major
and intermedia in the Mediterranean area and Iran. Thromb Haemost.
96:488–491. 2006.PubMed/NCBI
|
|
3
|
Butthep P, Bunyaratvej A, Funahara Y,
Kitaguchi H, Fucharoen S, Sato S and Bhamarapravati N: Alterations
in vascular endothelial cell-related plasma proteins in
thalassaemic patients and their correlation with clinical symptoms.
Thromb Haemost. 74:1045–1049. 1995.PubMed/NCBI
|
|
4
|
Butthep P, Bunyaratvej A, Funahara Y,
Kitaguchi H, Fucharoen S, Sato S and Bhamarapravati N: Possible
evidence of endothelial cell activation and disturbance in
thalassemia: An in vitro study. Southeast Asian J Trop Med Public
Health. 28 (Suppl 3):S141–148A. 1997.PubMed/NCBI
|
|
5
|
Vinchi F, Sparla R, Passos ST, Sharma R,
Vance SZ, Zreid HS, Juaidi H, Manwani D, Yazdanbakhsh K, Nandi V,
et al: Vasculo-toxic and pro-inflammatory action of unbound
haemoglobin, haem and iron in transfusion-dependent patients with
haemolytic anaemias. Br J Haematol. 193:637–658. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Satitthummanid S, Uaprasert N, Songmuang
SB, Rojnuckarin P, Tosukhowong P, Sutcharitchan P and Srimahachota
S: Depleted nitric oxide and prostaglandin E2 levels are
correlated with endothelial dysfunction in β-thalassemia/HbE
patients. Int J Hematol. 106:366–374. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Phongpao K, Pholngam N, Chokchaichamnankit
D, Nuamsee K, Praneetponkang R, Ounjai P, Paiboonsukwong K,
Siwaponanan P, Pattanapanyasat K, Svasti J, et al: Proteomic
profiling of circulating β-thalassaemia/haemoglobin E
extra-cellular vesicles reveals that association with
immunoglobulin induces membrane vesiculation. Br J Haematol.
204:2025–2039. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pattanapanyasat K, Gonwong S, Chaichompoo
P, Noulsri E, Lerdwana S, Sukapirom K, Siritanaratkul N and
Fucharoen S: Activated platelet-derived microparticles in
thalassaemia. Br J Haematol. 136:462–471. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kheansaard W, Phongpao K, Paiboonsukwong
K, Pattanapanyasat K, Chaichompoo P and Svasti S: Microparticles
from β-thalassaemia/HbE patients induce endothelial cell
dysfunction. Sci Rep. 8(13033)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kato GJ and Taylor JG VI: Pleiotropic
effects of intravascular haemolysis on vascular homeostasis. Br J
Haematol. 148:690–701. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chaichompoo P, Kumya P, Khowawisetsut L,
Chiangjong W, Chaiyarit S, Pongsakul N, Sirithanaratanakul N,
Fucharoen S, Thongboonkerd V and Pattanapanyasat K:
Characterizations and proteome analysis of platelet-free
plasma-derived microparticles in β-thalassemia/hemoglobin E
patients. J Proteomics. 76 Spec No.:239–250. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles.
7(1535750)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Welsh JA, Van Der Pol E, Arkesteijn GJA,
Bremer M, Brisson A, Coumans F, Dignat-George F, Duggan E, Ghiran
I, Giebel B, et al: MIFlowCyt-EV: A framework for standardized
reporting of extracellular vesicle flow cytometry experiments. J
Extracell Vesicles. 9(1713526)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Feliers D, Chen X, Akis N, Choudhury GG,
Madaio M and Kasinath BS: VEGF regulation of endothelial nitric
oxide synthase in glomerular endothelial cells. Kidney Int.
68:1648–1659. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Suvachananonda T, Wankham A, Srihirun S,
Tanratana P, Unchern S, Fucharoen S, Chuansumrit A, Sirachainan N
and Sibmooh N: Decreased nitrite levels in erythrocytes of children
with β-thalassemia/hemoglobin E. Nitric Oxide. 33:1–5.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837,
837a-837d. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Donadee C, Raat NJ, Kanias T, Tejero J,
Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, et al:
Nitric oxide scavenging by red blood cell microparticles and
cell-free hemoglobin as a mechanism for the red cell storage
lesion. Circulation. 124:465–476. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brewster LM, Bain AR, Garcia VP, Fandl HK,
Stone R, DeSouza NM, Greiner JJ, Tymko MM, Vizcardo-Galindo GA,
Figueroa-Mujica RJ, et al: Global REACH 2018: Dysfunctional
extracellular microvesicles in Andean highlander males with
excessive erythrocytosis. Am J Physiol Heart Circ Physiol.
320:H1851–H1861. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brewster LM, Coombs GB, Garcia VP, Hijmans
JG, DeSouza NM, Stockelman KA, Barak OF, Mijacika T, Dujic Z,
Greiner JJ, et al: Effects of circulating extracellular
microvesicles from spinal cord-injured adults on endothelial cell
function. Clin Sci (Lond). 134:777–789. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yingchoncharoen T, Rakyhao T, Chuncharunee
S, Sritara P, Pienvichit P, Paiboonsukwong K, Sathavorasmith P,
Sirirat K, Sriwantana T, Srihirun S and Sibmooh N: Inhaled
nebulized sodium nitrite decreases pulmonary artery pressure in
β-thalassemia patients with pulmonary hypertension. Nitric Oxide.
76:174–178. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sasiprapha T, Pussadhamma B, Sibmooh N,
Sriwantana T, Pienvichit P, Chuncharunee S and Yingchoncharoen T:
Efficacy and safety of inhaled nitrite in addition to sildenafil in
thalassemia patients with pulmonary hypertension: A 12-week
randomized, double-blind placebo-controlled clinical trial. Nitric
Oxide. 120:38–43. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kukongviriyapan V, Somparn N, Senggunprai
L, Prawan A, Kukongviriyapan U and Jetsrisuparb A: Endothelial
dysfunction and oxidant status in pediatric patients with
hemoglobin E-beta thalassemia. Pediatr Cardiol. 29:130–135.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aggeli C, Antoniades C, Cosma C,
Chrysohoou C, Tousoulis D, Ladis V, Karageorga M, Pitsavos C and
Stefanadis C: Endothelial dysfunction and inflammatory process in
transfusion-dependent patients with beta-thalassemia major. Int J
Cardiol. 105:80–84. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Siriworadetkun S, Thubthed R, Thiengtavor
C, Paiboonsukwong K, Khuhapinant A, Fucharoen S, Pattanapanyasat K,
Vadolas J, Svasti S and Chaichompoo P: Elevated levels of
circulating monocytic myeloid derived suppressor cells in
splenectomised β-thalassaemia/HbE patients. Br J Haematol.
191:e72–e76. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tantawy AA, Adly AA, Ismail EA and Habeeb
NM: Flow cytometric assessment of circulating platelet and
erythrocytes microparticles in young thalassemia major patients:
Relation to pulmonary hypertension and aortic wall stiffness. Eur J
Haematol. 90:508–518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Carciero L, Di Giuseppe G, Di Piazza E,
Parand E, Soldovieri L, Ciccarelli G, Brunetti M, Gasbarrini A,
Nista EC, Pani G, et al: The interplay of extracellular vesicles in
the pathogenesis of metabolic impairment and type 2 diabetes.
Diabetes Res Clin Pract. 216(111837)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Athira AP, Sreekanth S, Chandran A and
Lahon A: Dual role of extracellular vesicles as orchestrators of
emerging and reemerging virus infections. Cell Biochem Biophys: Sep
3, 2024 (Epub ahead of print).
|
|
28
|
Li L, Li F, Bai X, Jia H, Wang C, Li P,
Zhang Q, Guan S, Peng R, Zhang S, et al: Circulating extracellular
vesicles from patients with traumatic brain injury induce
cerebrovascular endothelial dysfunction. Pharmacol Res.
192(106791)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
An R, Man Y, Cheng K, Zhang T, Chen C,
Wang F, Abdulla F, Kucukal E, Wulftange WJ, Goreke U, et al: Sickle
red blood cell-derived extracellular vesicles activate endothelial
cells and enhance sickle red cell adhesion mediated by von
Willebrand factor. Br J Haematol. 201:552–563. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Atipimonpat A, Siwaponanan P, Khuhapinant
A, Svasti S, Sukapirom K, Khowawisetsut L and Pattanapanyasat K:
Extracellular vesicles from thalassemia patients carry
iron-containing ferritin and hemichrome that promote cardiac cell
proliferation. Ann Hematol. 100:1929–1946. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Klaihmon P, Phongpao K, Kheansaard W,
Noulsri E, Khuhapinant A, Fucharoen S, Morales NP, Svasti S,
Pattanapanyasat K and Chaichompoo P: Microparticles from
splenectomized β-thalassemia/HbE patients play roles on
procoagulant activities with thrombotic potential. Ann Hematol.
96:189–198. 2017.PubMed/NCBI View Article : Google Scholar
|