Introduction
Fluoride (F) is an element that is naturally present
in the environment, and World Health Organization (2017) guidelines
state that the allowable concentration of F in drinking water is
1.5 ppm. However, in some areas, such as India, where groundwater
containing high concentrations of F is supplied as drinking water,
concentrations can exceed 48 ppm (1), and this is associated with widespread
skeletal fluorosis and mottling of the teeth, which are serious
public health problems. In addition, 25 countries fluoridate their
tap water to prevent tooth decay (GOV.UK, 2022). In
recent years, there has been concern that F exposure during
pregnancy in countries where people consume fluoridated tap water
or groundwater with high F concentrations may reduce the
intelligence quotients (IQs) of children, and increase their risks
of memory and learning disorders, attention deficit hyperactivity
disorder and autism spectrum disorder (ASD) (2-4).
In general, it is considered that both environmental
and genetic factors are involved in the etiology of developmental
abnormalities (5), and if F
exposure causes brain dysfunction, it is possible that it is a
significant environmental factor. In some European Union member
states, the fluoridation of tap water was banned between the 1970s
and 1990s, and it has been reported that the incidence of ASDs is
low in these countries (2,6). Worldwide, ~240 million children have
developmental disorders (7). In
particular, the prevalence of ASD is ~1/100, and this has rapidly
increased over the past 20 years, such that these disorders have
become a serious problem (WHO, 2023).
In an epidemiologic study of pregnant women living
in areas of Canada with fluoridated or non-fluoridated drinking
water, the IQ scores of boys were found to decrease as their
urinary F concentrations increased (3). In a study of Indian adolescents who
were consuming well water with F concentrations of 5-10 ppm, their
IQ scores, attention, concentration, verbal memory and spatial
memory were found to decrease with increasing F concentration
(8). Most of the F absorbed into
the body accumulates in the bones and teeth (1,9), but
there is concern that trace amounts may cross the blood-brain
barrier and accumulate in brain tissue, where it is neurotoxic
(10). Furthermore, it has been
suggested that when women are exposed to F during pregnancy, the
concentrations of F in the placenta, plasma/serum and umbilical
cord blood increase in direct proportion to its consumption
(11,12). The placenta not only transports
nutrients and gases, but also contains neurotransmitters such as
serotonin, dopamine and norepinephrine/epinephrine, and there is
concern that exposure to various risk factors may affect fetal
brain development and be involved in the development of ASD
(13). If F crosses the blood-brain
barrier, it accumulates in brain tissue, and although it is not
involved in the synthesis of the neurotransmitter, it can impair
their synthesis. As a result, brain development may be impaired,
and subsequent behavior may be affected (14). Water fluoridation has been discussed
worldwide, and although no definitive conclusions have been
reached, F has been suggested to be a neurotoxin (15,16).
Previous studies of experimental animals have shown nerve damage,
neurodegeneration, a lack of muscle coordination, chronic fatigue,
attention deficits and memory impairments caused by the
accumulation of F in brain regions associated with long-term
exposure to high concentrations (50-100 ppm) of F (17-21).
In the present study, the effects of F on brain
function were evaluated, including the characteristics of ASD, by
chronically exposing mice to relatively low concentrations of F
during pregnancy and subsequently, and the relationships between
the results of behavioral testing and the levels of brain
neurotransmitters were evaluated.
Materials and methods
Animals and treatment
Male and female 21-day-old ICR mice (F0) were
purchased from Sankyo Laboratory Services, Inc. They were housed in
an air-conditioned room under a 12/12-h light/dark cycle (lights
off at 20:00; rearing temperature at 24˚C), with males and females
under standard rearing conditions, three per cage. Information on
the F0 mice is shown in Table I.
The purchased mice were randomly allocated to a control group
consuming tap water and sodium fluoride (Nacalai Tesque, Inc.)
exposure groups consuming 15 mg F ions/l (15 ppm) or 30 mg F ions/l
(30 ppm). The sodium fluoride was dissolved in ultrapure water
(Organo Corp.). Female and male F0 mice were exposed to the sodium
fluoride-containing drinking water from 3 weeks of age. F0 mice
were mated at 8 weeks-old and continued to consume tap water or
water containing sodium fluoride during pregnancy and until weaning
on postnatal day 21. After weaning, the pups (F1 mice) were exposed
to the same concentrations of F as F0 through their drinking water.
In the present study, behavioral testing and brain analysis were
performed exclusively on male F1 offspring. Only male F1 mice were
included in the analysis because the prevalence of ASD in males is
higher than in females (22).
Behavioral tests began at 8 weeks of age to assess
neurodevelopmental effects and concluded by 10 weeks. After
testing, the F1 mice were euthanized, and their brains were
collected. Neurotransmitter concentrations in the cerebellum,
midbrain and hippocampus were measured using liquid
chromatography-tandem mass spectrometry (LC/MS/MS). Exposure to
sodium fluoride continued for the F1 mice until euthanasia. This
choice is in line with the research content, which focuses on the
neurodevelopmental consequences associated with ASD. The
experimental protocol was approved by the Juntendo University
Center for Biomedical Resources (approval no. 1231; Tokyo, Japan).
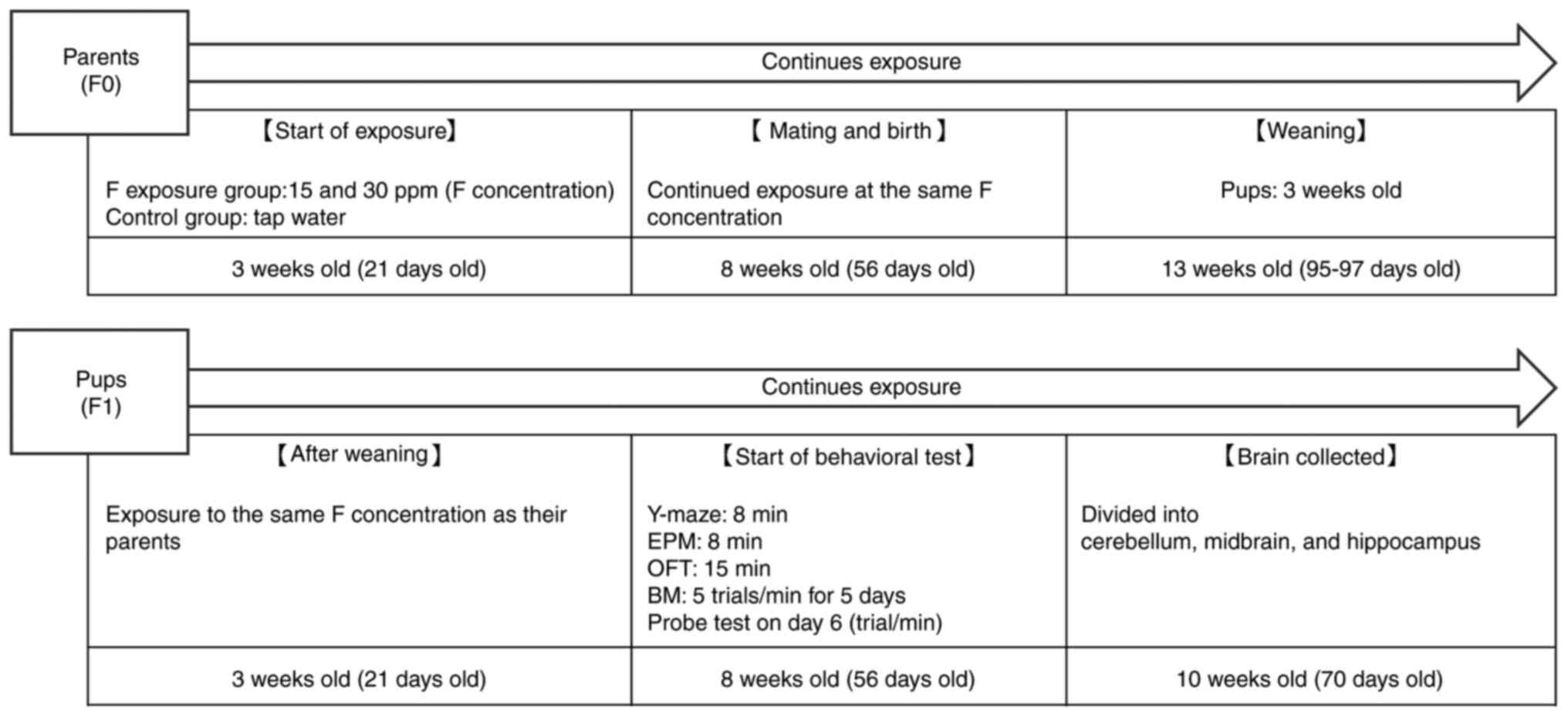
The experimental procedure, including sodium fluoride exposure,
behavioral testing, and euthanasia/brain collection, for the F0 and
F1 mice is shown in Fig. 1.
 | Table IInformation of F0 mice (weight, food,
water, and number of births). Data represents the mean ± SEM (n=3)
for body weight, food, and water intake, and the number of births
represents the sum of each group. |
Table I
Information of F0 mice (weight, food,
water, and number of births). Data represents the mean ± SEM (n=3)
for body weight, food, and water intake, and the number of births
represents the sum of each group.
| | Body weight of
21day-old | Daily Food intake
per animal | Daily water intake
per animal |
|---|
| Male | | | |
|
Control | 20.1±0.2 | 3.1±0.3 | 4.4±1.2 |
|
15 ppm | 20.2±0.1 | 3.3±0.3 | 3.9±1.5 |
|
30 ppm | 20.1±0.1 | 3.3±0.2 | 4.1±1.8 |
| Female | | | |
|
Control | 20.3±0.1 | 3.2±0.2 | 4.5±1.1 |
|
15 ppm | 20.1±0.2 | 3.1±0.4 | 4.4±0.7 |
|
30 ppm | 20.2±0.2 | 3.0±0.3 | 4.0±1.3 |
| Number of
births | | | |
| | Male | Female | |
| Control | 11 | 12 | |
| 15 ppm | 11 | 17 | |
| 30 ppm | 18 | 15 | |
Behavioral testing for developmental
disorders
Behavioral characteristics of developmental
disorders were evaluated in 8-week-old male mice (n=10 for each of
the control, 15 ppm F, and 30 ppm F groups) using the four
behavioral tests described below. These behaviors were
automatically recorded using a CCD camera and DVTrack Video
Tracking System (Compact VAS/DV; Muromachi Kikai Co., Ltd.). In
addition, behaviors in the Barnes maze (BM) were assessed visually
after recording. To avoid interference between tests, each set of
apparatus was washed with 70% ethyl alcohol after each test.
Y-maze
Voluntary behavior was evaluated using a Y-maze
(Muromachi Kikai Co). The Y-maze apparatus was made of gray
polyvinyl chloride and consisted of three arms, with 120˚ angles
between adjacent arms. Each arm was 4x41.5x10 cm [width (W) x
length (L) x height (H)] in size. The spontaneous alternation rate
and spontaneous locomotor activity (locomotor activity) were
measured over 8 min.
Elevated plus maze (EPM)
Anxiety-like behavior was evaluated using an EPM
(Panlab, S.L., Harvard Apparatus). The apparatus was made of
methacrylic resin and aluminum, with black floors and gray walls.
The EPM was placed 45 cm above the floor and consisted of open and
closed arms, each of size 6x29.5 cm [W x depth], with an H of 15 cm
for the open arm. The number of entries into the open arm and the
time spent in the open arm were measured over 8 min.
BM
Hippocampus-dependent spatial learning and memory
were evaluated using a BM (Panlab, S.L.). The BM consisted of
boxes, a white circular platform (92 cm in diameter) placed 90 cm
above the ground, with 20 equally-spaced holes of 5 cm in diameter
through which fake target boxes and a single black escape box could
be accessed. The latter served as the goal throughout the training
period and memory test. The training consisted of five trials of 1
min per day for 5 consecutive days, during which the time taken to
reach the escape box (latency) and the number of errors made were
measured. On the sixth day, a 1-min test was performed to evaluate
memory retention.
Open field test (OFT)
Exploratory and anxiety-like behaviors were
evaluated using an OFT (Muromachi Kikai Co.). It was made of gray
polyvinyl chloride, was 50x50 cm in size, and had walls 40 cm high.
The distance traveled (cm), the traveling time (sec), the time
spent in the central zone (s), the mean speed (cm/sec), and the
grooming frequency were measured over 15 min. Several visible
objects were placed in plain sight of the mouse to cue spatial
learning.
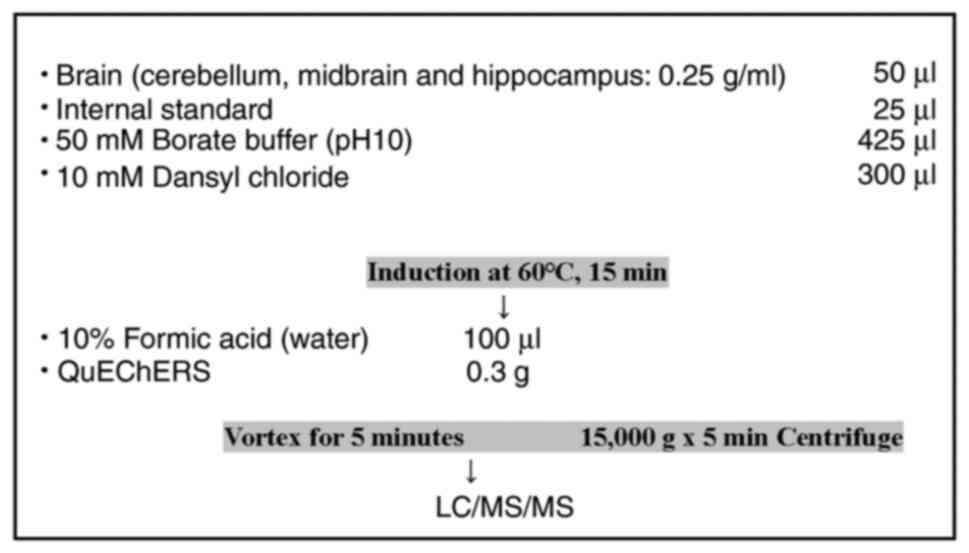
Measurement of levels of
neurotransmitters
At the end of the behavioral study (at 10 weeks of
age), male F1 mice were euthanized by cervical dislocation, and
their brains were collected. The brains were then divided into the
cerebellum, midbrain, and hippocampus and stored at -80˚C. The
cerebellum, midbrain, and hippocampus masses were measured, then
these components were homogenized in a 10-mM hydrochloric acid
solution and diluted to a final concentration of 0.25 g/ml and
centrifuged at 10,000 x g for 5 min, then the supernatants were
collected. After pretreatment, the cerebellum, midbrain and
hippocampus were analyzed for their glycine (Gly; pmol/g),
gamma-aminobutyric acid (GABA; pmol/g), glutamic acid (Glu;
pmol/g), tryptophan (Trp; nmol/g), tyrosine (Tyr; nmol/g),
homovanillic acid (HVA; pmol/g), 5-hydroxytryptamine (5-HT;
pmol/g), 3,4-dihydroxyphenylacetic acid (DOPAC; pmol/g), dopamine
(DA; pmol/g) and noradrenaline (NA; pmol/g) contents using liquid
chromatography-mass spectrometry (LC-MS/MS). LC-MS/MS analyses were
conducted using an LCMS-8045 mass spectrometer equipped with a
Nexera UHPLC and a SIL-30AC autosampler (Shimadzu Corporation).
Chromatographic separation was achieved using a SunShell C18 column
(2.1x150 mm, 2.6-µm particles; ChromaNik Technologies, Inc.) fitted
with a SecurityGuard Ultra C18 (2.1x2 mm, 2-µm particles;
Phenomenex; https://www.phenomenex.com/) guard column. The mobile
phase consisted of 10 mM ammonium formate buffer (pH 3.6) and
acetonitrile. The total assay time was 25 min and the following
multistep gradient was used: 0-5 min, 15-80% B (linear gradient);
5-10 min, 80-95% B (linear gradient); 10-20 min, 95% B (isocratic);
20-20.1 min, 95-15% B (linear gradient); 20.1-25 min, 15% B
(isocratic). The mobile phase flow rate was 0.2 ml/min, the column
temperature was 40˚C, and the injection volume was 5 µl.
Electrospray ionization was employed in both positive and negative
ion modes, and selective reaction monitoring was used for analyte
quantification. The interface voltage was set at 4.0 kV. Drying and
nebulizing gases were supplied at flow rates of 10.0 and 3.0 l/min,
respectively. The temperature was set to 250˚C, and the heat block
temperature was set at 400˚C (23)
(Fig. 2).
Statistical analysis
The Shapiro-Wilk test was used to assess the
normality of each dataset. For normally distributed continuous
data, one-way analysis of variance (ANOVA) was used to compare the
mean values of groups, and if a significant difference was
detected, the Tukey-Kramer post hoc test was used to
identify specific differences. For variables that were not normally
distributed, the Kruskal-Wallis test was used to compare the three
groups, and following the identification of a significant result,
pairwise comparisons were conducted using the Dunn-Bonferroni
post hoc test. The significance level (α) was set at 0.05,
and P-values for the post hoc tests were adjusted using the
Bonferroni correction to account for multiple comparisons.
Spearman's rank correlation coefficients were calculated to assess
the relationships between behavioral test results and the levels of
neurotransmitters. No prior sample size calculation was performed,
but a post hoc power analysis was conducted to evaluate the
ability of the analysis to detect differences in the mean values
obtained for each behavioral test of groups of 10 mice. This
analysis indicated a statistical power of 1-β=0.22, assuming an
effect size of f=0.30 and a significance level of α=0.05. All
statistical analyses were conducted using SPSS version 29 (IBM
Corp.).
Results
Effects of prenatal sodium fluoride
exposure on body mass
Data confirmed normal distribution (P<0.05). At
weaning (3 weeks old), the body masses were 21.7±0.8 g for the
control group (n=10), 20.9±0.6 for the 15-ppm group (n=10), and
15.5±0.8 for the 30-ppm group (n=10). That of the 30-ppm group was
significantly lower than those of the control and 15-ppm groups
(P<0.001). Before euthanasia (10 weeks old), the body masses of
the mice were as follows: control group, 40.8±0.8 g; 15-ppm group,
42.8±0.4 g; and 30-ppm group, 41.2±2.8 g; with no significant
differences.
Effects of prenatal sodium fluoride
exposure on developmental disease-like behavior
Data confirmed normal distribution (P<0.05). The
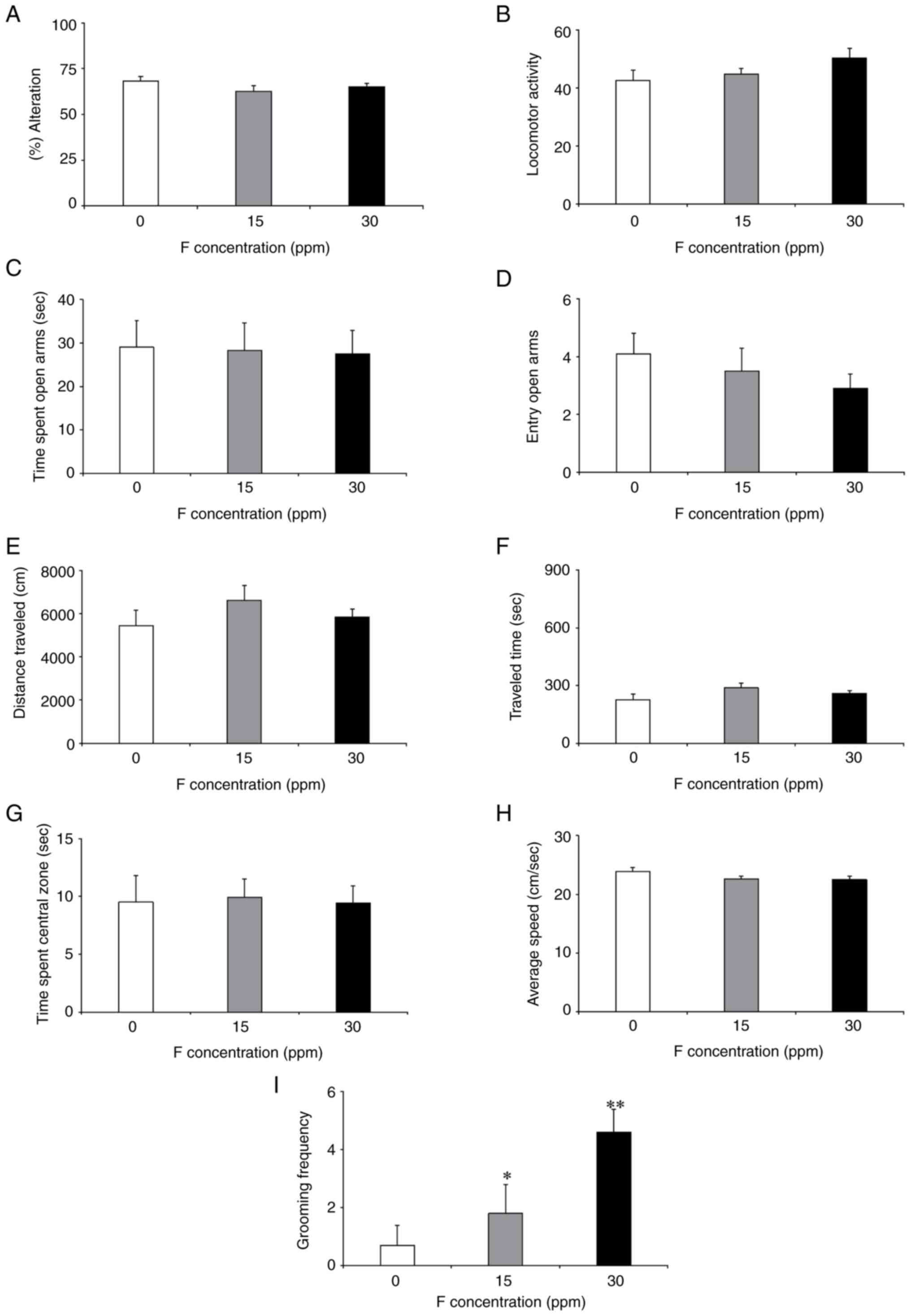
Y-maze, EPM and OFT results are shown in Fig. 3A-I. There were no significant
differences between the F-exposure and control groups in the Y-maze
(Fig. 3A and B) or in the EPM (Fig. 3C and D). In the OFT, there were significantly
higher grooming frequencies in the 15 and 30-ppm groups than in the
control group (Fig. 3I). However,
there were no significant differences in the other parameters
associated with the OFT (Fig.
3E-H). The results of the BM analysis are shown in Fig. 4A-D. During the 5-day training
period, the 15 and 30-ppm groups showed significantly longer
latencies and more errors than the control group (Fig. 4A and B). In the test, the 15 and 30-ppm groups
also exhibited significantly longer latencies and more errors
(Fig. 4C and D).
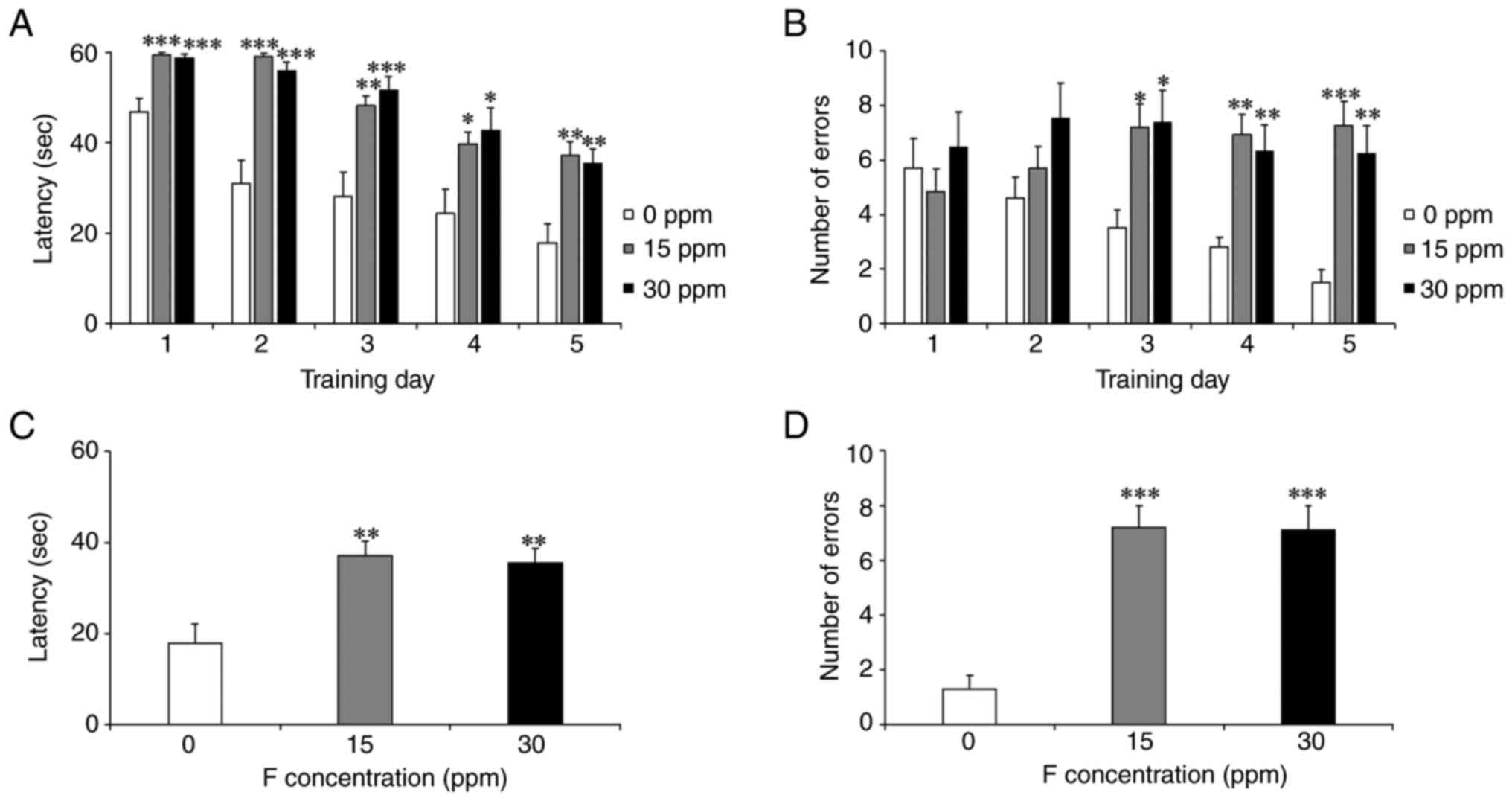 | Figure 4Effect of sodium fluoride exposure on
developmental disease-like behavior. The number of errors and
latency in the 5-day training and probe test in the Barnes maze
were compared between the control and fetal period sodium fluoride
exposure groups. Data represents the mean ± SEM (n=10). An ANOVA
was conducted, and post hoc comparisons were performed using the
Tukey-Kramer test. (A and B) During the 5 days of training, the 15
and 30 ppm groups required significantly longer time to latency
compared with the control group (0 ppm vs. 15 ppm: D1; P<0.001,
D2; P<0.001, D3; P<0.01, D4; P<0.05, D5; P<0.01, 0 ppm
vs. 30 ppm: D1; P<0.001, D2; P<0.001, D3; P<0.001, D4;
P<0.05, D5; P<0.01, and significantly more errors (0 ppm vs.
15 ppm: D3; P<0.05, D4; P<0.001, D5; P<0.001, 0 ppm vs. 30
ppm: D3; P<0.05, D4; P<0.01, D5; P<0.001. (C and D) In the
probe test, both the 15 and 30 ppm groups had significantly longer
latencies (0 ppm vs. 15 ppm; P<0.01, 0 ppm vs. 30 ppm;
P<0.01) and more errors than the control group (0 ppm vs. 15
ppm; P<0.001, 0 ppm vs. 30 ppm; P<0. 001.
*P<0.05, **P<0.01 and
***P<0.001. |
Effects of fetal sodium fluoride
exposure on subsequent neurotransmitters
Levels of neurotransmitters have been measured as
indicators of developmental disorders; therefore, neurotransmitters
were also measured (Table II). The
levels of Gly, Glu, Trp and Tyr in the cerebellum; Gly, HVA, NA and
DA in the midbrain; and Gly and HVA in the hippocampus showed a
normal distribution (P<0.05). Therefore, ANOVA was performed,
followed by the Tukey-Kramer test as a post hoc analysis. On the
other hand, the Kruskal-Wallis test was used for GABA, HVA, 5-HT
and NA in the cerebellum; GABA, Glu, Trp, Tyr, Dopac, 5-HT and NA
in the midbrain' GABA, Glu, Trp, Tyr, Dopac, 5-HT and NA in the
hippocampus; and DA, as normality was not observed (P>0.05). For
post hoc analysis, the Dunn-Bonferroni test was performed to
determine significant differences between groups whereas the
Kruskal-Wallis test yielded statistically significant results. The
cerebellar Glu was significantly lower in the 15 and 30 ppm groups
compared with the control group (P<0.01).
 | Table IIEffects of fetal period exposure to
sodium fluoride on neurotransmitters. |
Table II
Effects of fetal period exposure to
sodium fluoride on neurotransmitters.
| | Gly | GABA | Glu | Trp | Tyr | HVA | DOPAC | 5-HT | DA | NA |
|---|
| Cerebellum (mean ±
SEM) | | | | | | | | | | |
|
0 ppm | 1.1±0.1 | 2.0±0.5 | 3.9±0.8 | 27.3±4.5 | 132.7±18.5 | 89.0±28.7 | 14.2±3.1 | 111.6±58.4 | Not detected | 130.5±19.6 |
|
15 ppm | 1.4±0.2 | 2.7±0.6 |
1.4±0.2a | 34.1±7.5 | 155.0±28.9 | 94.5±28.5 | 15.8±2.0 | 78.7±60.8 | | 94.1±6.8 |
|
30 ppm | 1.3±0.3 | 2.5±0.7 |
1.3±0.3a | 40.1±9.3 | 155.2±30.5 | 93.1±26.9 | 11.2±2.8 | 17.7±12 | | 104.9±6.2 |
| Midbrain (mean ±
SEM) | | | | | | | | | | |
|
0 ppm | 1.6±0.2 | 2.4±0.5 | 2.1±0.6 | 37.3±6.9 | 164.9±26.8 | 278.5±69.2 | 384.3±72.9 | 214.8±39.6 | 21.4±5.2 | 326.1±52.6 |
|
15 ppm | 2±0.3 | 4.1±0.9 | 2.6±1 | 53.1±12.8 | 222.9±46 | 352.0±81 | 304.3±62.2 | 87.5±69.6 | 22.7±6.7 | 154.6±15.5 |
|
30 ppm | 1.9±0.3 | 3.4±0.8 | 2.9±0.7 | 49.1±13.1 | 202.8±48.2 | 305.8±54.2 | 318.2±62.2 | 200.2±42.3 | 14.1±2.4 | 235.8±42.7 |
| Hippocampal (mean ±
SEM) | | | | | | | | | | |
|
0 ppm | 1.1±0.1 | 2.4±0.4 | 4.4±1 | 28.7±3.9 | 157.7±17.1 | 211.2±36.8 | 314.3±124.5 | 285.4±37.9 | 39.4±14.7 | 269.7±49.2 |
|
15 ppm | 1.3±0.1 | 3.2±0.6 | 5.9±1.8 | 34.1±5.2 | 175.0±23.4 | 264.7±58.8 | 198.2±35.9 | 225.9±44.5 | 13.9±5.4 | 171.4±19.5 |
|
30 ppm | 1.2±0.2 | 2.9±0.8 | 6.8±1.7 | 30.8±6.4 | 153.2±24.9 | 206.4±41.7 | 206.4±55.7 | 232.5±29.1 | 24.8±6.5 | 257.8±17.8 |
Correlations between behavioral test
outcomes and levels of brain neurotransmitters
Although the mechanisms of ASD are not fully
understood, human studies have reported decrease in the
neurotransmitters 5-HT, DA (24),
Glu and GABA (25). In addition, it
has been reported that the effects of sodium fluoride exposure on
the behavior of experimental animals and humans include a decrease
in cognitive function (26,27), anxiety-like and depressive behavior
(28). If ASD-like behavior is
being observed, it was considered that it might be related to
neurotransmitters; thus the relationship between the two was
calculated. The characteristics of ASD include delayed social
interaction, communication and repetitive behaviors. In addition,
it has been reported that the levels of the following
neurotransmitters in the brain are decreased with ASD: 5-HT
(abnormal control of emotions and social behavior), Glu (excitatory
neurotransmitter) (24), GABA
(inhibitory neurotransmitter) and DA (abnormal reward processing)
(25). If F-exposure is a factor in
the development of ASD, it was considered that there may be
abnormalities in neurotransmitters related to behavior, therefore
both behavior and neurotransmitters were analyzed. Therefore, the
effects of sodium fluoride exposure on behavior and
neurotransmitters were investigated using experimental animals
during the fetal period. As a result, it was found that exposure to
sodium fluoride affects behavior tests and neurotransmitters in the
brain. Neurotransmitters are chemicals that transmit information in
the brain, and are therefore involved in a variety of behaviors.
The identification of the effects of sodium fluoride exposure on
behavior (for example, memory, learning, anxiety-like behavior) and
levels of neurotransmitters provoked us to perform correlation
analysis to determine the extent to which these parameters were
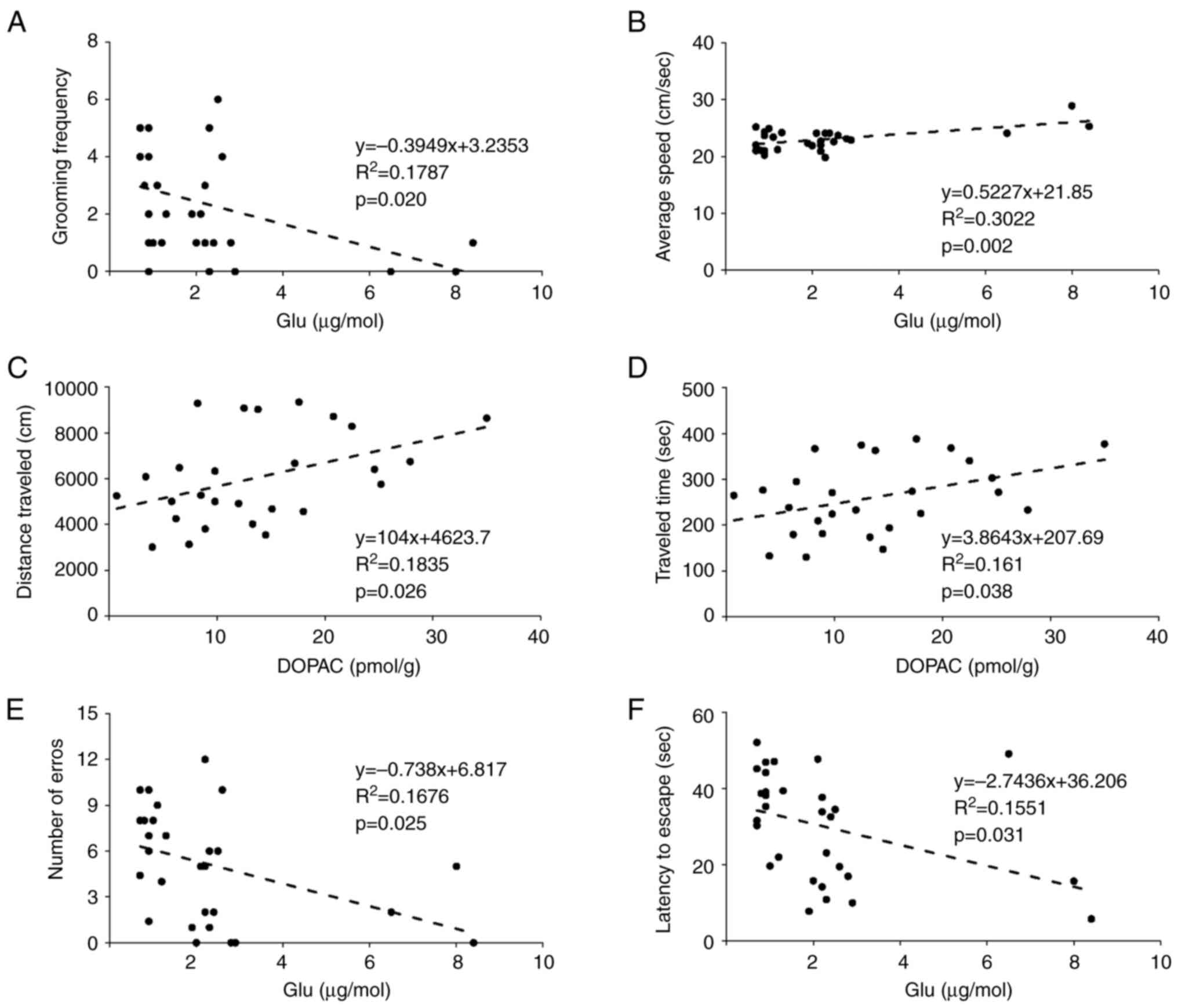
related. The correlations between the levels of cerebellar
neurotransmitters and behavioral test outcomes are demonstrated in
Fig. 5. There was a negative
correlation between grooming frequency in the OFT and the Glu
concentration (Fig. 5A). There were
also positive correlations between the mean speed and the Glu
concentration (Fig. 5B); the
distance traveled (Fig. 5C) and the
traveling time (Fig. 5D) during the
OFT with the DOPAC concentration. For the BM, there were
significant negative correlations of the number of errors (Fig. 5E) and the latency (Fig. 5F) with the Glu concentration. The
correlations between the levels of neurotransmitters in the
midbrain and the behavioral test outcomes are shown in Fig. 6. In the OFT, there were significant
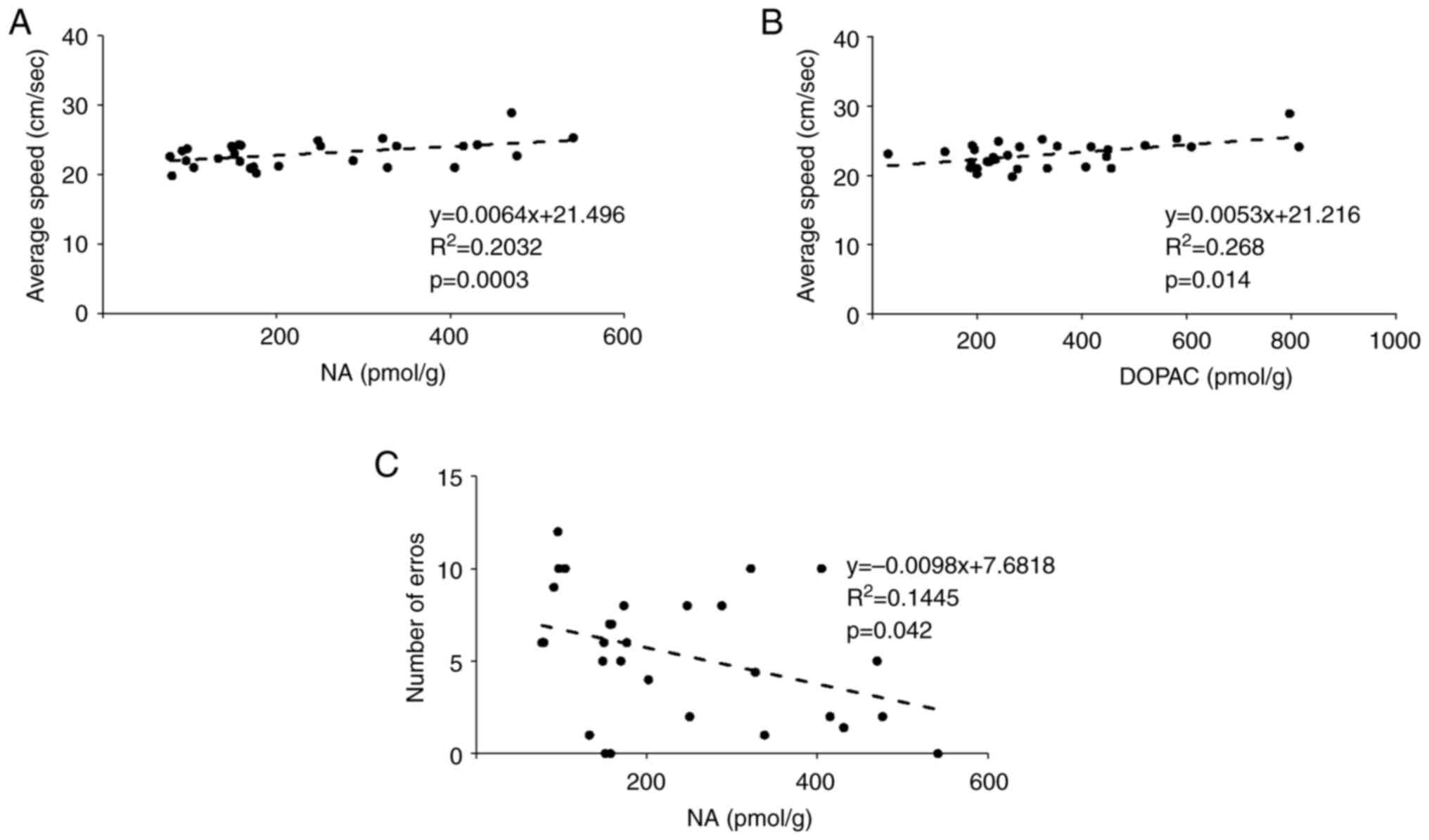
positive correlations of mean speed with the NA (Fig. 6A) and DOPAC (Fig. 6B) concentrations. In the BM, there
was a negative correlation between the number of errors and the NA
concentration (Fig. 6C). The
correlations between the levels of hippocampal neurotransmitters
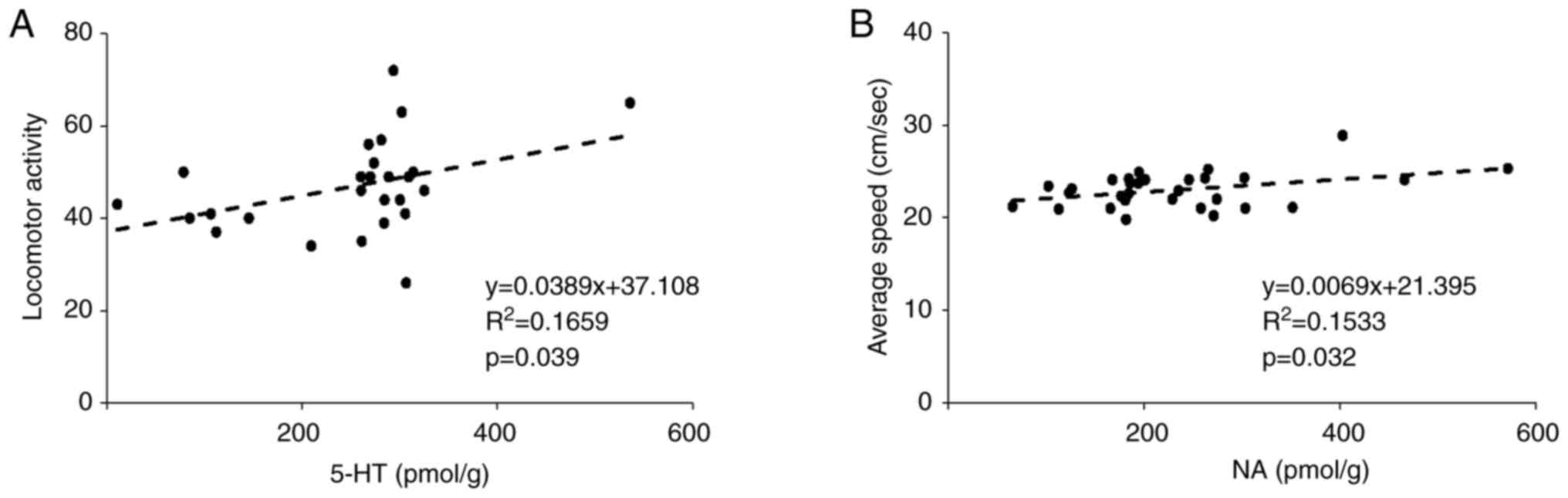
and the behavioral test outcomes are shown in Fig. 7. In the Y-maze, there was a positive
correlation between the 5-HT concentration and locomotor activity
(Fig. 7A). In addition, in the OFT,
there was a positive correlation between the NA concentration and
average speed (Fig. 7B).
Discussion
At present, there is particular concern about the
possibility of damage to the central nervous system being caused by
excitotoxicity resulting from exposure to F (29). However, the mechanisms underlying
the relationship between exposure to F and brain dysfunction remain
unclear. In the present study, it was aimed to determine whether
exposure to sodium fluoride in utero causes subsequent brain
dysfunction, such as ASD. To this end, an experiment was conducted
in mice to characterize the relationships between exposure to
sodium fluoride in utero and subsequently, the behavior of
the mice in adulthood, and the neurotransmitters that determine
such behavior. Behavioral testing is an important means of
evaluating neurobiological function, but these tests are known to
be susceptible to individual differences, environmental factors and
stress, and therefore can yield variable results (30). For this reason, it can be difficult
to accurately assess neurophysiologic changes and the underlying
molecular mechanisms using behavioral testing alone. The use of a
combination of behavioral testing and neurotransmitter measurements
makes it possible to identify the neurobiological mechanisms
underlying the observed behavior and correct for the variability in
behavioral test outcomes (31,32).
This combination provides multilayered information that cannot be
obtained from a single behavioral test and improves the
reproducibility of findings (33).
The main causes of brain dysfunction, including ASD, are considered
to involve complex interactions of genetic and environmental
factors (34,35). The characteristics of ASD include
delayed social interaction, communication and repetitive behaviors
(36). In addition, it has been
reported that the levels of the following neurotransmitters in the
brain are decreased with ASD: 5-HT (abnormal control of emotions
and social behavior), Glu (excitatory neurotransmitter), GABA
(inhibitory neurotransmitter) and DA (abnormal reward processing)
(24). If F exposure is a factor in
the development of ASD, then there may be abnormalities in the
neurotransmitters that are related to behavior. It was found that
low Glu concentrations in the cerebella of the mice were associated
with more anxiety-like behavior, less locomotor activity and poorer
cognitive function (Fig. 5A,
B, E and F).
The cerebellum has neural circuits that connect to the amygdala and
prefrontal cortex, and it is considered that it affects emotion and
anxiety through interactions with these regions (37,38).
Glu is an excitatory neurotransmitter and mediates signal
transmission to the deep cerebellar nuclei. The low Glu
concentration may have reduced the transmission of information from
the cerebellum to the prefrontal cortex and amygdala, thereby
affecting emotion, cognitive function and locomotor activity
(39,40). In addition, Glu contributes to
synaptic plasticity, such as long-term potentiation and long-term
depression, and it has been reported that a low Glu concentration
inhibits these processes, leading to poorer learning and adaptive
behavior (41,42). In ASD and schizophrenia,
abnormalities in the cerebellum have been reported to cause anxiety
and emotional instability (43).
The results of the present and previous studies suggest that the
cerebellum plays important roles, not only in locomotor activity,
but also in the control of anxiety and emotion. In addition, it was
found that the lower the DOPAC concentration in the cerebellum is,
the more impaired the locomotor activity is (Fig. 5C and D). However, further investigation is
needed regarding the lower locomotor activity associated with a low
DOPAC concentration, because there have been no studies to date
regarding low DOPAC concentrations in the cerebellum.
It was found that low concentrations of NE and DOPAC
in the midbrain were associated with poorer motor function
(Fig. 6A and B). It has previously been reported that
low NE and DA concentrations in the midbrain, as well as low
concentrations of their metabolites, such as DOPAC, are associated
with poorer locomotor activity (44). Furthermore, low NA concentrations in
the midbrain have been shown to be associated with a larger number
of errors in the BM (Fig. 6C).
Furthermore, low NA concentrations in the midbrain, and
particularly in the locus coeruleus (LC), are associated with
impaired memory and cognitive function (45). The LC is the main source of
norepinephrine in the brain and plays an important role in the
regulation of various cognitive processes (46,47).
In the present study, the correlations obtained between locomotor
activity and levels of neurotransmitter concentrations suggested
that poor locomotor activity may be the result of low
concentrations of 5-HT and NA (Fig.
7A and B). This suggests that
the concentrations of 5-HT and NA, which are involved in emotion
and cognitive function, may also contribute to motor control. The
effects of 5-HT on emotion and cognitive function have been widely
reported, and it is considered that these may also affect locomotor
activity (48,49). NA has been reported to promote
neurogenesis and synaptic plasticity in the hippocampus (50,51),
and there have also been reports that hippocampal neurogenesis is
related to motor learning (52).
Thus, the present results suggest that low hippocampal
concentrations of 5-HT and NA may indirectly affect locomotor
activity via cognition and emotion. Correlation analysis of
the relationships between the levels of neurotransmitters and
behavioral data generated relatively weak correlations (the R²
values were mostly <0.3). For correlation coefficients <0.3,
the statement ‘a trend was indicated’ is used because it is a weak
correlation. Indeed, a single neurotransmitter does not determine
behavior, but instead interacts with other neurotransmitters,
hormones, environmental factors, learning experiences and genetics
to function in this way (53).
Individual differences, environmental conditions, technical errors
in the measurement of behavioral parameters, and the levels of
neurotransmitters may have weakened these correlations (54). In the future, it will be necessary
to construct more precise models and evaluate the interactions
between neurotransmitters and behavior, considering multiple
variables. In addition, the current research results showed that
chronic exposure of the fetus to sodium fluoride caused weight loss
after weaning. In a study in which pregnant rats were exposed to F,
it was reported that F passed through the placental barrier and
accumulated in the amniotic fluid and fetal plasma, that the
osmotic pressure of the amniotic fluid decreased on day 20 of
gestation, and that the rate of delayed fetal development was high
in F0 fetuses (55). It has been
suggested that F1s after weaning may decrease in weight because of
F exposure to fetal development (55). However, the mechanism remains
unknown.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by MEXT KAKENHI (grant
no. JP19K07808).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MH conceptualized the study, developed methodology,
conducted investigation, wrote the original draft and acquired
funding. YI conducted investigation, and wrote, reviewed and edited
the manuscript. AS and TT wrote, reviewed and edited the
manuscript, supervised the study, and confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The animal experiments were approved (approval no.
1241) by the Juntendo University Center for Biomedical Resources
(Tokyo, Japan) and were performed by according to appropriate
ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Srivastava S and Flora SJS: Fluoride in
drinking water and skeletal fluorosis: A Review of the global
impact. Curr Environ Health Rep. 7:140–146. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Strunecka A and Strunecky O: Chronic
fluoride exposure and the risk of autism spectrum disorder. Int J
Environ Res Public Health. 16(3431)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Green R, Lanphear B, Hornung R, Flora D,
Martinez-Mier EA, Neufeld R, Ayotte P, Muckle G and Till C:
Association between maternal fluoride exposure during pregnancy and
IQ scores in offspring in Canada. JAMA Pediatr. 173:940–948.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bashash M, Marchand M, Hu H, Till C,
Martinez-Mier EA, Sanchez BN, Basu N, Peterson KE, Green R, Schnaas
L, et al: Prenatal fluoride exposure and attention deficit
hyperactivity disorder (ADHD) symptoms in children at 6-12 years of
age in Mexico City. Environ Int. 121(Pt 1):658–666. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nilsson EE, Sadler-Riggleman I and Skinner
MK: Environmentally induced epigenetic transgenerational
inheritance of disease. Environ Epigenet. 4(dvy016)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Elsabbagh M, Divan G, Koh YJ, Kim YS,
Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C, et
al: Global prevalence of autism and other pervasive developmental
disorders. Autism Res. 5:160–179. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Olusanya BO, Smythe T, Ogbo FA, Nair MKC,
Scher M and Davis AC: Global prevalence of developmental
disabilities in children and adolescents: A systematic umbrella
review. Front Public Health. 11(1122009)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khairkar P, Palicarp SM, Kamble A, Alladi
S, Thomas S, Bommadi R, Mohanty S, Reddy R, Jothula KY, Anupama K,
et al: Outcome of systemic fluoride effects on developmental
neurocognitions and psychopathology in adolescent children. Indian
J Pediatr. 88(1264)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kuru R, Balan G, Yilmaz S, Taslı PN, Akyuz
S, Yarat A and Sahin F: The level of two trace elements in carious,
non-carious, primary, and permanent teeth. Eur Oral Res. 54:77–80.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Żwierełło W, Maruszewska A,
Skórka-Majewicz M and Gutowska I: Fluoride in the central nervous
system and its potential influence on the development and
invasiveness of brain tumours-a research hypothesis. Int J Mol Sci.
24(1558)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Abduweli Uyghurturk D, Goin DE,
Martinez-Mier EA, Woodruff TJ and DenBesten PK: Maternal and fetal
exposures to fluoride during mid-gestation among pregnant women in
northern California. Environ Health. 19(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thippeswamy HM, Nanditha Kumar M, Girish
M, Prashanth SN and Shanbhog R: Linear regression approach for
predicting fluoride concentrations in maternal serum, urine and
cord blood of pregnant women consuming fluoride containing drinking
water. Clin Epidemiol Global Health. 10(100685)2021.
|
|
13
|
Bartos M, Gumilar F, Gallegos CE, Bras C,
Dominguez S, Cancela LM and Minetti A: Effects of perinatal
fluoride exposure on short- and long-term memory, brain antioxidant
status, and glutamate metabolism of young rat pups. Int J Toxicol.
38:405–414. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
National Toxicology Program (NTP): NTP
monograph on the state of the science concerning fluoride exposure
and neurodevelopment and cognition: a systematic review. NTP,
Research Triangle Park, NC, 2024.
|
|
15
|
Morabia A: Community water fluoridation:
Open discussions strengthen public health. Am J Public Health.
106:209–210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McGrady MG, Ellwood RP and Pretty IA:
Water fluoridation as a public health measure. Dent Update.
37:658–660, 662-664. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu F, Zhang Y, Trivedi A, Jiang X, Chandra
D, Zheng J, Nakano Y, Abduweli Uyghurturk D, Jalai R, Onur SG, et
al: Fluoride related changes in behavioral outcomes may relate to
increased serotonin. Physiol Behav. 206:76–83. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bittencourt LO, Dionizio A, Ferreira MKM,
Aragão WAB, de Carvalho Cartágenes S, Puty B, do Socorro Ferraz
Maia C, Zohoori FV, Buzalaf MAR and Lima RR: Prolonged exposure to
high fluoride levels during adolescence to adulthood elicits
molecular, morphological, and functional impairments in the
hippocampus. Sci Rep. 13(11083)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ran LY, Xiang J, Zeng XX, Tang JL, Dong
YT, Zhang F, Yu WF, Qi XL, Xiao Y, Zou J, et al: Integrated
transcriptomic and proteomic analysis indicated that neurotoxicity
of rats with chronic fluorosis may be in mechanism involved in the
changed cholinergic pathway and oxidative stress. J Trace Elem Med
Biol. 64(126688)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reddy YP, Tiwari S, Tomar LK, Desai N and
Sharma VK: Fluoride-induced expression of neuroinflammatory markers
and neurophysiological regulation in the brain of wistar rat model.
Biol Trace Elem Res. 199:2621–2626. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pereira M, Dombrowski PA, Losso EM, Chioca
LR, Da Cunha C and Andreatini R: Memory impairment induced by
sodium fluoride is associated with changes in brain monoamine
levels. Neurotox Res. 19:55–62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zeidan J, Fombonne E, Scorah J, Ibrahim A,
Durkin MS, Saxena S, Yusuf A, Shih A and Elsabbagh M: Global
prevalence of autism: A systematic review update. Autism Res.
15:778–790. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Iwasaki Y, Matsumoto H, Okumura M, Inoue
H, Kaji Y, Ando C and Kamei J: Determination of neurotransmitters
in mouse brain using miniaturized and tableted QuEChERS for the
sample preparation. J Pharm Biomed Anal. 217(114809)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Murayama C, Iwabuchi T, Kato Y, Yokokura
M, Harada T, Goto T, Tamayama T, Kameno Y, Wakuda T, Kuwabara H, et
al: Extrastriatal dopamine D2/3 receptor binding, functional
connectivity, and autism socio-communicational deficits: A PET and
fMRI study. Mol Psychiatry. 27:2106–2113. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saleh MG, Prescot A, Chang L, Cloak C,
Cunningham E, Subramaniam P, Renshaw PF, Yurgelun-Todd D, Zöllner
HJ, Roberts TPL, et al: Glutamate measurements using edited MRS.
Magn Reson Med. 91:1314–1322. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu F, Ma J, Zhang H, Liu P, Liu YP, Xing
B and Dang YH: Fluoride exposure during development affects both
cognition and emotion in mice. Physiol Behav. 124:1–7.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fiore G, Veneri F, Di Lorenzo R, Generali
L, Vinceti M and Filippini T: Fluoride exposure and ADHD: A
systematic review of epidemiological studies. Medicina (Kaunas).
59(797)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li X, Zhang J, Niu R, Manthari RK, Yang K
and Wang J: Effect of fluoride exposure on anxiety- and
depression-like behavior in mouse. Chemosphere. 215:454–460.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ottappilakkil H, Babu S, Balasubramanian
S, Manoharan S and Perumal E: Fluoride induced neurobehavioral
impairments in experimental animals: A brief review. Biol Trace
Elem Res. 201:1214–1236. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Izídio GS, Lopes DM, Spricigo L Jr and
Ramos A: Common variations in the pretest environment influence
genotypic comparisons in models of anxiety. Genes Brain Behav.
4:412–419. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Berridge CW and Waterhouse BD: The locus
coeruleus-noradrenergic system: Modulation of behavioral state and
state-dependent cognitive processes. Brain Res Brain Res Rev.
42:33–84. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Robinson TE and Berridge KC: Review. The
incentive sensitization theory of addiction: Some current issues.
Philos Trans R Soc Lond B Biol Sci. 363:3137–3146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
McCall C and Singer T: The animal and
human neuroendocrinology of social cognition, motivation and
behavior. Nat Neurosci. 15:681–688. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Lord C, Elsabbagh M, Baird G and
Veenstra-Vanderweele J: Autism spectrum disorder. Lancet.
392:508–520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Landrigan PJ: What causes autism?
Exploring the environmental contribution. Curr Opin Pediatr.
22:219–225. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hodges H, Fealko C and Soares N: Autism
spectrum disorder: Definition, epidemiology, causes, and clinical
evaluation. Transl Pediatr. 9 (Suppl 1):S55–S65. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Eden AS, Schreiber J, Anwander A, Keuper
K, Laeger I, Zwanzger P, Zwitserlood P, Kugel H and Dobel C:
Emotion regulation and trait anxiety are predicted by the
microstructure of fibers between amygdala and prefrontal cortex. J
Neurosci. 35:6020–6027. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gold AL, Shechner T, Farber MJ, Spiro CN,
Leibenluft E, Pine DS and Britton JC: Amygdala-cortical
connectivity: Associations with anxiety, development, and threat.
Depress Anxiety. 33:917–926. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rudolph S, Badura A, Lutzu S, Pathak SS,
Thieme A, Verpeut JL, Wagner MJ, Yang YM and Fioravante D:
Cognitive-affective functions of the cerebellum. J Neurosci.
43:7554–7564. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pal MM: Glutamate: The master
neurotransmitter and its implications in chronic stress and mood
disorders. Front Hum Neurosci. 15(722323)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
An L and Sun W: Prenatal melamine exposure
impairs spatial cognition and hippocampal synaptic plasticity by
presynaptic and postsynaptic inhibition of glutamatergic
transmission in adolescent offspring. Toxicol Lett. 269:55–64.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fendt M, Imobersteg S, Peterlik D,
Chaperon F, Mattes C, Wittmann C, Olpe HR, Mosbacher J, Vranesic I,
van der Putten H, et al: Differential roles of mGlu(7) and mGlu(8)
in amygdala-dependent behavior and physiology. Neuropharmacology.
72:215–223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mapelli L, Soda T, D'Angelo E and Prestori
F: The cerebellar involvement in autism spectrum disorders: From
the social brain to mouse models. Int J Mol Sci.
23(3894)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cramb KML, Beccano-Kelly D, Cragg SJ and
Wade-Martins R: Impaired dopamine release in Parkinson's disease.
Brain. 146:3117–3132. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen Y, Chen T and Hou R: Locus coeruleus
in the pathogenesis of Alzheimer's disease: A systematic review.
Alzheimers Dement (N Y). 8(e12257)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Borodovitsyna O, Flamini M and Chandler D:
Noradrenergic modulation of cognition in health and disease. Neural
Plast. 2017(6031478)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sara SJ: The locus coeruleus and
noradrenergic modulation of cognition. Nat Rev Neurosci.
10:211–223. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jenkins TA, Nguyen JC, Polglaze KE and
Bertrand PP: Influence of tryptophan and serotonin on mood and
cognition with a possible role of the gut-brain axis. Nutrients.
8(56)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Thorstensen JR, Henderson TT and Kavanagh
JJ: Serotonergic and noradrenergic contributions to motor cortical
and spinal motoneuronal excitability in humans. Neuropharmacology.
242(109761)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Brown PL, Shepard PD, Elmer GI, Stockman
S, McFarland R, Mayo CL, Cadet JL, Krasnova IN, Greenwald M,
Schoonover C and Vogel MW: Altered spatial learning, cortical
plasticity and hippocampal anatomy in a neurodevelopmental model of
schizophrenia-related endophenotypes. Eur J Neurosci. 36:2773–2781.
2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pae CU, Marks DM, Han C, Patkar AA and
Steffens D: Does neurotropin-3 have a therapeutic implication in
major depression? Int J Neurosci. 118:1515–1522. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hong SM, Liu Z, Fan Y, Neumann M, Won SJ,
Lac D, Lum X, Weinstein PR and Liu J: Reduced hippocampal
neurogenesis and skill reaching performance in adult Emx1 mutant
mice. Exp Neurol. 206:24–32. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Richards SEV and Van Hooser SD: Neural
architecture: From cells to circuits. J Neurophysiol. 120:854–866.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Institute of Medicine Committee on
Assessing Interactions Among Social B and Genetic Factors in H: The
National Academies Collection: Reports funded by National
Institutes of Health. In: Genes, behavior, and the social
environment: Moving beyond the nature/nurture debate. Hernandez LM
and Blazer DG (eds). National Academies Press (US), National
Academy of Sciences, Washington (DC), 2006.
|
|
55
|
Rodriguez PJ, Goodwin Cartwright BM,
Gratzl S, Brar R, Baker C, Gluckman TJ and Stucky NL: Semaglutide
vs tirzepatide for weight loss in adults with overweight or
obesity. JAMA Intern Med. 184:1056–1064. 2024.PubMed/NCBI View Article : Google Scholar
|