Introduction
The intervertebral disc (IVD) degeneration is a
common disease in orthopedics, which is an important incentive of
low back pain. With the deepening of aging population degree, the
IVD degeneration showing an increasing incidence, gradually becomes
a principal factor to affect the quality of life and imposes
increasingly heavy economic burdens on the society (1,2). The
pathophysiologic mechanisms of IVD have not been fully understood.
Generally, it is regarded as a dysfunctional and cell-mediated
molecular degeneration process that is dependent on age,
environment and genetics (3-5).
Certain studies have shown that IVD degeneration is mainly
manifested on the decreasing number of IVD cells and the changes of
structure and function in extracellular matrix (ECM) (6,7). The
collagen and elastin are important components of ECM in the nucleus
pulposus (NP), which can produce water-insoluble proteins through
cross-linkages to stabilize ECM.
Lysyl oxidase (LOX), a copper-dependent amine
oxidase, insolubilizes ECM proteins to keep the stability of ECM by
initiating collagen and elastin cross-linkages (8). Hence, LOX plays a critical role in
cellular proliferation, development, maturation and apoptosis
(9,10). In humans, LOX gene has already been
detected in most tissues' cells and their stromal, and is
responsible for diverse biological functions, including
developmental regulation, tissue repair, tumor suppression, cell
mobility, gene transcriptional regulation, signal transduction and
cell adhesion (11,12). However, there have been only few
related studies between LOX and IVD degeneration. It has been
reported that LOX and its family members are expressed in human NP
(13). IVD degeneration includes
not only degeneration of NP cells, but also degeneration of ECM.
The decreases of collagen and elastin cross-linkages lead to the
changes of components and structure in ECM. The fibrosis,
increasing hardness and reducing ability of relieving and resisting
mechanical load can be found in IVD. Based on the important role of
LOX in maintaining the structure and function of ECM, the present
study aimed to identify the correlations between LOX and
clinicopathological factors and explore relationships between IVD
degeneration and LOX expression in human NP.
Materials and methods
Patients and categorization
These cases were prospectively recruited at the
Affiliated Southeast Hospital of Xiamen University from January
2018 to December 2018 for the purpose of the present study; 22
cases with lumbar IVD degeneration were designed to the observed
group, and the control group consisted of 4 young patients with the
need of surgically removing the IVD due to lumbar vertebra fracture
caused by sudden trauma (Table I).
Combined with the preoperative magnetic resonance imaging
examination results, patients were grouped based on Pfirrmann
grades of the IVD (14). Inclusion
criteria: i) Meet the diagnostic criteria for lumbar disc
herniation (LDH); ii) Have a Pfirrmann grade of II to IV, as
assessed by changes in signal intensity and intervertebral disc
height on magnetic resonance imaging; iii) The course of LDH
exceeds 12 weeks, with symptoms worsening or recurring after
systemic conservative treatment; iv) LDH pain severely affects
daily life or work; v) Symptoms of muscle paralysis or single nerve
or cauda equina palsy, such as bladder dysfunction, are present;
vi) Age >18 years. Exclusion criteria: i) Previous surgical
treatment for the same segmental intervertebral disc; ii)
Complicated with malignant tumors, tuberculosis, or other
consumptive diseases; iii) Suffering from severe osteoporosis, bone
deformities, or spondylitis; iv) Complicated with severe infections
or immune system diseases. The control group represented grade I
(group A), and the observed group was subdivided into 4 groups:
Grade II (group B), grade III (group C), grade IV (group D) and
grade V (group E). In the group A, there were 3 men and 1 woman,
aged from 15 to 19 years (16.5 years on average), with IVD segment
of L2-3 in 1 case and L3-4 in 3 cases. In the
group B, there were 3 men and 2 women, aged from 23 to 29 years
(31.2 years on average), with IVD degeneration segment of
L4-5 in 4 cases and L5S1 in 1
case. In the group C, there were 4 men and 2 women, aged from 32 to
49 years (40.8 years on average), with IVD degeneration segment of
L4-5 in 4 cases and L5S1 in 2
cases. In the group D, there were 4 men and 2 women, aged from 37
to 59 years (49.7 years on average), with IVD degeneration segment
of L4-5 in 4 cases and L5S1 in 2
cases. In the group E, there were 2 men and 3 women, aged from 48
to 68 years (60.6 years on average), with IVD degeneration segment
of L4-5 in 2 cases and L5S1 in 3
cases. Institutional review board approval (approval no. 20210303)
for the present study was obtained on March 3, 2021 by the Xiamen
University's Medical Ethical Committee (Zhangzhou, China). Informed
consent was obtained from each patient for participation in
compliance with the Helsinki declaration and its amendments.
 | Table IGeneral characteristics of
patients. |
Table I
General characteristics of
patients.
| | Group A | Group B | Group C | Group D | Group E |
|---|
| Pfirrmann grade | I | II | III | IV | V |
| Sex
(Female/Male) | 1/3 | 2/3 | 2/4 | 2/4 | 3/2 |
| Age (average,
year) | 16.5 | 31.2 | 40.8 | 49.7 | 60.6 |
| Intervertebral disc
segment
(L2-3/L3-4/L4-5/L5S1) | 1/3/0/0 | 0/0/4/1 | 0/0/4/2 | 0/0/4/2 | 0/0/2/3 |
Sample collection and detection
The removed IVD specimens were washed using sterile
normal saline for 3 times and NP was removed. One segment that was
put into the specimen bag was fixed in 10% neural buffered formalin
at room temperature for 48 h, and was embedded in paraffin after
routine steps of desiccation. For H&E and immunohistochemistry
(IHC) staining, 3 µm-thick sections were cut. Another segment was
put into a centrifuge tube and quickly preserved in liquid nitrogen
for the western blotting and reverse transcription-quantitative PCR
(RT-qPCR) experiment. All specimens were repeated three times to
test the reproducibility of the experimental results and reduce the
influence of accidental error.
H&E and IHC staining
A total of three sections were received from each
specimen for H&E and IHC staining. After routine H&E
staining, morphologic features of IVD tissues with different
degeneration degrees were observed under the light microscope. The
sections were put in an oven at 60˚C for 1 h. After dewaxing
(xylene, Maixin Fuzhou) and hydration (ethanol solutions, Maixin
Fuzhou), the removal of endogenous peroxidase (3% hydrogen peroxide
solution, at room temperature for 10 min, Lablead Biotech) and the
blockage (5% BSA, at room temperature for 1 h, Lablead Biotech) by
non-specific antigen were applied in the sections. The sections
were then incubated at 4˚C for 12 h with primary antibodies against
rabbit anti-human LOX polyclonal antibody (1:300; cat. no.
ab174316; Abcam). Then the sections were incubated at 37˚C for 30
min with secondary antibodies (undiluted; cat. no. Kit-5030; Maixin
Fuzhou) against biotin-linked goat-anti-rabbit IgG. The sections
with DAB staining and hematoxylin staining were analyzed using the
light microscope.
Western blotting
Total protein samples from NPs were extracted using
cell lysis buffer (undiluted; cat. no. W0013; Lablead Biotech) and
quantitative analysis was performed through ultraviolet
spectrophotometer, wherein the absorbance was measured at a
wavelength of 280 nm. Then, the protein samples were stored at
-20˚C. Equal amounts of protein, corresponding to 20 micrograms per
lane, were separated by SDS-PAGE gel electrophoresis using a 12%
acrylamide gel, and then transferred to PVDF membranes. After
blocking with 5% skim milk on shaker for 1 h at room temperature,
PVDF membranes were cut according to the molecular weight of target
genes proteins and reference genes proteins. The membranes were
incubated with primary antibodies against LOX (1:1,000; cat. no.
ab174316; Abcam) at 4˚C overnight and primary antibodies were
recycled later. After washing the membranes 3 times (10 min each)
with TBST 1X, PVDF membranes were incubated with the appropriate
HRP-conjugated secondary antibodies (undiluted; cat. no. Kit-5030;
Maixin Fuzhou) for 1 h at room temperature and secondary antibodies
were recycled later. Then, the membranes were washed 3 times (10
min each) with TBST 1X containing 0.1% (v/v) Tween 20. Freshly
enhanced chemiluminescent agent (ECL Reagent A and B; cat. no.
E1050; Lablead Biotech) was used (a mixture of A solution and B
solution by 1:1), which was evenly dripped on the membranes. After
being incubated at room temperature for 2 min, quick exposure and
development were carried out and gray analysis was performed using
Scion Image software (version: 4.0.3.2; Scion Corporation).
RT-qPCR
After homogenization, total mRNA was extracted from
NPs with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The RNA concentration and purity were measured,
with an optimal 260/280 nm absorbance ratio of 2.0, after which
reverse transcription from total RNA to cDNA was conducted using
the RevertAid RT Kit (cat. no. K1691; Thermo Fisher Scientific,
Inc.). Human-β-actin gene was selected as an endogenous control. A
total of 1 µl cDNA template was used for PCR amplification. The PCR
primers, designed for the target gene and synthesized by Sangon
Biotech Co., Ltd., were labeled with a fluorophore (Super Green I;
SYBR021; Lablead Biotech) to enable quantitative detection during
the PCR process. Thermocycling conditions were as follows:
Pre-denaturation at 94˚C for 5 min, denaturation at 94˚C for 30
sec, annealing at 60˚C for 30 sec, extension at 72˚C for 30 sec,
followed by 30 cycles; extension at 72˚C for 10 min. A total of 10
µl PCR amplification products were analyzed on a 1% agarose gel
electrophoresis and the gel analysis system was used for detection.
The radio of gray value between target genes and reference genes
represented the expression level of LOX. The
2-ΔΔcq method was used for
relative quantitative analysis (15). The PCR primers were as follows:
LOX forward, 5'-TGATGCCAACACCCAGAG-3' and reverse,
5'-TCATAACAGCCAGGACTCAAT-3'; human-β-actin forward,
5'-TGGGCATGGAGTCCTGTG-3' and reverse, 5'-TCTTCATTGTGCTGGGTG-3'.
Statistical analysis
All data were analyzed using statistical software
SPSS 20.0 (IBM Corp.). Measuring material accorded with normal
distribution were expressed as the mean ± standard deviation.
Comparisons of groups were achieved by one-way analysis of variance
(ANOVA) followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference. The linear
regression equation was set up for the association of the
expression of LOX protein with age and Pfirrmann grades.
Results
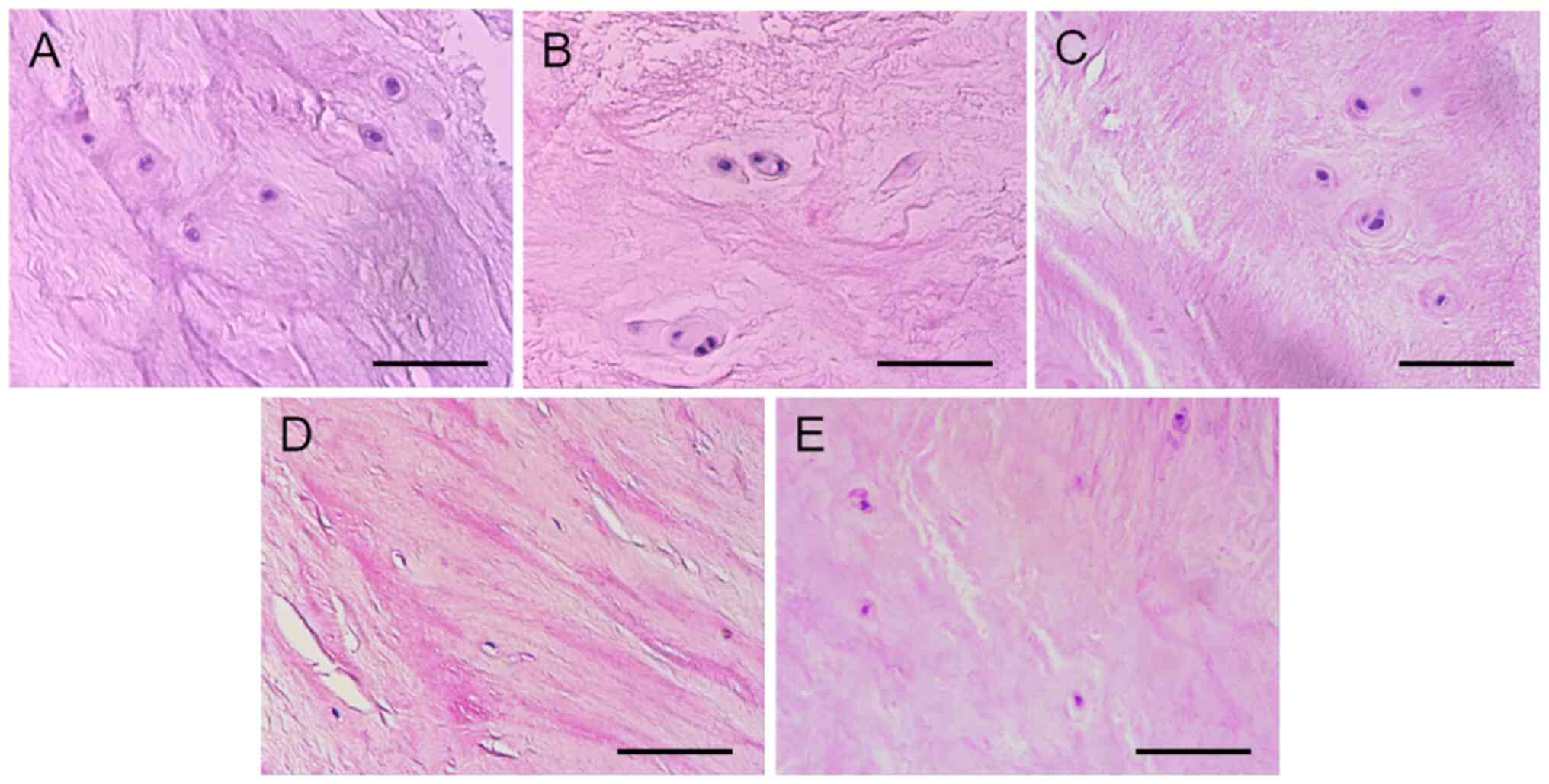
Normal NPs showed loose structure, complete matrix
components and arranged fiber structure. Numerous NPs cells were
distributed in the ECM. However, with dense and shrinking
structure, degenerated NPs showed incomplete matrix components,
fibroplasia and derangement. There were few NPs cells in the ECM
(Fig. 1).
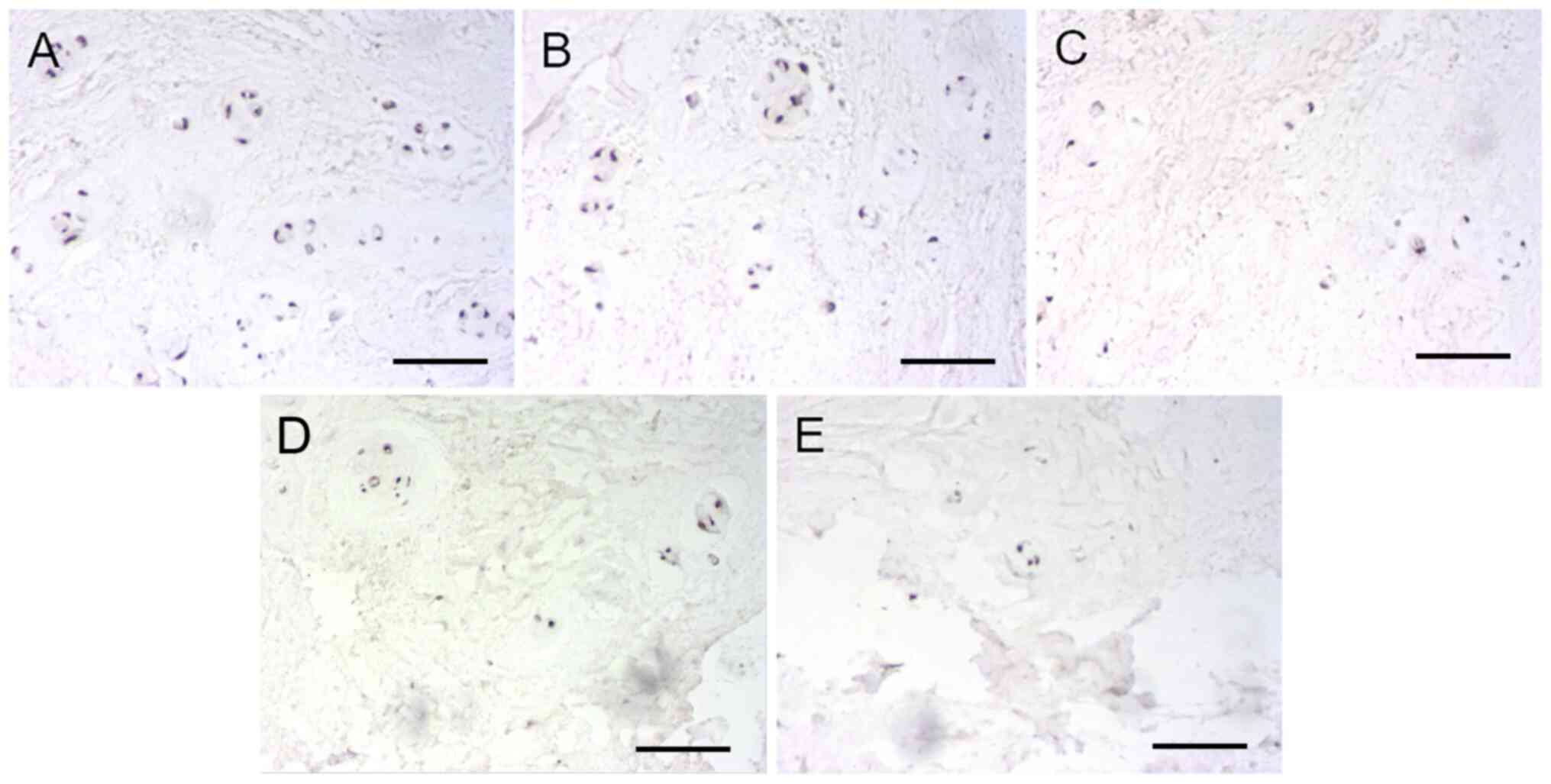
IHC detection revealed that LOX expression could be
found in both the observed group and the control group to a certain
extent. Data from all included patients were used to show the
association between age, degeneration grades and the ratio of
LOX-positive cells. In the control group, positive cells were
significantly expressed in NP and most of cytoplasm manifested tan
color, indicating that positive cells accounted for a large
proportion. Although there were also positive cells in the observed
group, the overall staining demonstrated lower intensity and the
number of positive cells was less than that in control group
(Fig. 2). Furthermore, the number
of positive cells decreased gradually according to the grouping
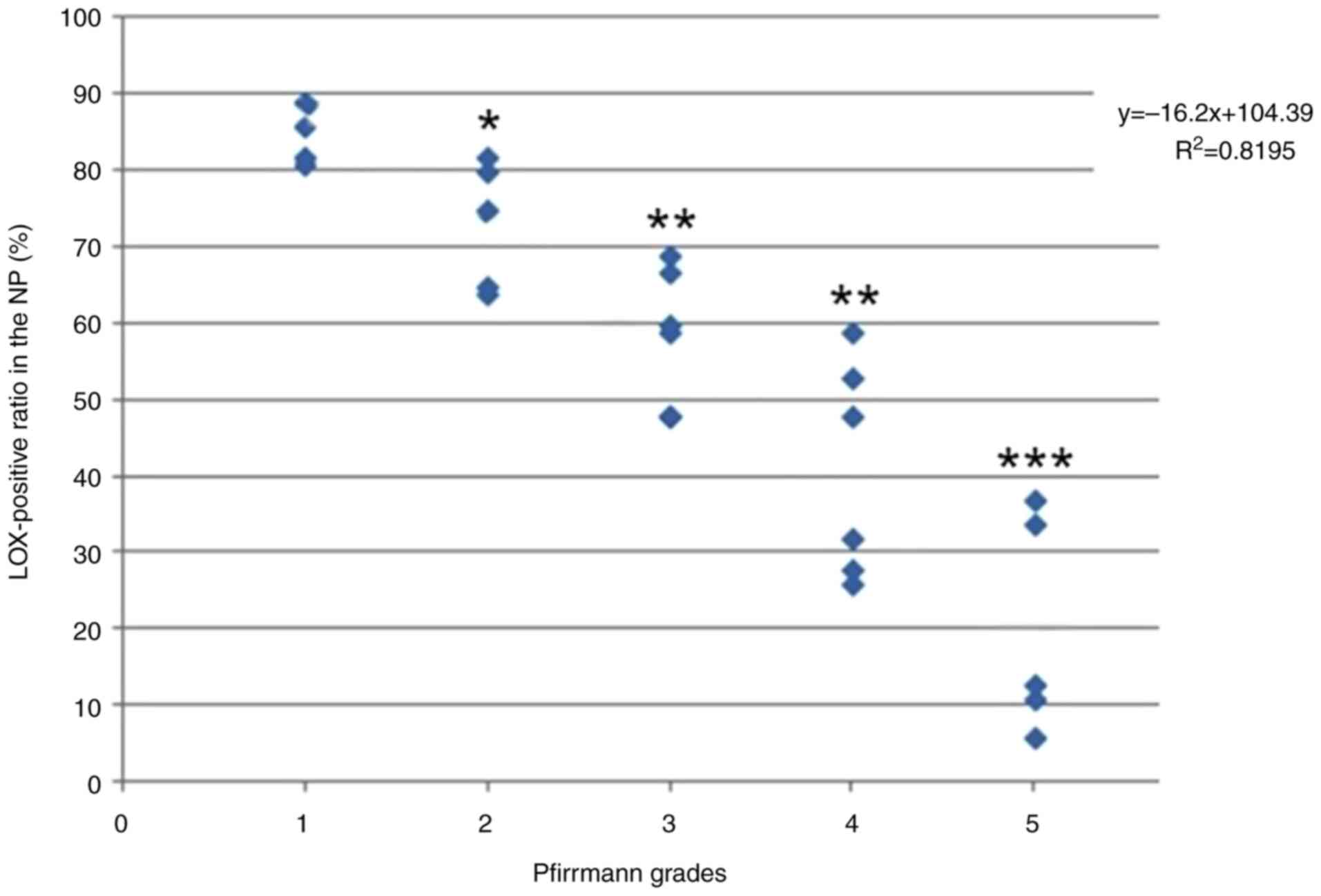
order of Group B, C, D and E. The positive rate of LOX expression
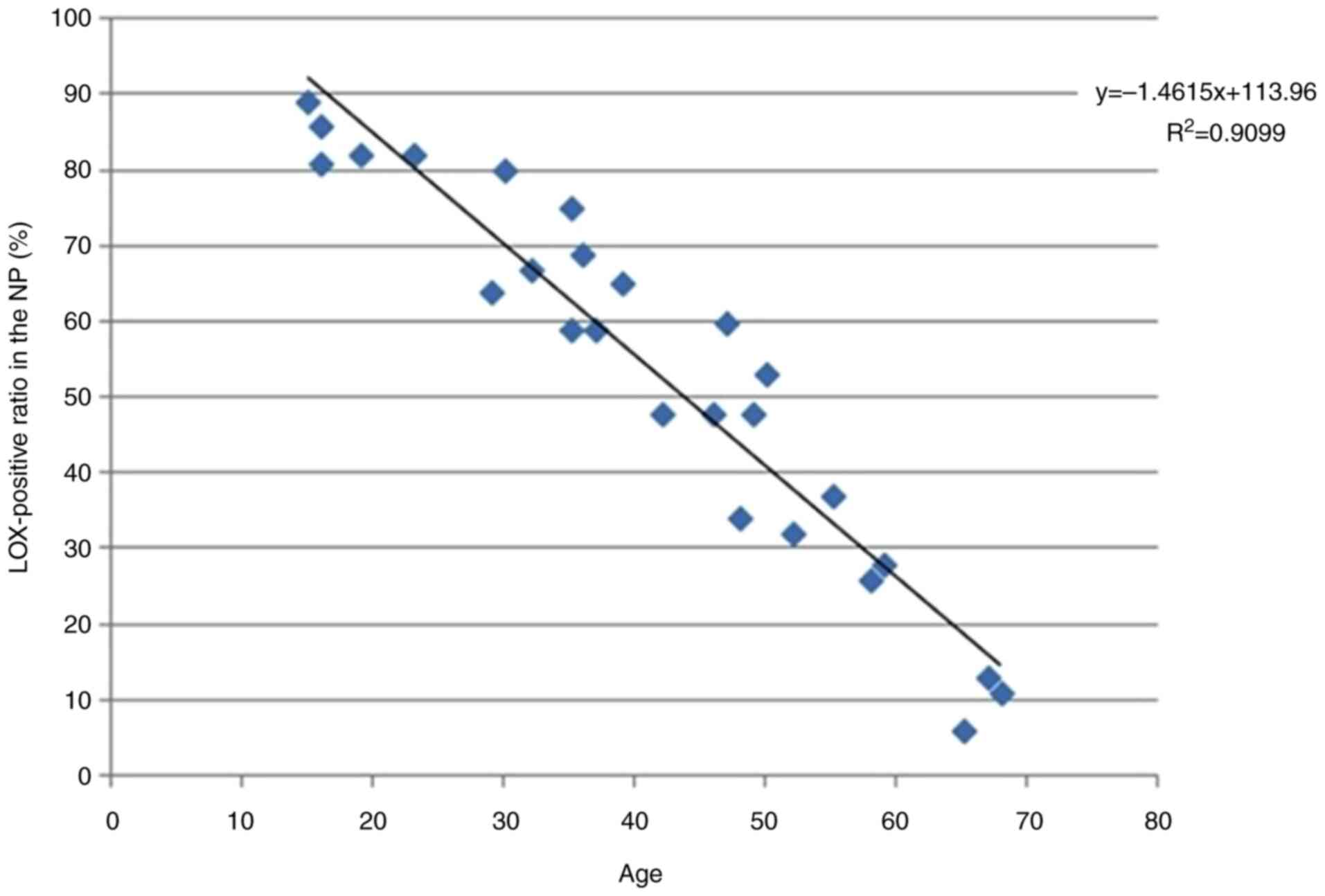
in NP cells negatively associated with Pfirrmann grades
(y=-16.2x+104.39, R2=0.8195; Fig. 3) and age (y=-1.4615x+113.96,
R2=0.9099; Fig. 4).
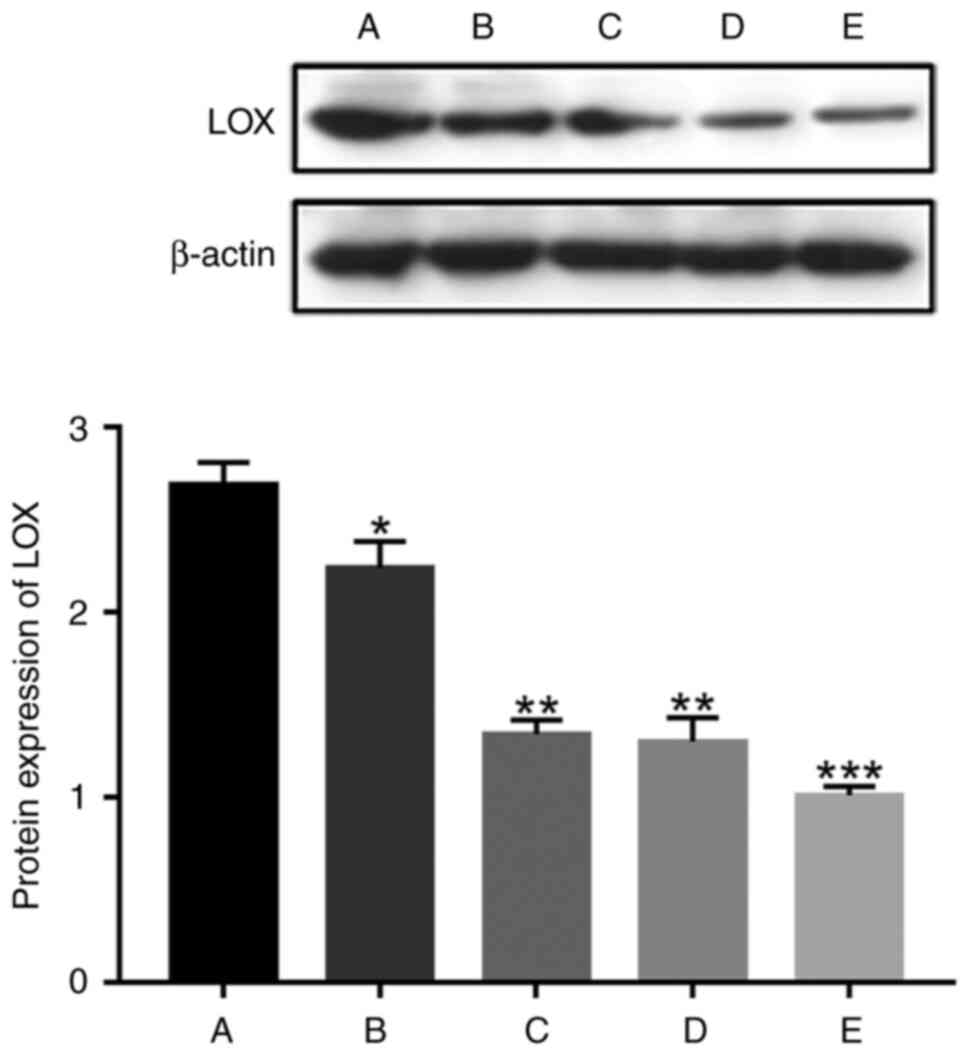
Detections by western blotting identified that the
protein expression of LOX from NPs in group A was 2.69±0.24, group
B was 2.24±0.32, group C was 1.34±0.19, group D was 1.30±0.32 and
group E was 1.01±0.12. Using ANOVA, it was found that there was a
significant difference in LOX protein expression among the five
groups (χ2=12.7, P=0.013). The protein expression of LOX
in group A was higher than that in groups B, C, D and E; the
protein expression of LOX in group B was higher than that in groups
C, D and E; the protein expression of LOX in both groups C and D
was higher than that in group E. With increasing IVD degeneration,
the protein expression of LOX in NP gradually decreased (Fig. 5).
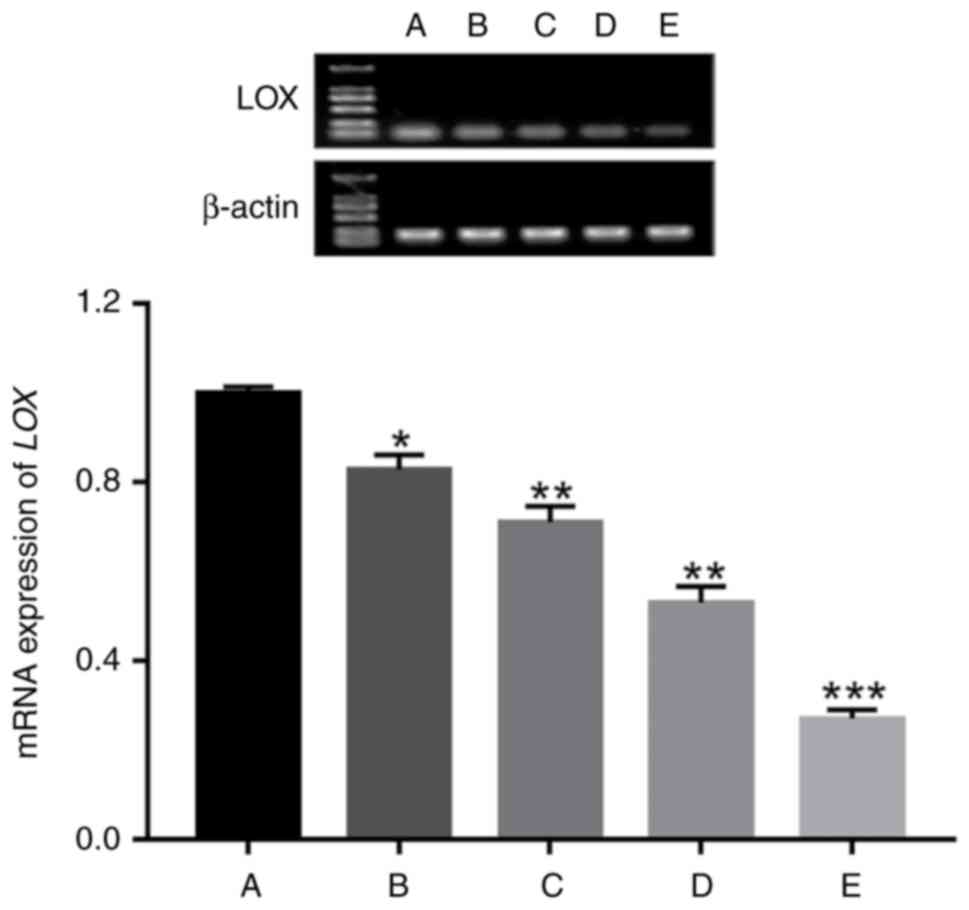
The results of RT-qPCR showed that the mRNA
expression of LOX from NPs in group A was 1.00±0.03, group B was
0.83±0.07, group C was 0.71±0.09, group D was 0.53±0.09 and group E
was 0.27±0.05. Using ANOVA, it was found that there was a
significant difference in LOX mRNA expression among the five groups
(χ2=15.357, P=0.004). The mRNA expression of LOX in
group A was higher than that in groups B, C, D and E; the mRNA
expression of LOX in group B was higher than that in groups C, D
and E; the mRNA expression of LOX in both group C and D was higher
than that in group E. With increasing IVD degeneration, the mRNA
expression of LOX in NP gradually decreased (Fig. 6).
Discussion
Deviant structure and function of spine caused by
the IVD degeneration would contribute to a range of disorders,
including IVD herniation, spondylolisthesis and spinal stenosis.
Patients with low back pain caused by these disorders' account for
a large proportion in outpatients and inpatients annually. A lot of
working time and medical expenses are spent on mitigating the pain,
which seriously affects the quality of life. It was previously
suggested that degenerative change of IVD is mainly on degeneration
of NP (16), and its histological
characteristics shows the decrease of cells and ECM, combined with
the hyperplasia and disorder of fiber components in ECM.
The biological functions of LOX are extensive, and
the expression of LOX can affect the structure and function of
numerous tissues. It has been revealed that the mice with LOX gene
knocking out will succumb after birth and the dissection results of
deadly newborn mice showed that the breakdown of pleuroperitoneal
membrane led to abdominal organs into the chest. Finally, tissue
staining technology and microscope examination demonstrated that
the deficiency of elastin and collagen cross-linkages in arteries
and other important tissues in newborn mice lacking LOX gene
resulted in weak tissue elasticity and poor mechanical strength,
which make numerous tissues and organs fail in resisting external
pressures (17). In a mouse model
of skin wound healing, Szauter et al (18) found that the mRNA expression of LOX
in skin tissues increased gradually in the early stage of wound
healing, using PCR technology. Cai et al (19) reported the same conclusion and they
identified that LOX could promote tendon regeneration, ligament
healing and cartilage reconstruction. It is inferred that LOX could
contribute to ECM remodeling and tissue repair through the
promotion of the collagen and elastin cross-linkages (20).
The results of the present study showed that the
positive rate of LOX expression in NP cells negatively associated
with Pfirrmann grades and age. The protein and mRNA expression of
LOX from NPs in the control group were significantly higher than
that in the observed group. In addition, with increasing IVD
degeneration, there was a decreasing tendency towards the
expression of LOX, the collagen and elastin cross-linkages, the
components of ECM, but the fiber components were hyperplasia. When
it comes to the NP, its hydration, tissue elasticity, mechanical
strength and the ability of dispersing and relieving mechanical
load were decreasing, but the hardness was increasing. With the
gradual loss of the IVD height, the longitudinal or transverse
pressures from the spine were mainly distributed in the surrounding
annulus fibrosus (AF), which contributed to radial and annular tear
of AF, the herniation of NP, and finally the compression of spinal
cord nerve (21). Additionally, the
decreases of LOX content also affected the regeneration and
remodeling of ECM. When NP could not be repaired timely and
effectively due to chemical or mechanical damages, the process of
degeneration of herniated NP was accelerating. Hence, it can be
deduced that the decreased expression of LOX might be the one of
important factors in the IVD degeneration.
There are certain limitations to the current study;
the molecular biology and the mechanism of LOX's role in IVD
degeneration were not explored, which need to be further
investigated. Furthermore, due to the small sample size of the
present study, larger clinical trials are needed to verify the
association between the expression of LOX in NP and age. As the
incidence of disc degeneration increases with the increase of age,
most of the patients with older fracture are accompanied by
different degrees of disc degeneration, which does not meet our
inclusion criteria. Therefore, in the present study, the age of
patients included in the normal group was younger than that in the
degenerative group. Although there were significant differences in
the age of the patients included in each group, the difference in
age did not affect the present conclusions, thus statistical tests
for age were not performed. Given that LOX is important in the
inflammatory phase, the increase of the LOX following skin injury
has been documented (10,22). Consequently, incorporating patients
with spinal trauma into the control group in our research could
potentially affect the results of LOX detection, representing a
limitation of the present study; the control group consisted of 4
young patients with the need of surgically removing the IVD due to
lumbar vertebra fracture caused by sudden trauma. Patients in the
control group had no history of low back pain and spinal canal
surgery and had pathological reports after operation showing normal
IVD. This method has also been reported (23).
In conclusion, the aforementioned results suggested
that the expression of LOX was closely associated with generation
of herniated NP and the decreases of LOX content might be a key
factor in the IVD degeneration. The attempt to increase the
expression level of LOX in NP or inhibit the decrease of LOX
content provides new ideas and methods for delaying and treating
the IVD degeneration.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 82172477 and 81600696), the
Natural Science Foundation of Fujian (grant nos. 2023J011844,
2023J011838 and 2019J01144) and the Fujian Medical University Union
Hospital Talent Research Launch Project (grant no. 2024XH001).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CC and WF performed experiments, analyzed data and
wrote the manuscript. CC carried out H&E and IHC analyses. ZS,
LL and CL performed western blotting and RT-qPCR. DL conceived the
study, designed and analyzed experiments and wrote the manuscript.
CC and DL confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval (approval no. XMUDN 2017-Y-017;
28/10/2017) for the study was granted by the Xiamen University's
Medical Ethical Committee (Zhangzhou, China). Informed consent was
obtained from all participants in compliance with the Helsinki
declaration and its amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evans CH and Huard J: Gene therapy
approaches to regenerating the musculoskeletal system. Nat Rev
Rheumatol. 11:234–242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sakai D and Grad S: Advancing the cellular
and molecular therapy for intervertebral disc disease. Adv Drug
Deliv Rev. 84:159–171. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Williams FM, Popham M, Sambrook PN, Jones
AF, Spector TD and MacGregor AJ: Progression of lumbar disc
degeneration over a decade: A heritability study. Ann Rheum Dis.
70:1203–1207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang R, Xu C, Zhong H, Hu B, Wei L, Liu N,
Zhang Y, Shi Q, Wang C, Qi M, et al: Inflammatory-sensitive CHI3L1
protects nucleus pulposus via AKT3 signaling during ntervertebral
disc degeneration. FASEB J. 34:3554–3569. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin D, Alberton P, Caceres MD, Prein C,
Clausen-Schaumann H, Dong J, Aszodi A, Shukunami C, Iatridis JC and
Docheva D: Loss of tenomodulin expression is a risk factor for
age-related intervertebral disc degeneration. Aging Cell.
19(e13091)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De Campos MF, de Oliveira CP, Neff CB,
Correa OM, Pinhal MA and Rodrigues LM: Studies of molecular changes
in intervertebral disc degeneration in animal model. Acta Ortop
Bras. 24:16–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trackman PC: Functional importance of
lysyl oxidase family propeptide regions. J Cell Commun Signal.
12:45–53. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lucero HA and Kagan HM: Lysyl oxidase: an
oxidative enzyme and effector of cell function. Cell Mol Life Sci.
63:2304–2316. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dobaczewski M, Gonzalez-Quesada C and
Frangogiannis NG: The extracellular matrix as a modulator of the
inflammatory and reparative response following myocardial
infarction. J Mol Cell Cardiol. 48:504–511. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Siddikuzzaman , Grace VM and
Guruvayoorappan C: Lysyl oxidase: A potential target for cancer
therapy. Inflammo-pharmacology. 19:117–129. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Teppo S, Sundquist E, Vered M, Holappa H,
Parkkisenniemi J, Rinaldi T, Lehenkari P, Grenman R, Dayan D,
Risteli J, et al: The hypoxic tumor microenvironment regulates
invasion of aggressive oral carcinoma cells. Exp Cell Res.
319:376–389. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li X, Wang X, Hu Z, Chen Z, Li H, Liu X,
Yong ZY, Wang S, Wei Z, Han Y, et al: Possible involvement of the
oxLDL/LOX-1 system in the pathogenesis and progression of human
intervertebral disc degeneration or herniation. Sci Rep.
7(7403)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kohyama K, Saura R, Doita M and Mizuno K:
Intervertebral disc cell apoptosis by nitric oxide: Biological
understanding of intervertebral disc degeneration. Kobe J Med Sci.
46:283–295. 2000.PubMed/NCBI
|
|
17
|
Hornstra IK, Birge S, Starcher B, Bailey
AJ, Mecham RP and Shapiro SD: Lysyl oxidase is required for
vascular and diaphragmatic development in mice. J Biol Chem.
278:14387–14393. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Szauter KM, Cao T, Boyd CD and Csiszar K:
Lysyl oxidase in development, aging and pathologies of the skin.
Pathol Biol (Paris). 53:448–456. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cai L, Xiong X, Kong X and Xie J: The role
of the lysyl oxidases in tissue repair and remodeling: A concise
review. Tissue Eng Regen Med. 14:15–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akagawa M and Suyama K: Characterization
of a model compound for the lysine tyrosylquinone cofactor of lysyl
oxidase. Biochem Biophys Res Commun. 281:193–199. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sowa G, Coelho P, Vo N, Bedison R, Chiao
A, Davies C, Studer R and Kang J: Determination of annulus fibrosus
cell response to tensile strain as a function of duration,
magnitude, and frequency. J Orthop Res. 29:1275–1283.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fushida-Takemura H, Fukuda M, Maekawa N,
Chanoki M, Kobayashi H, Yashiro N, Ishii M, Hamada T, Otani S and
Ooshima A: Detection of lysyl oxidase gene expression in rat skin
during wound healing. Arch Dermatol Res. 288:7–10. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Y, Han S, Kong M, Tu Q, Zhang L and
Ma X: Single-cell RNA-seq analysis identifies unique chondrocyte
subsets and reveals involvement of ferroptosis in human
intervertebral disc degeneration. Osteoarthritis Cartilage.
29:1324–1334. 2021.PubMed/NCBI View Article : Google Scholar
|