Introduction
Cresols are organic compounds, also known as
methylphenols, which have various biological effects:
Dinitro-o-cresol induces cell death but does not change oxidase
expression in soybean cells (1) and
causes cytotoxicity via apoptosis in LNCaP prostate cancer cells
(2). Additionally,
3-methyl-4-nitrophenol induces toxic effects in rat renal tubular
epithelial cells (3). Para
(p)-cresol is found in various essential oils from plants such as
cloves, cinnamon and basil, contributing to their unique scent
(4,5). p-Cresol has been shown to affect
proliferation, viability, differentiation and glucose uptake in
3T3-L1 adipocytes (6) and induce
cytotoxicity in polymorphonuclear cells (7). Furthermore, p-cresol is rapidly
absorbed and excreted, primarily into urine as conjugate
metabolites in rats (8). However,
it is still unclear how p-cresol affects physiology in human
glioblastoma.
Changes in concentration of calcium ions
(Ca2+) inside cells, known as intracellular
Ca2+ concentration ([Ca2+]i), play
a crucial role in regulating various cell processes associated with
cell death (9,10). Cells use several mechanisms to
regulate [Ca2+]i both globally and at the
subcellular level. One of the mechanisms involves G-protein-coupled
receptors, which activate phospholipase C (PLC) to release
Ca2+ from intracellular stores and influence
Ca2+ entry across the plasma membrane (9,10).
Understanding the mechanisms underlying compound-induced increases
in intracellular Ca2+ is crucial to comprehend the
biological effects on the cell.
To the best of our knowledge, there is a scarcity of
literature that discusses the effect of cresol-related compounds in
Ca2+ signaling. 4-chloro-m-cresol is the most studied
cresol and is commonly used as an inhibitor of sarcoendoplasmic
reticulum calcium ATPase Ca2+ pumps (11,12).
Non-specific effects of 4-chloro-m-cresol may cause Ca2+
flux and respiratory burst in human neutrophils (13). Additionally, 4-chloro-m-cresol
increases myoplasmic free Ca2+ concentration and force
of contraction in mouse skeletal muscle (14) and inhibits voltage-gated potassium
(K+) channels at the rat calyx of Held (15). Since p-cresol has a similar
structure to 4-chloro-m-cresol, p-cresol may affect Ca2+
homeostasis in cell models.
p-Cresol has been implicated in various pathological
conditions due to its cytotoxic effects on different cell types,
such as 3T3-L1 adipocytes (6) and
polymorphonuclear cells (7). To the
best of our knowledge, however, no studies have examined its impact
on the cytotoxicity in human glioma cells. Glioma, particularly
glioblastoma multiforme (GBM), is known for its aggressive nature
and poor prognosis (16-18).
The 5-year survival rate for GBM is less than 10%, with a median
survival time of approximately 15 months following diagnosis
(16-18).
The incidence of GBM varies by region, but it is generally reported
at 3-4 cases/100,000 individuals annually (16-18).
This data highlights the urgent need for effective therapeutic
strategies to improve outcomes for patients. Understanding the
molecular and cellular mechanisms underlying glioma pathophysiology
has driven extensive research (16-18).
Exploring environmental and metabolic factors influencing glioma
progression is key for identifying novel therapeutic targets.
Understanding how p-cresol influences glioma cell
biology may reveal novel insights into the environmental factors
contributing to glioma progression, as well as potential biomarkers
for glioma prognosis and therapeutic targets. Prevalence of glioma
has gradually increased, with current incidence rates reported at
~6 cases per 100,000 individuals annually in North America and
Europe over the past decade (16-18).
Despite advancements in surgical techniques, radiation therapy and
chemotherapy, the overall efficacy of current treatment options
remains limited, particularly for aggressive forms such as GBM
(16-18).
Given these challenges, exploring novel therapeutic avenues,
including the impact of essential oil components such as p-cresol,
could have practical applications in improving glioma treatment
outcomes. This may contribute to developing more effective
strategies for managing glioma progression and improving patient
outcomes.
Ca2+ signaling serves a key role in
various cellular processes, including proliferation, migration and
apoptosis. Because these processes contribute to tumor growth,
invasion, and resistance to treatment, they are particularly
relevant in cancer biology (9,10). In
glioblastoma, dysregulated Ca2+ signaling is implicated
in tumor progression and resistance to therapy. Elevated
intracellular Ca2+ levels activate various downstream
pathways, leading to changes in gene expression, metabolic activity
and cell behavior (9,10). PLC is a key enzyme in the
Ca2+ signaling pathway, catalyzing hydrolysis of
phosphatidylinositol 4,5-bisphosphate (PIP2) to generate
inositol 1,4,5-trisphosphate (IP3) and diacylglycerol
(DAG). IP3 binds to its receptors on the endoplasmic
reticulum, leading to release of Ca2+ into the
cytoplasm. When agonists activate cells, PLC is stimulated, leading
to the breakdown of PIP2 into IP3 and DAG
(9). Increased DAG concentration
activates protein kinase C (PKC), while IP3 binds
IP3 receptor in the endoplasmic reticulum, causing
Ca2+ release from internal stores (9). This increase in
[Ca2+]i triggers downstream signaling
pathways that contribute to glioblastoma cell survival and
proliferation (9,10). Understanding the specific roles of
PLC isoforms in Ca2+ signaling may reveal novel
therapeutic targets for glioblastoma. Targeting dysregulated
PLC-mediated pathways may enhance the efficacy of existing
treatments or lead to the development of novel therapeutic
strategies (9,10).
Although p-cresol has been shown to promote
blood-brain barrier (BBB) integrity and cross BBB in vivo
(19), it is unknown how p-cresol
affects [Ca2+]i in human glioblastoma. The
present study aimed to investigate this effect using DBTRG-05MG
human glioblastoma cells, a commonly used model for glioblastoma
research (20-22).
Fluorescent Ca2+-sensitive dye fura-2-AMwas used to
measure changes in [Ca2+]i in response to
p-cresol. Additionally, the study explored the effect of p-cresol
on cell viability.
Materials and methods
Chemicals
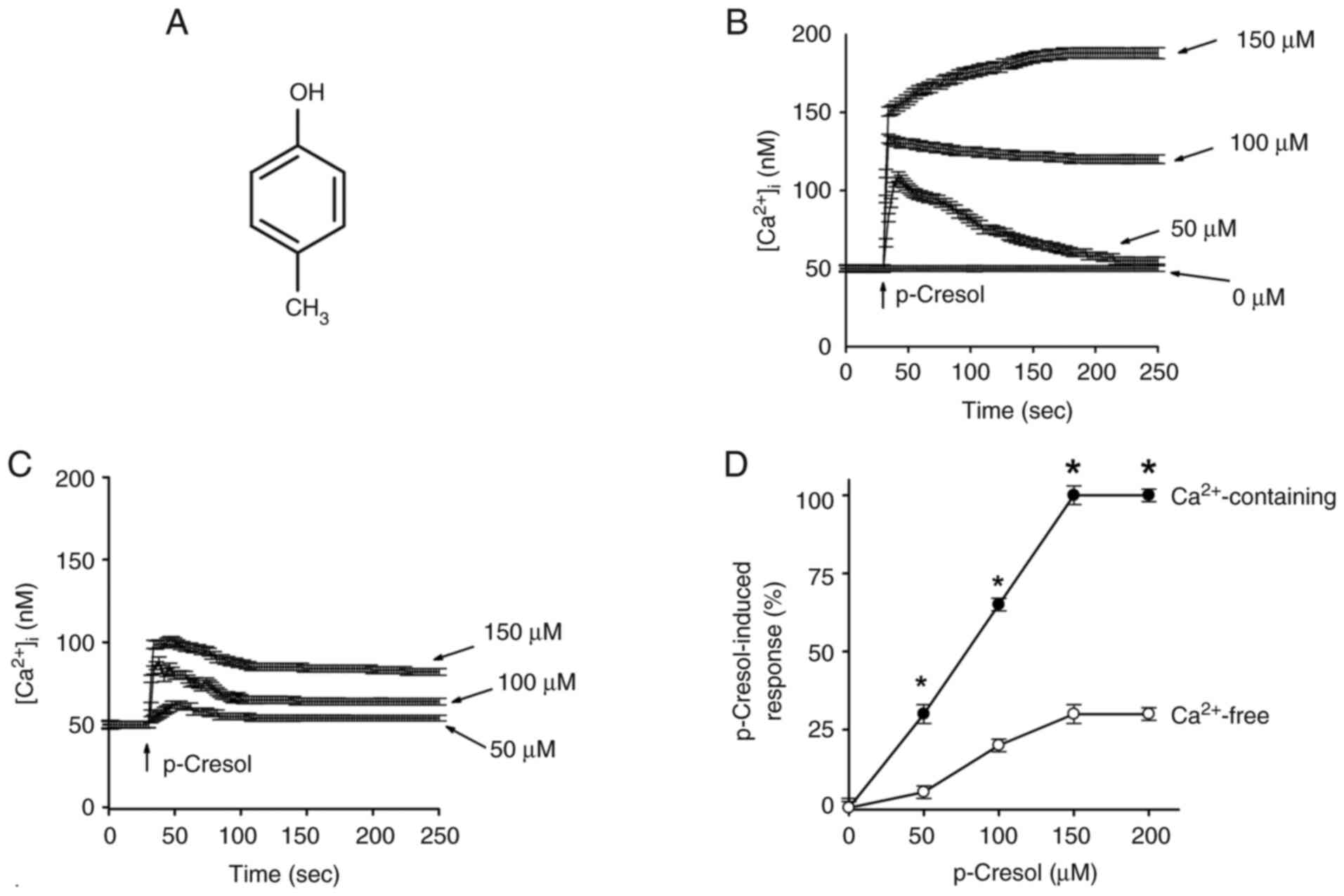
p-Cresol (Fig. 1A),
WST-1, nifedipine (voltage-gated Ca2+ channel blocker),
SKF96365 (store-operated Ca2+ entry modulator),
GF109203X (a PKC inhibitor), thapsigargin (an inhibitor of the
endoplasmic reticulum Ca2+ pump), ATP, and U73122 (a PLC
inhibitor) were obtained from Sigma-Aldrich (Merck KGaA).
Fura-2-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N'N'-tetraacetic acid
(BAPTA-AM), and 2-aminoethoxydiphenyl borate (2-APB) were obtained
from Molecular Probes. Finally, the reagents for cell culture,
including RPMI-1640 medium, fetal bovine serum (FBS), penicillin,
and streptomycin, were obtained from Gibco (Thermo Fisher
Scientific, Inc.).
Cell culture
DBTRG-05MG human glioblastoma cells from Bioresource
Collection and Research Center (Taiwan) were cultured in RPMI-1640
medium with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100
µg/ml streptomycin, all obtained from Gibco (Thermo Fisher
Scientific, Inc.), at 37˚C in a humidified atmosphere containing 5%
carbon dioxide (CO2).
Solutions used in
[Ca2+]i measurements
The Ca2+-containing medium at pH 7.4
contained the following components and concentrations: NaCl (140
mM), KCl (5 mM), MgCl2 (1 mM), CaCl2 (2 mM),
HEPES (10 mM), and glucose (10 mM). The Ca2+-free medium
had the same components, with CaCl2 replaced by 2 mM
EGTA. p-Cresol was dissolved in ethanol to make a 0.1 M stock
solution. Other chemicals were dissolved in water, ethanol or DMSO.
The impact of organic solvent concentration (≤0.1%) on cell
viability and basal [Ca2+]i was determined
through control experiments. Cells were exposed to the same
concentration of the solvent (without p-cresol or other
treatments).
[Ca2+]i
measurement
The cells were grown to 80-90% confluence on 6 cm
dishes, trypsinized, and suspended in Ca2+-containing or
free medium at a concentration of 1x106 cells/ml. The
seeding density for subsequent experiments was 1x105
cells/well in 24-well plates or 5x105 cells/well in
6-well plates. Following addition of 2 µM fura-2-AM for 30 min at
25˚C, cells were washed twice with Ca2+-containing
medium before being suspended in Ca2+-containing medium
at a concentration of 1x107 cells/ml. Fura-2-AM
fluorescence measurements were performed in a water-jacketed
cuvette at 25˚C. The cuvette contained 1 ml medium and
5x105 cells. The fluorescence was monitored with a
Shimadzu RF-5301PC spectrofluorophotometer. For calibration of
[Ca2+]i, Triton X-100 (0.1%) and
CaCl2 (5 mM) were added to obtain the maximal fura-2
fluorescence. Ca2+ chelator EGTA (10 mM) was added to
chelate Ca2+ to obtain the minimal fura-2 fluorescence.
The fura-2-loaded cells were excited alternately at 340 and 380 nm
and emission was recorded at 510 nm. The fluorescence ratio
(F340/F380) was used to estimate [Ca2+]i. The
fluorescence ratio was calibrated using standard solutions with
known Ca2+ concentrations and ionophore treatments to
determine minimum and maximum fluorescence ratios (23). EC50 (half-maximal
effective concentration) was calculated. Hill equation was used to
describe dose-response relationships.
SKF96365 (5 µM) and 2-APB (20 µM), store-operated
Ca2+ entry modulators, and GF109203X (2 µM), a PKC
inhibitor, were applied at 37˚C throughout the experiment assessing
p-cresol-induced changes in [Ca2+]i.
Nifedipine, a voltage-gated Ca2+ channel blocker, was
used at a concentration of 10 µM, Thapsigargin (1 µM), ATP (10 µM)
as a PLC agonist, U73122 (2 µM) as a PLC inhibitor, and U73343 (2
µM), an inactive analog of U73122, were all included in the
experiments to evaluate their effects on intracellular
Ca2+ dynamics. All treatments were conducted at a
temperature of 37˚C and the duration of each treatment was 30
sec.
Mn2+ measurement
The experiment involved quenching of fura-2
fluorescence by Mn2+ in medium containing
Ca2+ and 50 µM MnCl2. MnCl2 was
added to the cell suspension in the cuvette 30 sec before
fluorescence recording. Data was recorded at an excitation signal
of 360 nm (Ca2+-insensitive) and an emission signal of
510 nm at 1-sec intervals, as previously described (24).
Cell viability assay
Cell viability assay was conducted following the
manufacturer's instructions. Following treatment with p-cresol at
50, 100, 150, 200 and 250 µM at 37˚C for 24 h, WST-1 (10 µM) was
added and cells were incubated at 37˚C for 30 min. Cells were
treated with 5 µM BAPTA-AM for 1 h at 37˚C before incubating with
p-cresol. The absorbance was determined using an ELISA reader at a
wavelength of 450 nm, with a reference wavelength of 620 nm for
background correction. In the control, cells treated with the
vehicle (organic solvent at ≤0.1%) without p-cresol or BAPTA-AM
were used as the baseline for normalization.
Statistical analysis
Data were analyzed using GraphPad Prism version 9.0
(GraphPad Software, Inc.; Dotmatics). Statistical significance was
determined using one-way ANOVA followed by Tukey's multiple
comparison post hoc test. Tests for normality (Shapiro-Wilk test)
and homogeneity of variances (Levene's test) were performed to
ensure the assumptions of ANOVA were met. Data are presented as the
mean ± SD of three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
p-Cresol increases
[Ca2+]i
In Ca2+-containing and -free medium,
p-cresol at concentrations between 50 and 150 µM increased
[Ca2+]i in a concentration-dependent manner
(Fig. 1B and C). The cells were found to have a
viability >95% after 20 min. The cell viability of >95% was
determined using the trypan blue exclusion assay. Control
experiments confirmed that the organic solvent at a concentration
≤0.1% did not impact cell viability or basal
[Ca2+]i (data not shown). EC50
value was 70±2 in a Ca2+-containing and 70±3 µM in
Ca2+-free medium by fitting to a Hill equation (Fig. 1D).
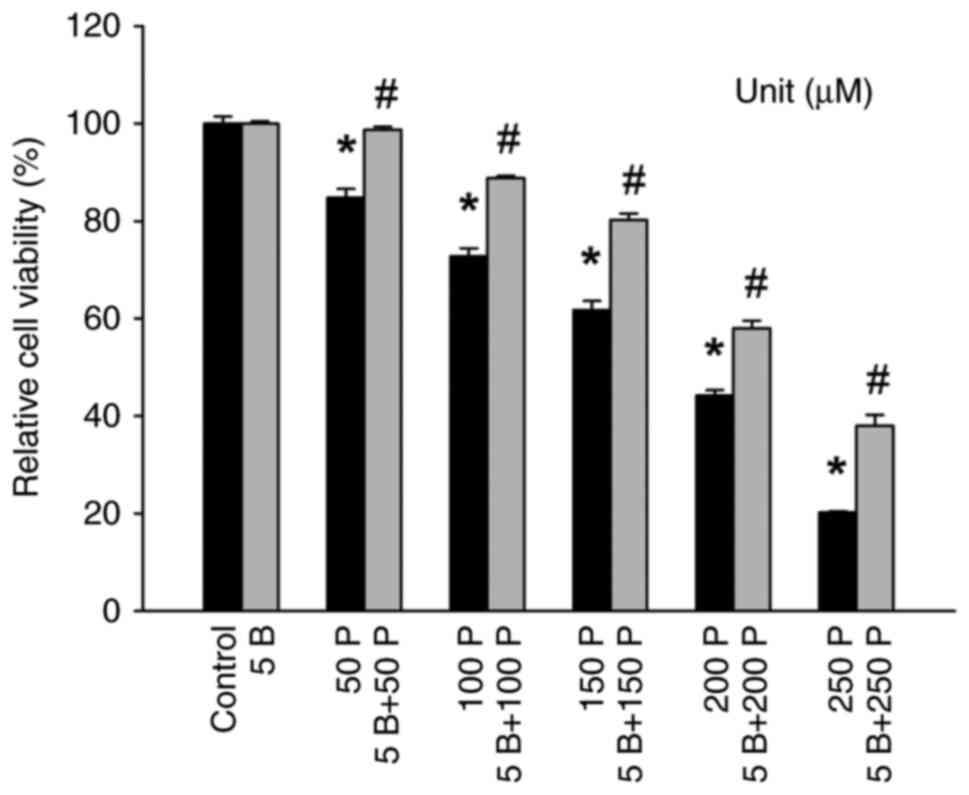
Effect of BAPTA-AM on reversing
p-cresol-induced cell death
Following exposure of DBTRG-05MG cells to p-cresol,
a significant and long-lasting increase in
[Ca2+]i was observed (Fig. 1). Since unregulated
[Ca2+]i can impact cell viability (9,10), the
effect of p-cresol on cell viability was investigated. There was a
decrease in cell viability in the presence of 50-250 µM p-cresol
(Fig. 2). The intracellular
Ca2+ chelator BAPTA-AM (25) was used to prevent
[Ca2+]i increases during p-cresol treatment.
Treatment with 5 µM BAPTA-AM effectively prevented increases in
cytosolic Ca2+ levels induced by 50-150 µM p-cresol,
indicating successful chelation of intracellular Ca2+.
Additionally, 5 µM BAPTA-AM did not alter the baseline cell
viability, demonstrating its specific action on Ca2+
signaling without affecting overall cell health. BAPTA-AM reversed
p-cresol-induced decreases in cell viability (Fig. 2).
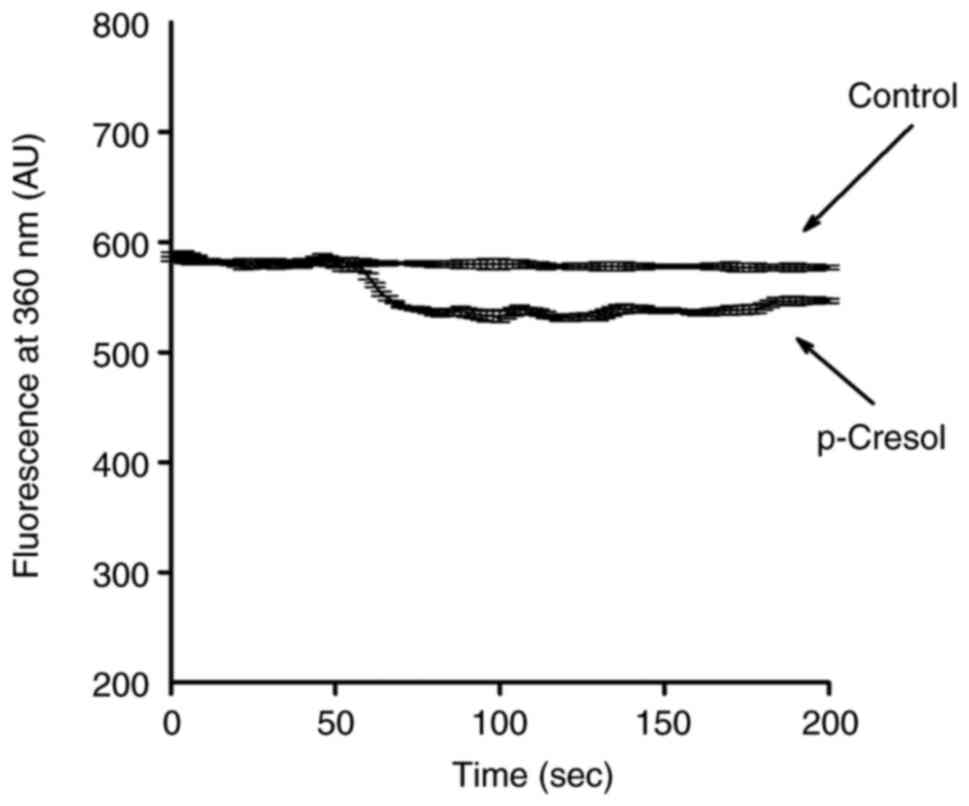
p-Cresol induces Mn2+
influx
Experiments were conducted to confirm that increases
in [Ca2+]i in response to p-cresol involved
influx of Ca2+. Mn2+ enters cells using
mechanisms similar to those of Ca2+ but quenches
fluorescence of the dye fura-2 at all excitation wavelengths
(24). Therefore, quenching of
fura-2 fluorescence when excited at the Ca2+-insensitive
excitation wavelength of 360 nm by Mn2+ suggests
involvement of Ca2+ influx. As the Ca2+
response induced by p-cresol peaked at 150 µM, subsequent
experiments used 150 µM p-cresol as a control; 150 µM p-cresol
triggered an immediate decrease in the 360 nm excitation signal,
reaching a maximum value of 62±2 arbitrary units at 100 sec
(Fig. 3). This indicated
participation of Ca2+ influx in p-cresol-induced
increases in [Ca2+]i.
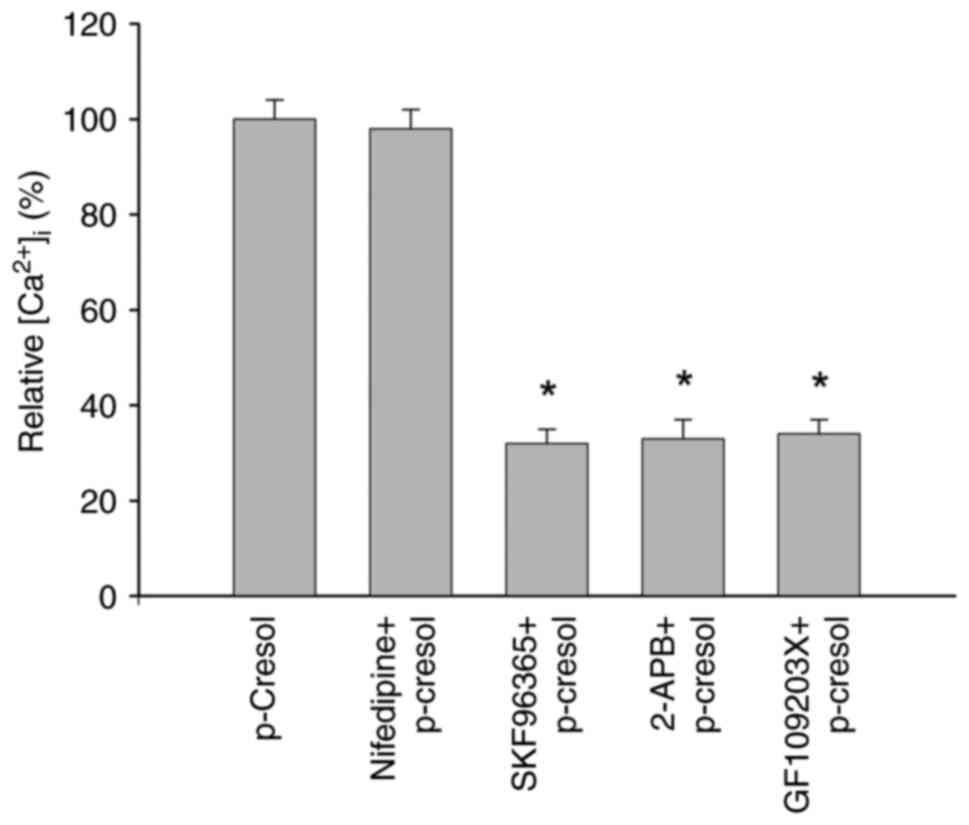
Pathways of p-cresol-induced
Ca2+ entry
Experiments were carried out to investigate the
Ca2+ entry pathway underlying the p-cresol-induced
increases in [Ca2+]i. SKF96365 (5 µM) and
2-APB (20 µM), store-operated Ca2+ entry modulators
(26,27) and GF109203X (2 µM), a PKC inhibitor
(28), inhibited p-cresol-induced
increases in [Ca2+]i by 65-70%, but
nifedipine (a voltage-gated Ca2+ channel blocker, 10 µM)
(29) did not (Fig. 4).
Source of p-cresol-induced
Ca2+ release
The endoplasmic reticulum is the primary
Ca2+ store in most types of cell, including DBTRG-05MG
cells (9,10). Therefore, the present study
investigated the role of the endoplasmic reticulum in
p-cresol-induced Ca2+ release in DBTRG-05MG cells in
Ca2+-free medium to eliminate the involvement of
Ca2+ influx. Addition of 1 µM thapsigargin (30), an inhibitor of the endoplasmic
reticulum Ca2+ pump, resulted in
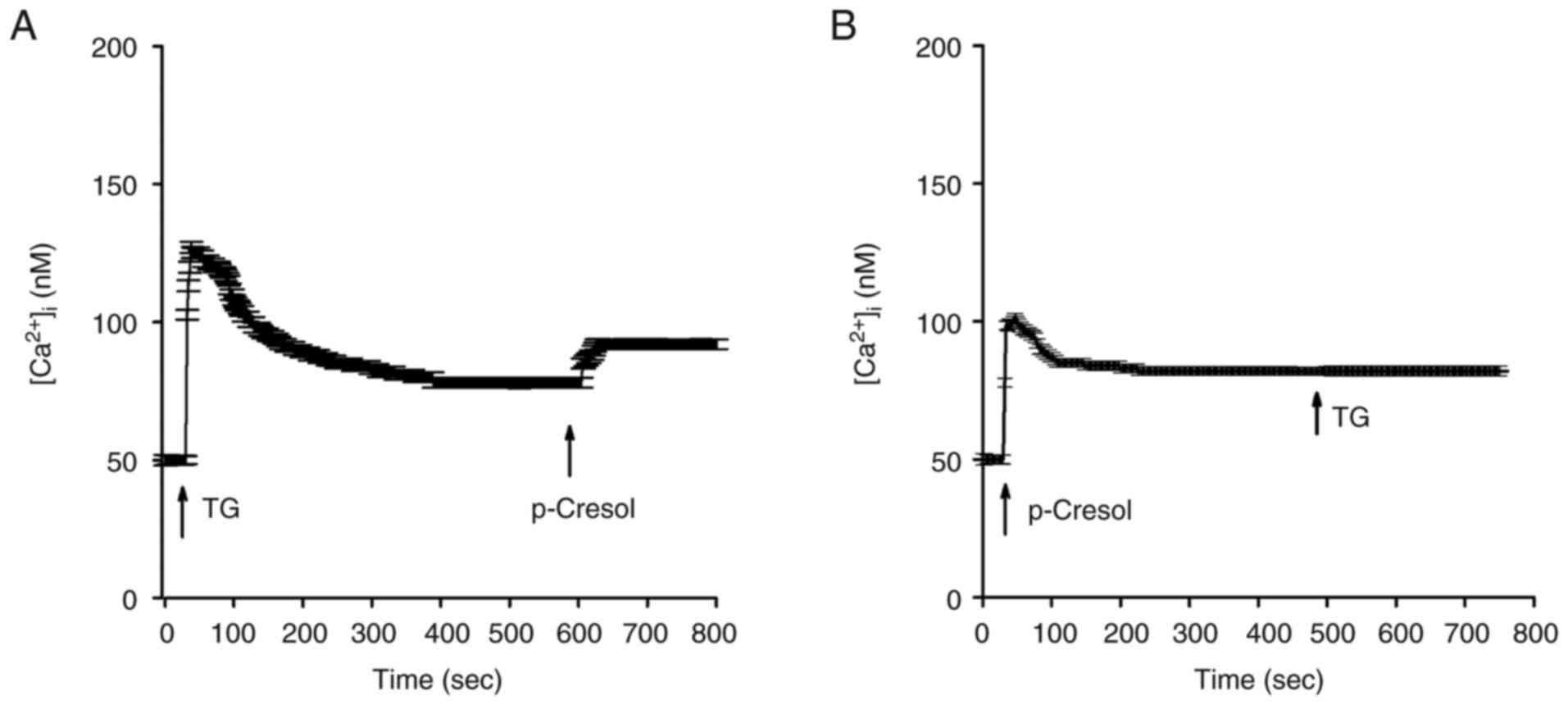
[Ca2+]i rises of 75±2 nM (Fig. 5A). Subsequent addition of 150 µM
p-cresol induced [Ca2+]i rises of 10±2 nM.
Following p-cresol-induced [Ca2+]i rises,
addition of 1 µM thapsigargin at 500 sec failed to induce further
[Ca2+]i rises (Fig. 5B).
Role of PLC in p-cresol-induced
[Ca2+]i rises
The enzyme PLC serves a key role in regulating
release of Ca2+ from Ca2+ stores (9,10). PLC
inhibitor U73122(31) was applied
to investigate if activation of this enzyme was necessary for
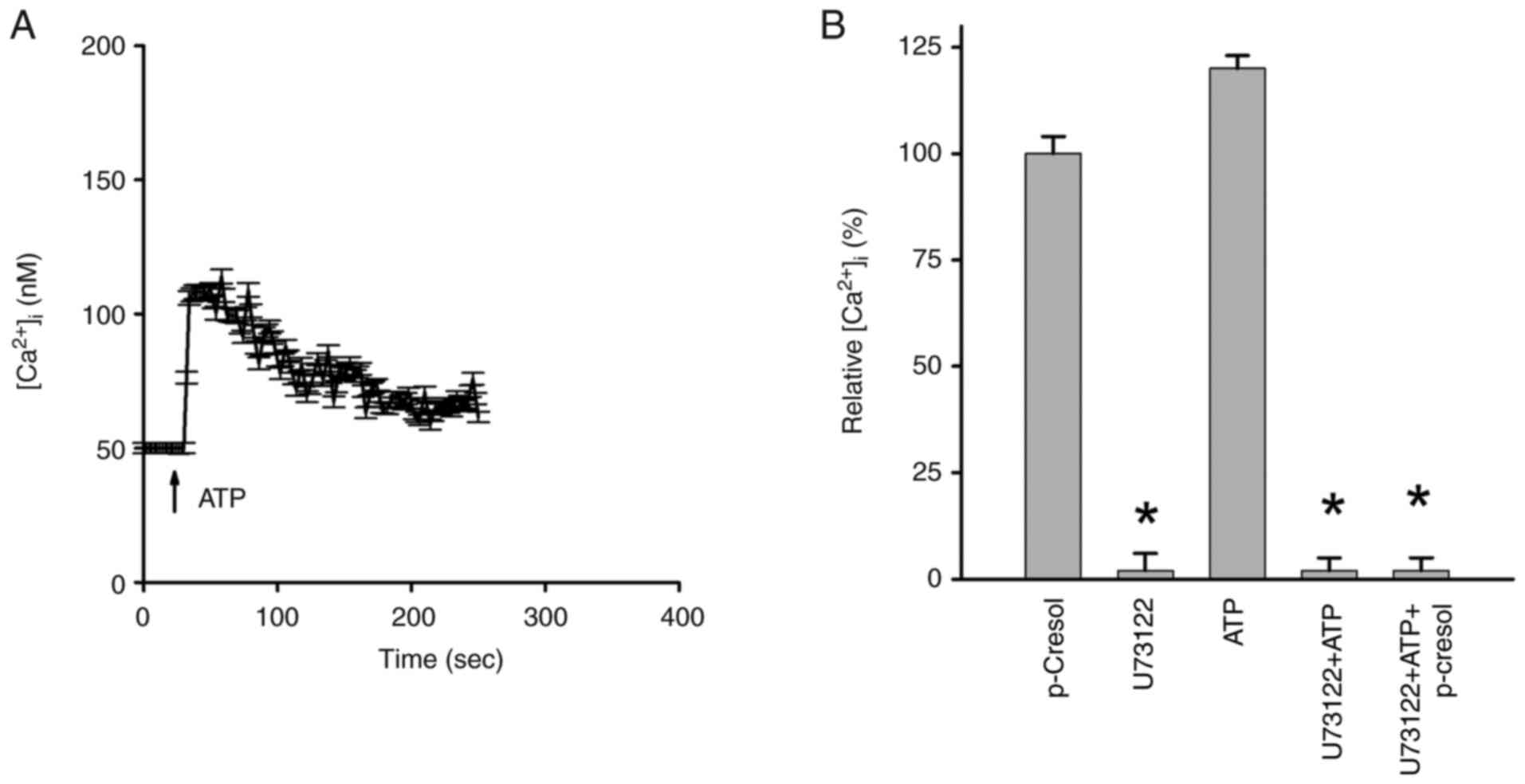
p-cresol-induced Ca2+ release. ATP induced
[Ca2+]i rise of 55±2 nM (Fig. 6A). Since ATP is a PLC-dependent
agonist that induces [Ca2+]i rises in most
types of cell (32), it was used to
study the inhibitory effect of U73122 on PLC activity. Incubation
with 2 µM U73122 did not affect basal [Ca2+]i
but eliminated ATP-induced [Ca2+]i rises,
suggesting effective suppression of PLC activity (Fig. 6B). Additionally, incubation with 2
µM U73122 did not alter basal [Ca2+]i but
abolished 150 µM p-cresol-induced [Ca2+]i
rises. However, U73343 (negative control), an analog of U73122, did
not cause inhibition (data not shown).
Discussion
Ca2+ signaling is key in regulating
physiological processes in human cells, including glioblastoma
cells (9,10). Previous studies have shown that the
cresol-related compound 4-chloro-m-cresol affects Ca2+
homeostasis in different cell models, such as human neutrophils
(13) and mouse skeletal muscle
(14). To the best of our
knowledge, however, the present study is the first to demonstrate
that p-cresol causes [Ca2+]i rises in human
glioblastoma cells. p-Cresol between 50 and 150 µM induced a
concentration-dependent increase in [Ca2+]i.
The mechanism underlying this increase may involve depleting
intracellular Ca2+ stores and causing Ca2+
influx from the extracellular environment. p-Cresol-induced
[Ca2+]i rises are significantly reduced in
the absence of extracellular Ca2+, which suggests that
Ca2+ influx occurred continuously throughout the
stimulation period. This indicates that p-cresol triggers
Ca2+ influx from extracellular sources, likely through
mechanisms such as store-operated Ca2+ entry or other
channels sensitive to changes in membrane potential or receptor
activation.
p-cresol is toxic to glioblastoma cells. Excessive
Ca2+ in cells can lead to detrimental changes in cell
viability, including apoptosis, necrosis, and impaired cellular
functions. These effects occur due to disrupted Ca2+
homeostasis, which can activate enzymes like proteases and
phosphatases, destabilize mitochondrial function, and induce
oxidative stress, ultimately contributing to cell death or
dysfunction (9,10). Ca2+ mobilization causes
Ca2+ influx across the plasma membrane via
store-operated Ca2+ entry (9,10). If
elevation in [Ca2+]i is prolonged, or
regulation of [Ca2+]i is abnormal, it can
lead to cell death. The present data suggest that p-cresol-induced
cell death depended on the rise in [Ca2+]i.
Furthermore, increased [Ca2+]i levels may
affect Ca2+-dependent downstream responses, leading to
changes in cell physiology (for example, activation of
calmodulin-dependent enzymes, modulation of ion channel activity,
regulation of gene expression through Ca2+-responsive
transcription factors like NFAT (nuclear factor of activated
T-cells), and triggering of apoptotic pathways via activation of
caspases (9,10). These responses highlight the
critical role of Ca2+ signaling in regulating cellular
processes such as metabolism, proliferation, and cell fate
determination (9,10).
p-Cresol causes entry of Ca2+ by
stimulating store-operated Ca2+ entry. The depletion of
intracellular Ca2+ stores induces this entry (9,10).
p-cresol-induced [Ca2+]i rises were inhibited
by specific compounds, namely SKF96365 and 2-APB, which are often
used as modulators of store-operated Ca2+ entry in
various types of cell, such as cortical neurons, myofibroblasts, T
lymphocytes, and rat sensory neurons (26,27,33,34).
The present data showed that these modulators inhibited
p-cresol-induced [Ca2+]i rises. On the other
hand, nifedipine, a voltage-gated Ca2+ channel blocker
(20), did not inhibit
p-cresol-induced [Ca2+]i rises, which
suggested that p-cresol-induced Ca2+ entry occurred via
a store-operated Ca2+ pathway.
Ca2+ stores in thapsigargin-sensitive
endoplasmic reticulum serve a dominant role in p-cresol-induced
Ca2+ release. PLC produces IP3 and DAG when
activated, activating PKC (9,10). To
examine the effect of modulation of PKC activity on
p-cresol-induced [Ca2+]i rises, PKC inhibitor
GF109203X was used. PKC is involved in signaling pathways that
regulate Ca2+ dynamics; inhibiting PKC with GF109203X
attenuated or blocked the increase in [Ca2+]i
typically triggered by p-cresol Additionally, the release of
Ca2+ was PLC-dependent, as shown by the abolition of
release when PLC activity was decreased by the inhibitor
U73122.
The present results showed a significant increase
in [Ca2+]i following p-cresol treatment. This
suggested that p-cresol disrupted Ca2+ homeostasis,
potentially by enhancing Ca2+ influx or inhibiting
Ca2+ efflux mechanisms. The increase in Ca2+
levels may be attributed to the activation of store-operated
Ca2+ entry channels or inhibition of
Ca2+-ATPases on the endoplasmic reticulum (9,10).
There was a dose-dependent decrease in cell viability with p-cresol
treatment, which aligned with disruptions in Ca2+
signaling. Dysregulated Ca2+ signaling can lead to
mitochondrial dysfunction, triggering cell death pathways (9,10). The
decrease in cell viability supports this mechanistic pathway, where
p-cresol-induced Ca2+ dysregulation results in cell
death. The present results provide more robust and comprehensive
understanding of how p-cresol affects Ca2+ signaling in
glioma cells.
Exposing cells to 100 µM p-cresol for 24 h can
induce cell death due to its cytotoxic effects. This concentration
exceeds physiological levels typically found in plasma, which are
20-40 µM for cresol compounds in vivo (8,35).
Elevated plasma levels of cresol compounds, especially in patients
with liver or kidney disorders (8,35), may
further increase the concentration of p-cresol beyond normal
physiological ranges, exacerbating its cytotoxic effects. Although
the present study does not focus on liver or kidney disorders, this
information is relevant to understanding the potential cytotoxicity
and biological effects of p-cresol at concentrations that may
exceed physiological levels observed in healthy individuals. This
broader context helps interpret the impact of p-cresol on cellular
systems and provides insights into its potential health
implications under different physiological conditions. The
potential use of p-cresol or its derivatives in treating human
glioblastoma requires further exploration. The effect of p-cresol
should be studied in vivo. Furthermore, since increases in
[Ca2+]i levels affect numerous
Ca2+-coupled cellular processes, these include
activation of calmodulin-dependent enzymes involved in signal
transduction, modulation of ion channel activity influencing
membrane potential and neurotransmitter release, and initiation of
apoptotic pathways through activation of caspases, highlighting the
pivotal role of Ca2+ signaling in regulating diverse
cellular functions (9,10). The effect of p-cresol-induced
[Ca2+]i rises on other cellular responses
requires further exploration.
The present study had a limited sample size, which
may decrease statistical power and generalizability. A small sample
size increases risk of errors, potentially overlooking subtle but
biologically significant effects of p-cresol. Future studies should
increase the sample size to validate the present findings.
Furthermore, the present study utilized in vitro models
(human glioblastoma cell line) to investigate the effects of
p-cresol. In vitro models do not fully replicate the
complexity of in vivo tumor microenvironments, such as
presence of immune cells, extracellular matrix components and
dynamic interactions with other types of cell. While the present
study primarily addressed the short-term impacts, it is necessary
to understand how p-cresol influences cell behavior over extended
periods. Additional experiments should assess long-term effects of
p-cresol on glioblastoma cell survival and proliferation.
Proteomic and transcriptomic analyses should be
performed to uncover the precise molecular targets of p-cresol.
Mass spectrometry-based proteomics can identify protein
modifications and changes in expression in response to p-cresol
treatment. RNA sequencing can reveal gene expression and signaling
pathway alterations. Understanding the specific molecular pathways
p-cresol affects may identify novel therapeutic targets for
glioblastoma treatment. Furthermore, long-term exposure studies
using glioblastoma xenograft models in immunocompromised mice are
required. These studies may provide insight into the chronic
effects of p-cresol and its potential as a therapeutic agent in
vivo, as well as assess the safety and efficacy of p-cresol in
a more physiologically relevant context. Future research should
determine the detailed mechanism of p-cresol, explore its long-term
effects in vivo, investigate combination therapies with
existing chemotherapeutic agents or targeted therapies, expand its
application to other types of cancer, and develop optimized
derivatives to advance understanding and therapeutic potential of
p-cresol in cancer treatment.
Phosphoproteomics analysis should be performed to
identify signaling pathways activated downstream of Ca2+
influx in response to p-cresol treatment. This approach maps
phosphorylation changes in proteins involved in key signaling
pathways. Glioblastoma cells treated with p-cresol should be
analyzed using mass spectrometry-based phosphoproteomics to
identify differentially phosphorylated proteins. Bioinformatics
tools should be used to map these proteins to specific signaling
pathways. To provide a more holistic view of the biological effects
of p-cresol, additional experiments to assess its impact on
oxidative stress, autophagy, and mitochondrial function should be
performed to provide insights into the mechanisms underlying its
cytotoxic effects and uncover potential therapeutic targets for
glioblastoma treatment.
PLC serves a key role is in p-cresol-induced
Ca2+ signaling. To investigate the involvement of
specific PLC isoforms in p-cresol-induced Ca2+ release
in glioblastoma cells, molecular biology techniques such as gene
expression analysis and siRNA-mediated knockdown will be employed.
These methods aim to determine the expression levels and functional
significance of different PLC isoforms when glioblastoma cells are
treated with p-cresol. Identifying the PLC family and specific
isoform(s) involved will provide insights into the molecular
mechanisms underlying the dysregulation of p-cresol-induced
Ca2+ signaling. Additionally, the functional role of the
identified PLC isoforms in p-cresol-induced Ca2+ release
and its downstream effects on glioblastoma cell physiology will be
assessed. This will involve using pharmacological inhibitors or
activators specific to the identified PLC isoform(s) to modulate
its activity. The impact on intracellular Ca2+ dynamics,
as well as on cell viability, proliferation, and migration, will be
evaluated to understand the broader implications of PLC-mediated
signaling pathways in p-cresol-treated glioblastoma cells.
Functional analysis may elucidate the contribution of PLC
isoform(s) to p-cresol-induced Ca2+ signaling
alterations and provide mechanistic insights into glioblastoma
pathophysiology. This may determine involvement of specific PLC
isoforms in p-cresol-induced Ca2+ release and elucidate
their functional role in glioblastoma cells. These investigations
may provide valuable insight into the molecular mechanisms
underlying Ca2+ signaling dysregulation in glioblastoma
and uncover novel therapeutic targets for glioblastoma
treatment.
In conclusion, p-cresol induced Ca2+
entry in DBTRG-05MG human glioblastoma cells via a PKC-dependent,
store-operated mechanism. p-Cresol triggered release of
Ca2+ from the endoplasmic reticulum via a PLC-dependent
pathway. This led to cell death, which is initiated by a
Ca2+ signal. The impact of p-cresol on Ca2+
movement in glioblastoma cells should be investigated in
vitro and in vivo.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Department of
Pharmacy and Master Program, College of Pharmacy and Health Care,
Tajen University.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PHC, CLS, SHF, RS and WZL all contributed
substantially to the conception and design, acquisition of data, or
analysis and interpretation of data. PHC, CLS, SHF and RS confirm
the authenticity of all the raw data. WZL wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aranha MM, Matos AR, Teresa Mendes A, Vaz
Pinto V, Rodrigues CM and Arrabaça JD: Dinitro-o-cresol induces
apoptosis-like cell death but not alternative oxidase expression in
soybean cells. J Plant Physiol. 164:675–684. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mariot P, Prevarskaya N, Roudbaraki MM, Le
Bourhis X, Van Coppenolle F, Vanoverberghe K and Skryma R: Evidence
of functional ryanodine receptor involved in apoptosis of prostate
cancer (LNCaP) cells. Prostate. 43:205–214. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yue Z, She RP, Bao HH, Tian J, Yu P, Zhu
J, Chang L, Ding Y and Sun Q: Necrosis and apoptosis of renal
tubular epithelial cells in rats exposed to 3-methyl-4-nitrophenol.
Environ Toxicol. 27:653–661. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hsu JE, Lo SH, Lin YY, Wang HT and Chen
CY: Effects of essential oil mixtures on nitrogen metabolism and
odor emission via in vitro simulated digestion and in vivo growing
pig experiments. J Sci Food Agric. 102:1939–1947. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Saleh-E-In MM, Bhattacharyya P and Van
Staden J: Chemical composition and cytotoxic activity of the
essential oil and oleoresins of in vitro micropropagated Ansellia
africana Lindl: A Vulnerable Medicinal Orchid of Africa. Molecules.
26(4556)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tanaka S, Yano S, Sheikh AM, Nagai A and
Sugimoto T: Effects of uremic toxin p-cresol on proliferation,
apoptosis, differentiation, and glucose uptake in 3T3-L1 cells.
Artif Organs. 38:566–571. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
de Carvalho JT Jr, Dalboni MA, Watanabe R,
Peres AT, Goes MA, Manfredi SR, Canziani ME, Cendoroglo GS,
Guimaraes-Souza N, Batista MC and Cendoroglo M: Effects of
spermidine and p-cresol on polymorphonuclear cell apoptosis and
function. Artif Organs. 35:E27–E32. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Letertre MPM, Myridakis A, Whiley L,
Camuzeaux S, Lewis MR, Chappell KE, Thaikkatil A, Dumas ME,
Nicholson JK, Swann JR and Wilson ID: A targeted ultra performance
liquid chromatography-Tandem mass spectrometric assay for tyrosine
and metabolites in urine and plasma: Application to the effects of
antibiotics on mice. J Chromatogr B Analyt Technol Biomed Life Sci.
1164(122511)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Berridge MJ: Calcium signalling in health
and disease. Biochem Biophys Res Commun. 485(5)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bootman MD and Bultynck G: Fundamentals of
cellular calcium signaling: A Primer. Cold Spring Harb Perspect
Biol. 12(a038802)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Mousa F and Michelangeli F: Commonly
used ryanodine receptor activator, 4-chloro-m-cresol(4CmC), is also
an inhibitor of SERCA Ca2+ pumps. Pharmacol Rep.
61:838–842. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lin AH, Sun H, Paudel O, Lin MJ and Sham
JS: Conformation of ryanodine receptor-2 gates store-operated
calcium entry in rat pulmonary arterial myocytes. Cardiovasc Res.
111:94–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hauser CJ, Kannan KB, Deitch EA and
Itagaki K: Non-specific effects of 4-chloro-m-cresol may cause
calcium flux and respiratory burst in human neutrophils. Biochem
Biophys Res Commun. 336:1087–1095. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arai S, Ikeda M, Ide T, Matsuo Y, Fujino
T, Hirano K, Sunagawa K and Tsutsui H: Functional loss of DHRS7C
induces intracellular Ca2+ overload and myotube
enlargement in C2C12 cells via calpain activation. Am J Physiol
Cell Physiol. 312:C29–C39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Suzuki D, Hori T, Saitoh N and Takahashi
T: 4-Chloro-m-cresol, an activator of ryanodine receptors, inhibits
voltage-gated K+ channels at the rat calyx of Held. Eur
J Neurosci. 26:1530–1536. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baqri W, Rzadki K, Habbous S and Das S:
Treatment, healthcare utilization and outcomes in patients with
glioblastoma in Ontario: A 10-year cohort study. J Neurooncol.
168:473–485. 2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liang T, Gu L, Kang X, Li J, Song Y, Wang
Y and Ma W: Programmed cell death disrupts inflammatory tumor
microenvironment (TME) and promotes glioblastoma evolution. Cell
Commun Signal. 22(333)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Srivastava R, Dodda M, Zou H, Li X and Hu
B: Tumor Niches: Perspectives for targeted therapies in
glioblastoma. Antioxid Redox Signal. 39:904–922. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stachulski AV, Knausenberger TB, Shah SN,
Hoyles L and McArthur S: A host-gut microbial amino acid
co-metabolite, p-cresol glucuronide, promotes blood-brain barrier
integrity in vivo. Tissue Barriers. 11(2073175)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang KF, Liu CY, Huang YC, Hsiao CY and
Tsai NM: Downregulation of VEGFR2 signaling by cedrol abrogates
VEGF-driven angiogenesis and proliferation of glioblastoma cells
through AKT/P70S6K and MAPK/ERK1/2 pathways. Oncol Lett.
26(342)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ertilav K and Nazıroğlu M: Honeybee venom
melittin increases the oxidant activity of cisplatin and kills
human glioblastoma cells by stimulating the TRPM2 channel. Toxicon.
222(106993)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Akyuva Y and Nazıroğlu M: Silver
nanoparticles potentiate antitumor and oxidant actions of cisplatin
via the stimulation of TRPM2 channel in glioblastoma tumor cells.
Chem Biol Interact. 369(110261)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ashokan A and Aradhyam GK: Measurement of
intracellular Ca2+ mobilization to study GPCR signal
transduction. Methods Cell Biol. 142:59–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Absi M, Eid BG, Ashton N, Hart G and
Gurney AM: Simvastatin causes pulmonary artery relaxation by
blocking smooth muscle ROCK and calcium channels: Evidence for an
endothelium-independent mechanism. PLoS One.
14(e0220473)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Segal S and Yaniv Y:
Ca2+-Driven selectivity of the effect of the cardiotonic
steroid marinobufagenin on rabbit sinoatrial node function. Cells.
12(1881)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gruszczynska-Biegala J, Strucinska K,
Maciag F, Majewski L, Sladowska M and Kuznicki J: STIM
Protein-NMDA2 receptor interaction decreases NMDA-Dependent calcium
levels in cortical neurons. Cells. 9(160)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hsu WL, Hsieh YC, Yu HS, Yoshioka T and Wu
CY: 2-Aminoethyl diphenylborinate inhibits bleomycin-induced skin
and pulmonary fibrosis via interrupting intracellular
Ca2+ regulation. J Dermatol Sci. 103:101–108.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chiu KM, Lee MY, Lu CW, Lin TY and Wang
SJ: Plantainoside D reduces depolarization-evoked glutamate release
from rat cerebral cortical synaptosomes. Molecules.
28(1313)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tauskela JS, Brunette E, Aylsworth A and
Zhao X: Neuroprotection against supra-lethal ‘stroke in a dish’
insults by an anti-excitotoxic receptor antagonist cocktail.
Neurochem Int. 158(105381)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Couly S, Yasui Y, Foncham S, Grammatikakis
I, Lal A, Shi L and Su TP: Benzomorphan and non-benzomorphan
agonists differentially alter sigma-1 receptor quaternary
structure, as does types of cellular stress. Cell Mol Life Sci.
81(14)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hung Y, Chung CC, Chen YC, Kao YH, Lin WS,
Chen SA and Chen YJ: Klotho modulates pro-fibrotic activities in
human atrial fibroblasts through inhibition of phospholipase C
signaling and suppression of store-operated calcium entry.
Biomedicines. 10(1574)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu Z, Wu X, Wang Q, Li Z, Liu X, Sheng X,
Zhu H, Zhang M, Xu J, Feng X, et al: CD73-Adenosine A1R Axis
regulates the activation and apoptosis of hepatic stellate cells
through the PLC-IP3-Ca2+/DAG-PKC signaling pathway.
Front Pharmacol. 13(922885)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ishikawa J, Ohga K, Yoshino T, Takezawa R,
Ichikawa A, Kubota H and Yamada T: A pyrazole derivative, YM-58483,
potently inhibits store-operated sustained Ca2+ influx
and IL-2 production in T lymphocytes. J Immunol. 170:4441–4449.
2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shideman CR, Reinardy JL and Thayer SA:
gamma-Secretase activity modulates store-operated Ca2+
entry into rat sensory neurons. Neurosci Lett. 451:124–128.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cuoghi A, Caiazzo M, Bellei E, Monari E,
Bergamini S, Palladino G, Ozben T and Tomasi A: Quantification of
p-cresol sulphate in human plasma by selected reaction monitoring.
Anal Bioanal Chem. 404:2097–2104. 2012.PubMed/NCBI View Article : Google Scholar
|