Introduction
Worldwide, diabetes represents a major public health
challenge: Its prevalence among the population was 10.5% in 2021,
and this is projected to be 12.2% in 2045(1). The mortality and morbidity rates of
diabetes are associated with various chronic complications, of
which diabetic nephropathy (DN) is typical. This is one of the main
microvascular complications that lead to end-stage renal disease
(2,3). DN is also termed diabetic kidney
disease and it similarly poses one of the leading public health
challenges globally. DN is characterized by an increase in urine
albumin excretion (microalbuminuria) and/or a decreased glomerular
filtration rate in clinical practice (4,5). DN
may lead to progressive renal failure if not diagnosed and treated
in a timely manner, and its progression may lead to irreversible
kidney damage, a decreased quality of life and premature death
(6,7). At present, the conventional DN
treatment medications are angiotensin II receptor blockers (ARBs)
and angiotensin-converting enzyme inhibitors (ACEIs) (8). However, patients who are treated with
ARBs or ACEIs may be at high risk of diabetic ketoacidosis and
irreversible kidney damage due to the side effects of these
medications and their long-term use, which limits the clinical
usefulness of these drugs (9,10). At
present, no specific medications or treatment options are available
that are effective in slowing the progression of DN to kidney
failure; however, natural components remain an important resource
and research ‘hotspot’ in the field of developing novel drugs, as
they are able to continuously provide new chemical scaffolds, and
are of great benefit for the treatment of different diseases
(11). Therefore, the search for
safe and more effective natural components as therapeutic options
is urgently required.
According to the Chinese Pharmacopoeia (2020
edition), Astragalus is well recognized as a traditional
herb, and is a leguminous plant known as ‘Huangqi’ in China
(12). It has been shown that
Astragalus exerts a variety of biological effects, including
immune regulation, also acting as an anti-inflammatory antioxidant
and an antitumor agent, and it has been widely used in the
treatment of diabetes, cardiovascular diseases, respiratory
diseases, nervous system diseases, cancer and several other
diseases, according to previous studies (13-16).
Astragalus polysaccharides (APS; molecular formula,
C10H7ClN2O2S) (Fig. 1) are important natural active
compounds extracted from Astragalus, and this natural
component has been mainly used in studies in the preclinical stages
(17). Numerous studies have shown
that APS possesses a range of biological activities, including
regulating kidney injury, blood glucose and blood lipid levels, in
addition to functioning as an anticancer and anti-aging agent,
among which the treatment of DN via modulating kidney injury is the
most important (18-21).
The aforementioned preclinical findings were performed by different
research groups, however, which may lead to inconsistencies in
their conclusions. Hence, systematic reviews and meta-analyses
should logically be performed to integrate the findings, and to
minimize any bias (22). Through
integrating the previously obtained experimental data, the
possibility of applying preclinical findings to clinical settings
is increased, and the need for animal studies is thereby reduced
(with the attendant reduction in the numbers of animals needing to
be sacrificed) (23,24). To the best of the authors'
knowledge, however, a high-level, evidence-based study of the
therapeutic effects of APS on DN, together with an exploration of
the underlying potential mechanisms, has not been summarized to
date. Therefore, in the present study, it was aimed to undertake a
systematic review and meta-analysis to elucidate both the
pharmacological effects of APS on models of DN, and the potential
underlying biological mechanisms, through analyzing the original
observational data.
Materials and methods
The Preferred Reporting Items for Systematic reviews
and Meta-Analysis (PRISMA) were applied for the conception and
performance of this meta-analysis (25).
Search strategy and study
selection
Objective studies were identified through searching
for papers published between January 2007 and March 2024 in the
following databases: Web of Science (www.webofscience.com), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (www.embase.com) and China National Knowledge
Infrastructure (oversea.cnki.net/index/), without implementing any
language restrictions. Broader terms of mesh and free-text terms
were applied to identify target drugs and diseases:
‘Astragalus polysaccharide’, ‘Diabetic nephropathies’,
‘Diabetic Glomerulosclerosis’ and ‘Diabetic Kidney Disease’. If
necessary, the authors of the identified studies were contacted to
obtain more information.
Eligibility criteria
According to the patient/population, intervention,
comparison and outcomes principle, the following inclusion criteria
were applied: i) Regarding the participants, DN model animals were
selected; ii) concerning the intervention, the animals were treated
with APS; iii) comparisons were made between the treated animals
and the control groups, including untreated controls; and iv)
regarding the outcomes, the primary outcome indicators included
creatinine (CR), kidney to body weight ratio (KI), blood urea
nitrogen (BUN), urine protein (Upro) and fasting blood glucose
(FBG).
The exclusion criteria comprised the following: i)
Duplicate data or studies; ii) if the article was merely a case
report, clinical trial, opinion, abstract, review or an in
vitro trial; iii) no control group or treatment group was
provided, or the study was performed either without APS, or APS was
combined with other drugs; iv) no sample size or specific
statistical analysis method was shown; and v) the study did meet
the aforementioned inclusion criteria, but the full text was not
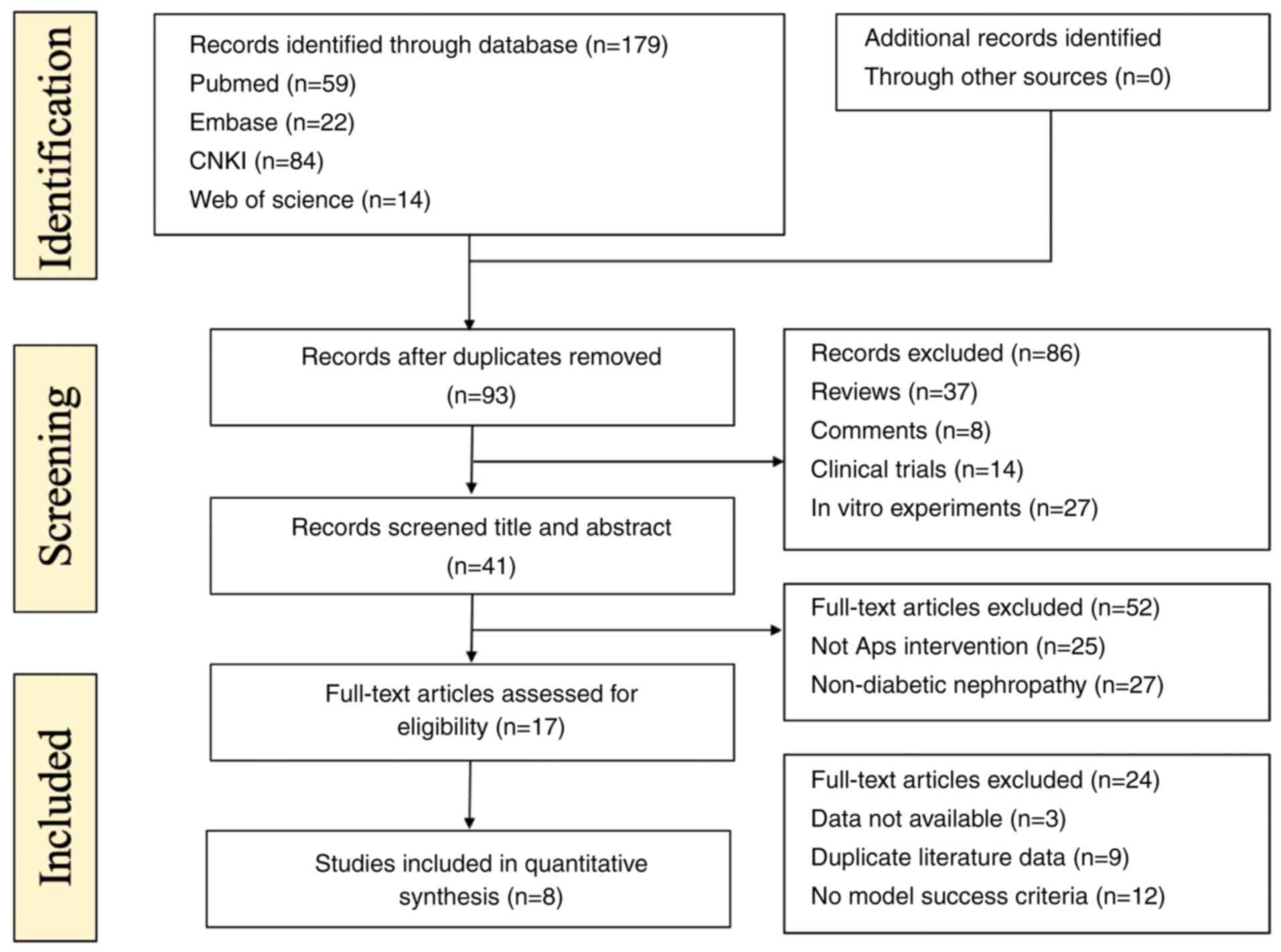
available (Fig. 2).
Extraction of data and quality
control
All selected documents were imported into Endnote
X9, and duplicates were removed. According to the inclusion and
exclusion criteria, two researchers performed the literature
searches independently. Both the title and the abstract were
checked to remove irrelevant literature, and the remaining studies
were then carefully examined by reading the full text. The
following relevant information from the selected studies was
extracted: i) The first author name and the publication date; ii)
the basic information, including species, group, body weight (BW)
and sample size; iii) the modeling methods and success criteria;
iv) the APS intervention route, APS dose and treatment duration;
and v) the outcome measures and statistical differences between the
different groups. An Excel database was created, and the data were
extracted from the target articles manually. The authors of the
relevant articles were contacted for their original data when the
results were presented graphically only; if no response was
received from the authors concerned, then WebPlotDigitizer 4.5
software (https://automeris.io/WebPlotDigitizer) was used to
quantify the graphic data. The last data point among the multiple
time points was subsequently extracted for meta-analysis. If
multiple doses of APS were applied in the treatment groups, the
highest group was selected for analysis. The formula ‘SD=SEM x
n1/2’ was employed to convert the data into SD where the data in
the text were shown as SEM (26).
The SYRCLE risk of bias tool for animal studies was applied to
examine the risk of bias in the included studies by two assessors
independently (27). The
assessments of measurement bias, selection bias, reporting bias,
implementation bias and other sources of bias were included. Any
disagreements were resolved with the corresponding author during
the quality evaluation.
Statistical analysis
The statistical software package STATA (15.1
edition; StataCorp LP) was applied for the statistical analyses. It
was ensured that the results included were continuous variables;
therefore, the total effect size was expressed in terms of
standardized mean differences (SMDs) and 95% confidence intervals
(CIs). P<0.05 of was considered to indicate a statistically
significant difference. The statistical heterogeneity was assessed
using I-Square (I2) analysis. If I2>50%
was considered to indicate statistically significant heterogeneity,
the random effects model was used, whereas the fixed effects model
was used in all other cases (28).
To explore the underlying causes of heterogeneity, subgroup and
sensitivity analyses were performed, including the indicators of
FBG, Cr, BUN and Upro. To perform the subgroup analysis, subgroups
were designated according to the following: i) the species (mice or
rats); ii) the treatment duration (≤8 weeks or >8 weeks); and
iii) the APS dose (<400 mg/kg/day or ≥400 mg/kg/day). Egger's
linear regression analysis and Begg's rank correlation analysis
were applied to evaluate the publication bias of FBG, Cr, BUN and
Upro. If publication biases were to be identified, then
trim-and-fill methods were applied. In order to better present the
effects of intervention duration and the APS dose on the results,
time-dose response relationship plots of FBG, Cr, BUN and Upro were
created. All groups with results P<0.05 were included in the
time-dose analysis for the multiple groups that participated in the
same study.
Results
Study selection
Conducting a search of the four major databases (Web
of Science, PubMed, Embase and CNKI), the existence of 179
potential target articles was disclosed, including 59 from PubMed,
14 from Web of Science, 22 from Embase and 84 from CNKI. After
consolidating the 179 articles and removing the duplicates, 93
records were retained. After performing two more screenings
according to the pre-set criteria, a final total of 8 articles met
the eligibility requirements, and were finally selected in the
current meta-analysis. The selection process of the articles is
shown in Fig. 2.
Features of the selected studies
A total of 8 studies were included, and the DN
models were based on either male rats or mice. In total, 226
animals were used, including 82 in the model group and 144 in the
treatment group. A total of 6 studies used Sprague Dawley rats
(184/226; 81.4%), 1 study used Wistar rats (30/226; 13.3%), and 1
study used db/db mice (12/226,5.3%). The animal weight was
mentioned in five of the studies, and age was mentioned in six of
the studies. Different doses of streptozotocin (STZ) were applied
during the construction of the DN model in 7 studies, among which
three of those studies used STZ combined with a high sugar, high
fat diet, with high fat feed to replicate the conditions of
diabetes. Spontaneous diabetic model (SDM) mice were used in 1
study. All studies considered FBG >11.1 mmol/l (range: 11.1~16.7
mmol/l) as the criterion to evaluate the success of the models,
with the exception of the study that used SDM model mice. The
minimum and the maximum duration of administration of APS were 6
and 12 weeks, respectively, whereas the minimum and the maximum
doses of APS were 25 mg/kg/day and 1,000 mg/kg/day, respectively.
Regarding the primary outcome measures, 6 studies recorded FBG
levels, 7 studies recorded BUN levels, 5 studies recorded Cr
levels, and 6 studies recorded Upro levels. A total of 3 studies
focused on inflammatory indicators, including IL-6, IL-1β and
TNF-α. Certain studies mentioned markers of oxidative stress, such
as malondialdehyde (MDA) and superoxide dismutase (SOD). The
detailed information concerning APS is shown in Table I, and the features of the present
study are shown in Table II.
 | Table IInformation of Astragalus
polysaccharides in each study. |
Table I
Information of Astragalus
polysaccharides in each study.
| First author,
year | Supplier | Purity (%) | (Refs.) |
|---|
| Chen et al,
2008 | Macklin Inc.
Ltd. | >98 | (18) |
| Liu et al,
2023 | Beijing Solarbio
Science & Technology Co., Ltd. | >90 | (30) |
| Meng et al,
2020 | Lanzhou Wotelaisi
Biological Co. Ltd. | >98 | (19) |
| Zhang et al,
2007 | Company of
Bencao | >95 | (20) |
| Peng et al,
2020 | SenxingBio | >70 | (33) |
| Guo et al,
2023 | Beijing Solarbio
Science & Technology Co., Ltd. | >90 | (21) |
| Wu et al,
2024 | MilliporeSigma | >99.8 | (32) |
| Mao et al,
2010 | Wosenbio | >70 | (29) |
 | Table IIBasic characteristics of the included
studies. |
Table II
Basic characteristics of the included
studies.
| First author/s,
year | Species (sex,
n=treatment/ model group, weight) | Modeling
method/standard | APS
(administration, drug dose, duration) | Outcomes | Intergroup
difference | (Refs.) |
|---|
| Chen et al,
2008 | db/db and db/m mice
(male,6/6,4-6 weeks) | Spontaneous
diabetic model | By intragastric,
200 mg/kg/d, 12 weeks | 1. Upro | P<0.05 | (18) |
| | | | | 2. FBG | P<0.01 | |
| | | | | 3. GTT | P <0.01 | |
| | | | | 4. BW | P<0.01 | |
| | | | | 5. BUN | P<0.01 | |
| Liu et al,
2023 | Sprague-Dawley rats
(male, 27/9, 252±2.7 g) | Intraperitoneal
injection of STZ (60 mg/kg), FBG >16.7 | By intragastric,
200 mg/kg/d, 12 weeks | 1. Upro | P<0.05 | (30) |
| | | | | 2. FBG | P<0.05 | |
| | | | | 3. GHb | P<0.05 | |
| | | | | 4. BUN | P<0.05 | |
| | | | | 5. Cr | P<0.05 | |
| | | | | 6. TC | P<0.05 | |
| | | | | 7. TG | P<0.05 | |
| | | | | 8. HDL | P<0.05 | |
| | | | | 9. LDL | P<0.05 | |
| | | | | 10. SOD | P<0.05 | |
| | | | | 11. MDA | P<0.05 | |
| Meng et al,
2020 | Sprague-Dawley rats
(male, 27/9, 252±2.7 g) | Intraperitoneal
injection of STZ (35 mg/kg), FBG >11.1 | By intragastric,
25/50/100 mg/kg/d, 8 weeks | 1. BW | P<0.01 | (19) |
| | | | | 2. FBG | P<0.05 | |
| | | | | 3. Upro | P<0.05 | |
| | | | | 4. BUN | P<0.05 | |
| | | | | 5. Cr | P<0.05 | |
| | | | | 6. α-SMA | P<0.05 | |
| | | | | 7. TGF-β | P<0.05 | |
| Zhang et al,
2007 | Sprague-Dawley rats
(male, 16/20, 220-240 g) | Intraperitoneal
injection of STZ (60 mg/kg), FBG >13.8 | By intragastric, 1
g/kg/d, 8 weeks | 1. TG | P<0.05 | (20) |
| | | | | 2. Cr | P<0.01 | |
| | | | | 3. HDL | P<0.05 | |
| | | | | 4. BUN | P<0.01 | |
| | | | | 5. TC | P<0.05 | |
| | | | | 6. FBG | P<0.01 | |
| | | | | 7. Upro | P<0.01 | |
| | | | | 8. NF-κB | P<0.01 | |
| | | | | 9. I-κB | P<0.01 | |
| | | | | 10. BW | P<0.01 | |
| Peng et al,
2020 | Sprague-Dawley rats
(male, 8/8, 220±20 g) | Intraperitoneal
injection of STZ (60 mg/kg), FBG >16.7 | By intragastric,
400 g/kg/d,8 weeks | 1. BW | P<0.05 | (33) |
| | | | | 2. FBG | P<0.05 | |
| | | | | 3. Upro | P<0.05 | |
| | | | | 4. BUN | P<0.05 | |
| | | | | 5. Cr | P<0.01 | |
| | | | | 6. nephrin | P<0.05 | |
| | | | | 7. podocin | P<0.05 | |
| Guo et al,
2023 | Sprague-Dawley rats
(male, 30/10, 220±20 g) | Intraperitoneal
injection of STZ (65 mg/kg), FBG >11.7 | By intragastric,
200/400 g/800/kg/d, 10 weeks | 1. BW | P<0.01 | (21) |
| | | | | 2. KI | P<0.01 | |
| | | | | 3. FBG | P<0.01 | |
| | | | | 4. BUN | P<0.01 | |
| | | | | 5. Cr | P<0.01 | |
| | | | | 6. Upro | P<0.01 | |
| | | | | 7. MCP-1 | P<0.01 | |
| | | | | 8. TGF-β1 | P<0.01 | |
| | | | | 9. IL-1β | P<0.01 | |
| | | | | 10. IL-6 | P<0.01 | |
| | | | | 11. TLR-4 | P<0.01 | |
| | | | | 12. NF-κB | P<0.01 | |
| Wu et al,
2024 | Sprague-Dawley rats
(male, 10/10, 200-220 g) | Intraperitoneal
injection of STZ (35 mg/kg), FBG >16.7 | By intragastric,
400 g/kg/d, 8 weeks | 1. BUN | P<0.05 | (32) |
| | | | | 2. Cr | P<0.05 | |
| | | | | 3. TC | P<0.05 | |
| | | | | 4. TG | P<0.05 | |
| | | | | 5. PI3K | P<0.05 | |
| | | | | 6. AKT | P<0.05 | |
| | | | | 7. Upro | P<0.05 | |
| | | | | 8. FBG | P<0.05 | |
| | | | | 9. IL-1β | P<0.05 | |
| Mao et al,
2010 | Wistar rats (male,
20/10, 200±20 g) | Intraperitoneal
injection of STZ (55 mg/kg), FBG >16.7 | By intragastric,
200/400 g/kg/d, 6 weeks | 1. BUN | P<0.01 | (29) |
| | | | | 2. FBG | P<0.01 | |
| | | | | 3. BW | P<0.01 | |
| | | | | 4. Cr | P<0.01 | |
| | | | | 5. KI | P<0.01 | |
| | | | | 6. Upro | P<0.01 | |
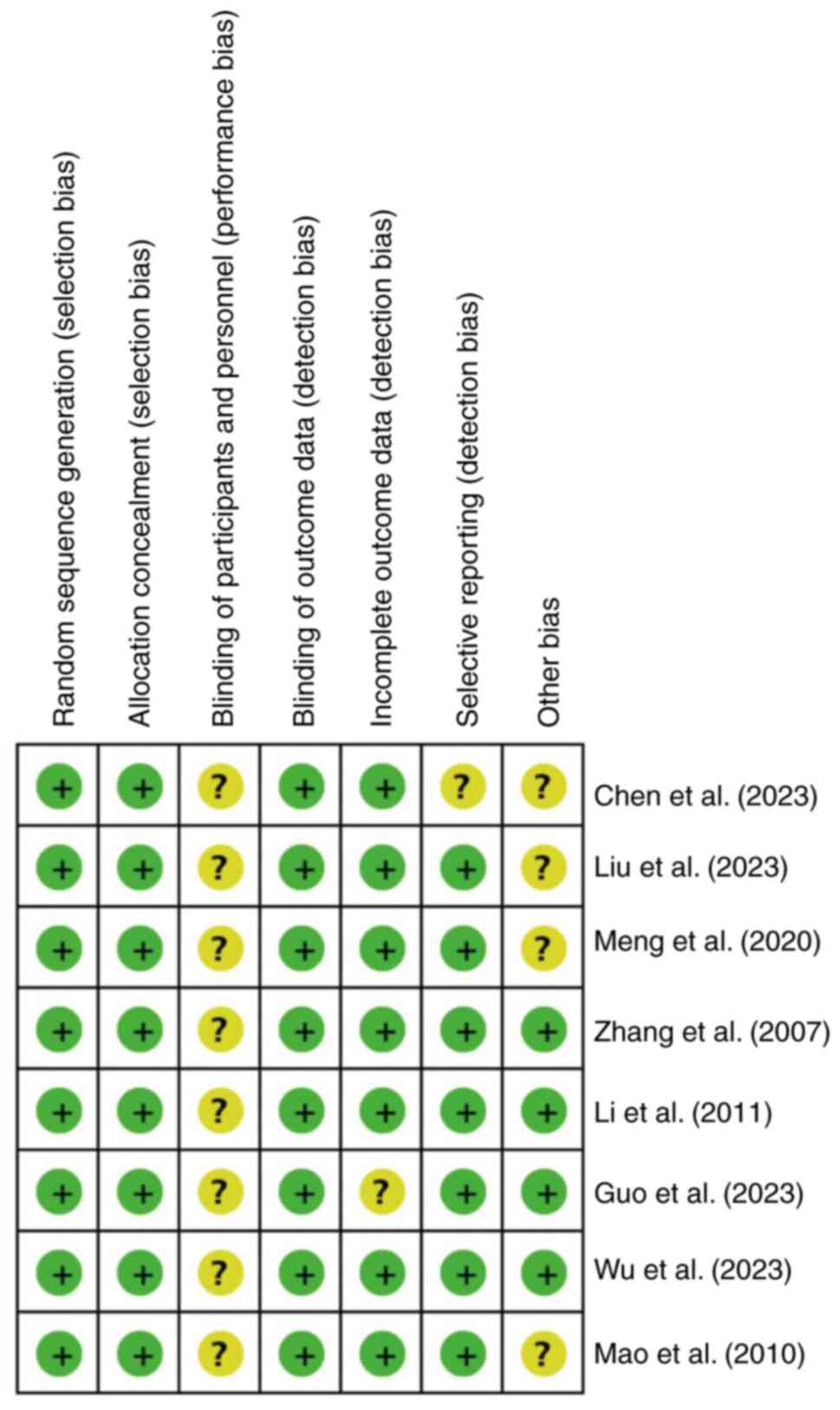
Quality of the included studies
As shown in Fig. 3,
the quality of the articles was evaluated strictly according to the
aforementioned criteria. The Jadad score results ranged from 5-7
points. One study (29) received 5
points, 2 studies (18,20) received 6 points, and 5 studies
(19,21,30-32)
received 7 points each (Table
III). In all the included studies, animals were grouped using a
randomized method. All studies demonstrated baseline
characteristics between the APS groups and the DN group. The
experimental setting was identical, and none of the studies
described whether the distribution of different groups was
adequately masked. In addition, whether or not the grouping and
setting of animals conformed to the principle of randomization was
also considered in the present study. Furthermore, all 8 articles
were randomized and described results with complete data, and no
selective reporting bias was observed. No other sources of bias
were identified in the present meta-analysis (Fig. 3).
 | Table IIIJadad Score of the Effects of
Astragalus polysaccharides on diabetic nephropathy regarding
the enrolled studies. |
Table III
Jadad Score of the Effects of
Astragalus polysaccharides on diabetic nephropathy regarding
the enrolled studies.
| Study ID | Randomized
grouping | Allocation
concealment | Baseline
characteristics | Identical
setting | Total score |
|---|
| Chen et al,
2023 | 2 | 1 | 1 | 1 | 6 |
| Liu et al,
2023 | 2 | 1 | 2 | 2 | 7 |
| Meng et al,
2020 | 2 | 2 | 2 | 1 | 7 |
| Zhang et al,
2007 | 2 | 1 | 2 | 1 | 6 |
| Peng et al,
2020 | 2 | 2 | 2 | 1 | 7 |
| Guo et al,
2023 | 2 | 2 | 2 | 1 | 7 |
| Wu et al,
2023 | 2 | 1 | 2 | 2 | 7 |
| Mao et al,
2010 | 2 | 1 | 1 | 1 | 5 |
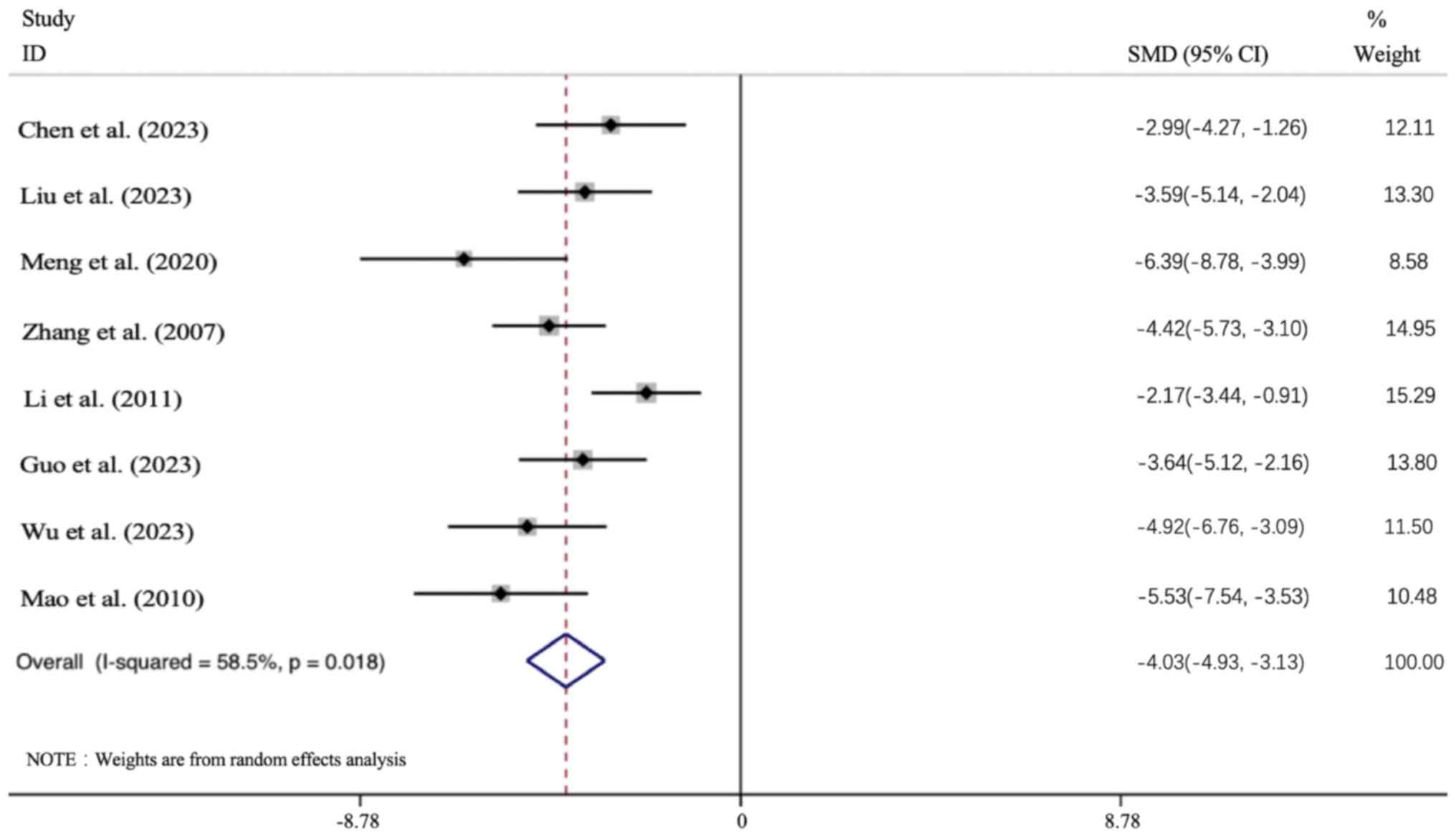
Primary outcomes. Effect of APS
intervention on FBG
All 8 articles presented the FBG data. In all cases,
the APS intervention group led to a significant improvement in the
FBG level [n=156; SMD: -4.029 (95% CI: -4.929 to -3.13), P<0.05;
heterogeneity: I2=58.5%, P<0.05; Fig. 4].
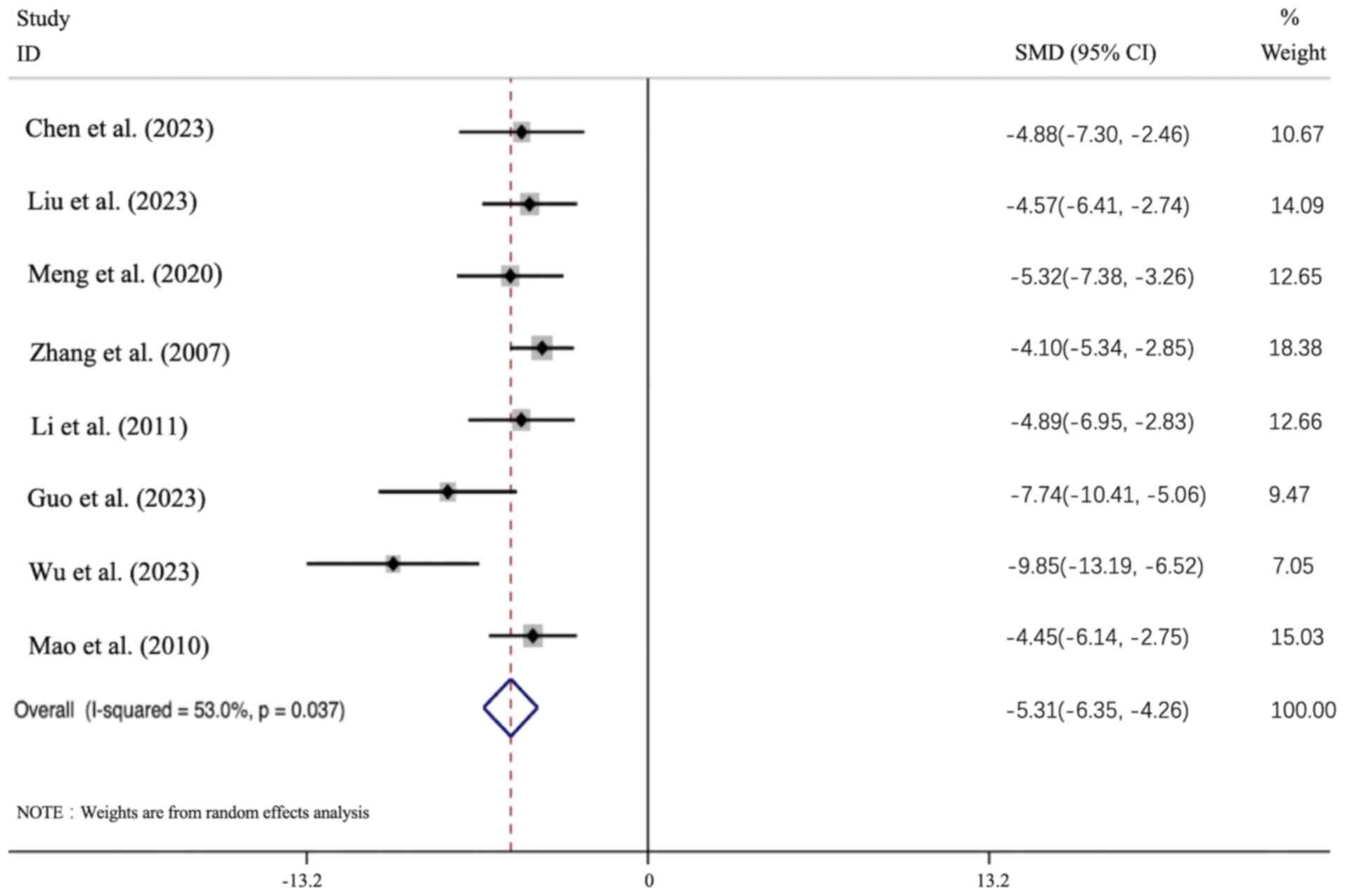
Effect of APS intervention on Cr. All 8
articles presented the Cr data, and the results of all these
studies revealed that the APS intervention group led to a
significant improvement in the Cr level [n=156; SMD: -5.037 (95%
CI: -6.353 to -4.26), P<0.05; heterogeneity: I2=53%,
P<0.01; Fig. 5].
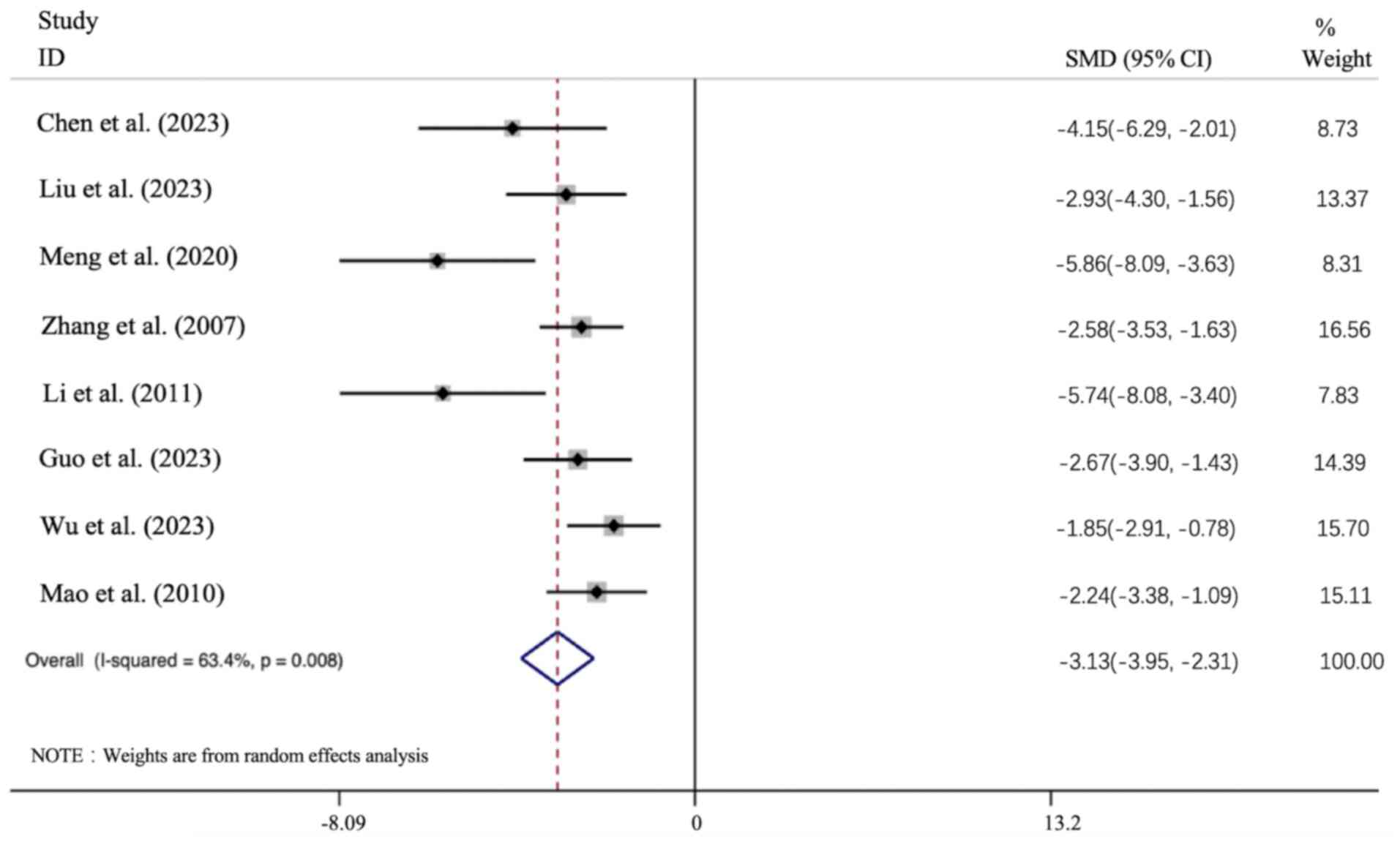
Effect of APS intervention on BUN. All 8
articles presented the BUN data, and the results of all these
studies showed that the APS intervention group led to a significant
improvement in the BUN level [n=156, SMD: -3.13 (95% CI: -3.954 to
-2.306), P<0.01; heterogeneity: I2=63.4%, P<0.01;
Fig. 6].
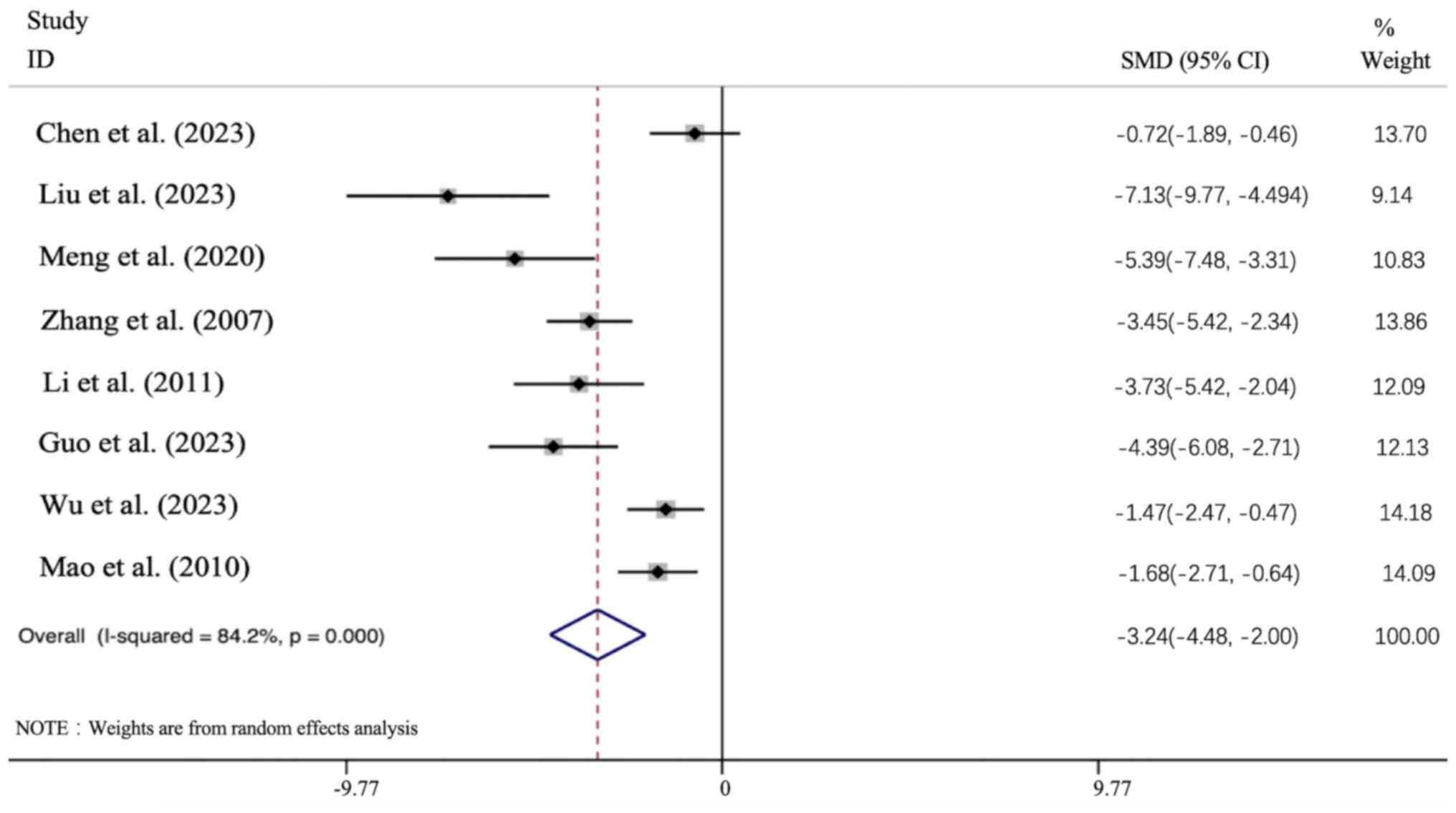
Effect of APS intervention on Upro. All 8
articles presented the Upro data, and the results of all these
studies demonstrated that the APS intervention group led to a
significant improvement in the Upro level [n=156; SMD: -3.241 (95%
CI: -4.479 to -2.003), P<0.01; heterogeneity:
I2=84.2%, P<0.01; Fig.
7].
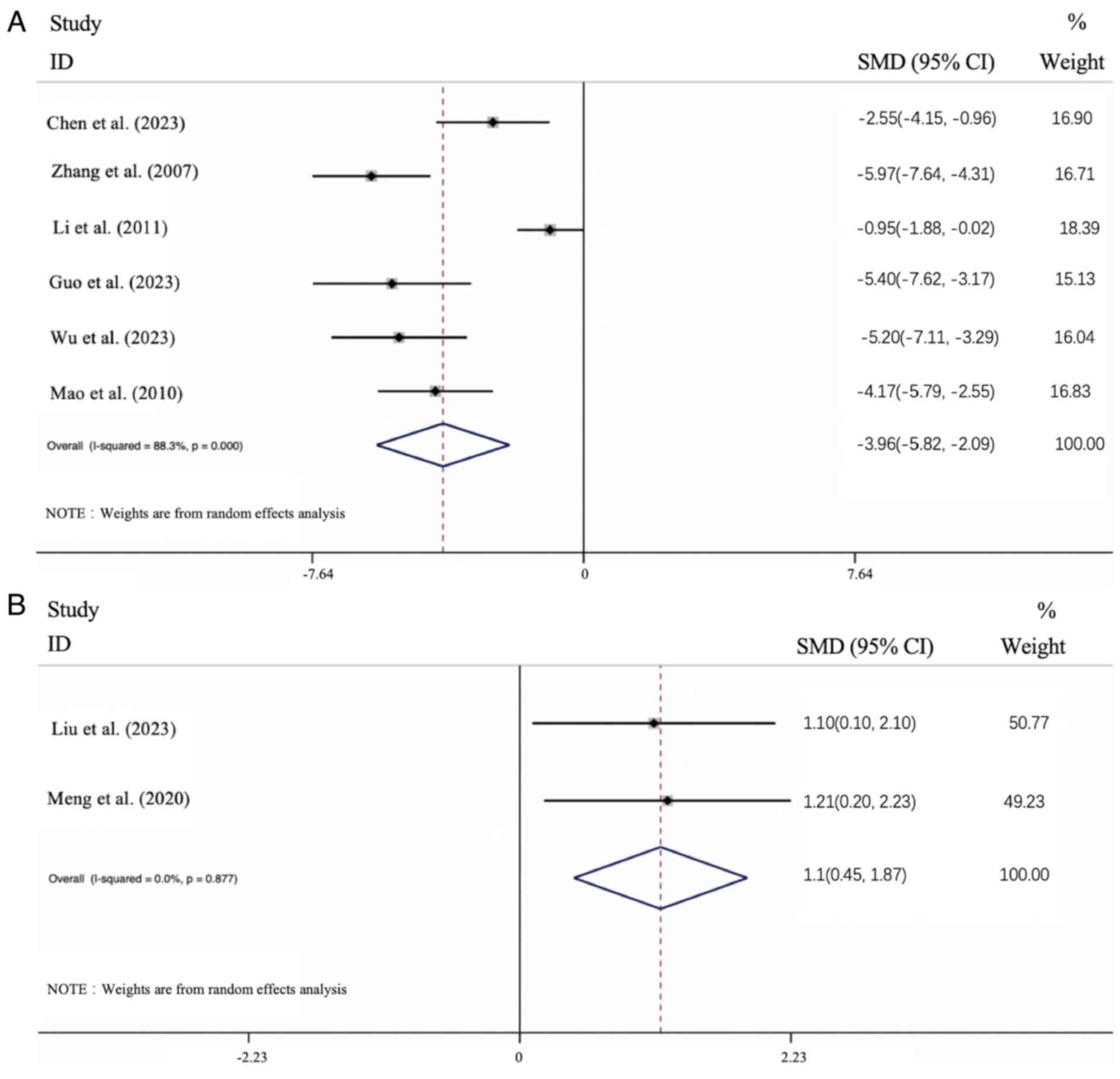
Effect of APS intervention on KI. Usually,
the kidney-to-body weight ratio remains relatively stable (33). An increase in the level of KI is
indicative of edema, congestion or hypertrophy in the kidney
tissue, whereas a decrease in KI suggests the presence of
degenerative changes, such as renal atrophy (34). Data on KI were provided in all
studies. The KI increased in the DN model in the 6 relevant
articles, and the results identified that APS treatment led to an
improvement in the KI level [n=120; SMD: 3.956 (95% CI: 5.825 to
2.087), P<0.01; heterogeneity: I2=88.3%, P<0.01;
Fig. 8A]. The KI was found to
decrease in the remaining 2 articles that described a DN model, and
the results showed that APS treatment led to an improvement in the
KI level [n=36; SMD: 1.058 (95% CI: 0.445 1.870), P<0.05;
heterogeneity: I2=0%, P>0.01; Fig. 8B]. Considered overall, APS treatment
led to an improvement in the KI level of the DN groups.
Secondary outcomes. Effect of APS
intervention on BW
The indicator of BW was provided in 6 of the
articles, which all described a decrease in the BW in the DN model,
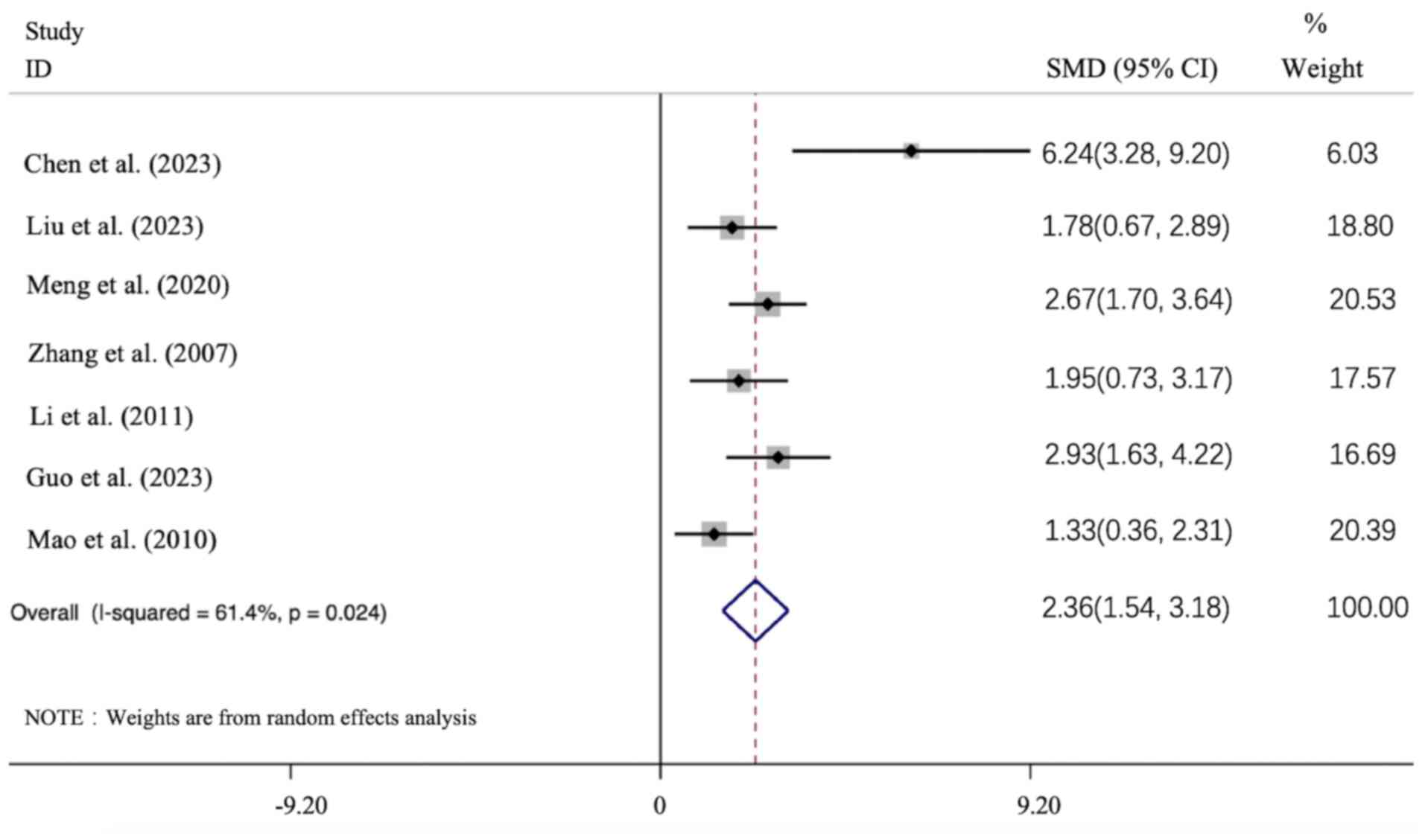
and the data indicated that APS intervention led to an improvement
in the BW level compared with the model group [n=118; SMD: 2.361
(95% CI: 1.545-3.178), P<0.05; heterogeneity:
I2=61.4%, P<0.01; Fig.
9].
Effect of APS intervention on total cholesterol
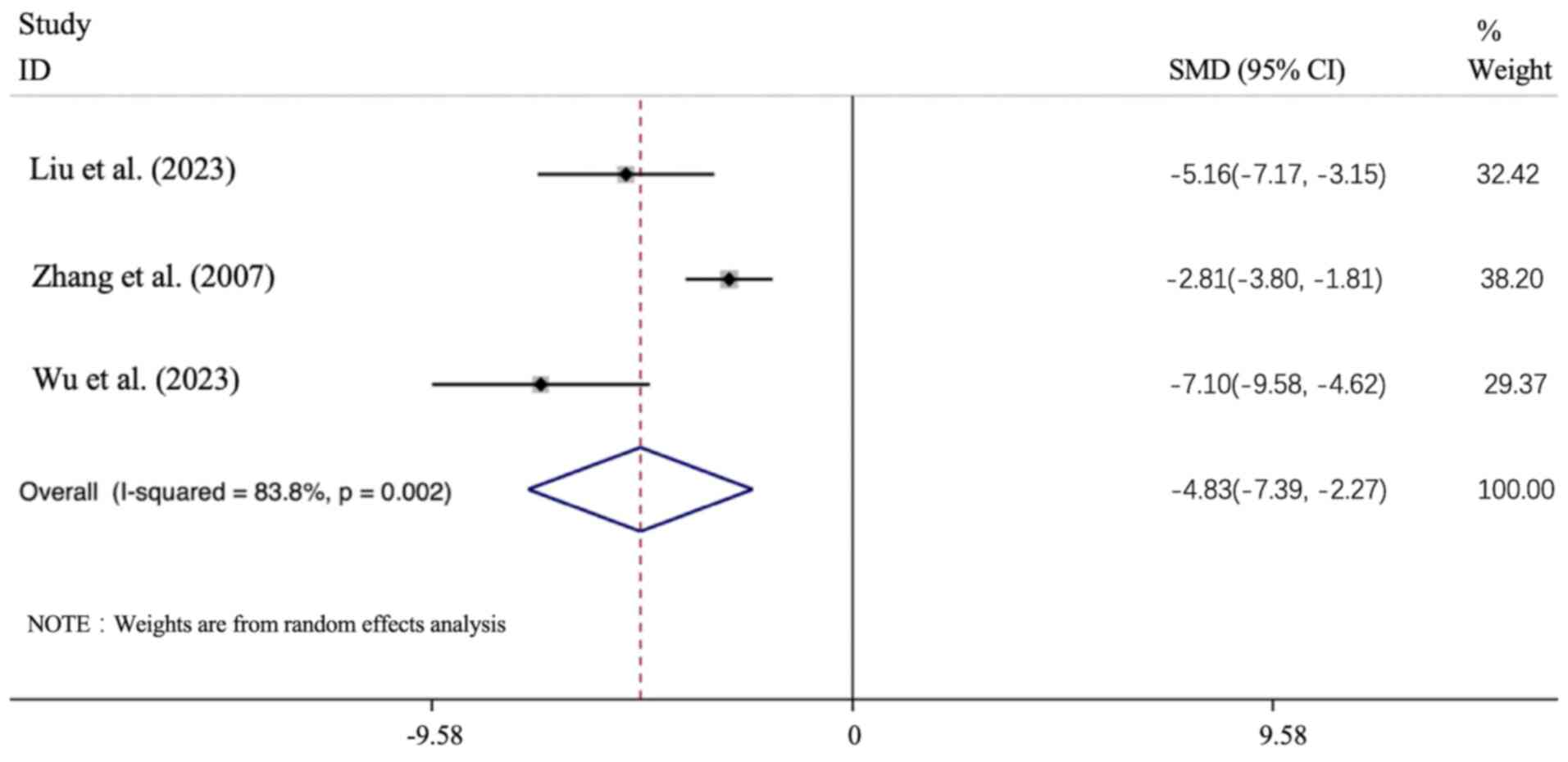
(TC). Only 3 articles employed TC as an outcome index. The
studies showed a TC increase in the DN model groups, whereas APS
intervention groups could improve the TC level (P<0.05). [n=70;
SMD: -4.832 (95% CI: -7.394 to -2.27), P<0.01; heterogeneity:
I2=83.8%, P<0.01; Fig.
10].
Sensitivity and subgroup analysis
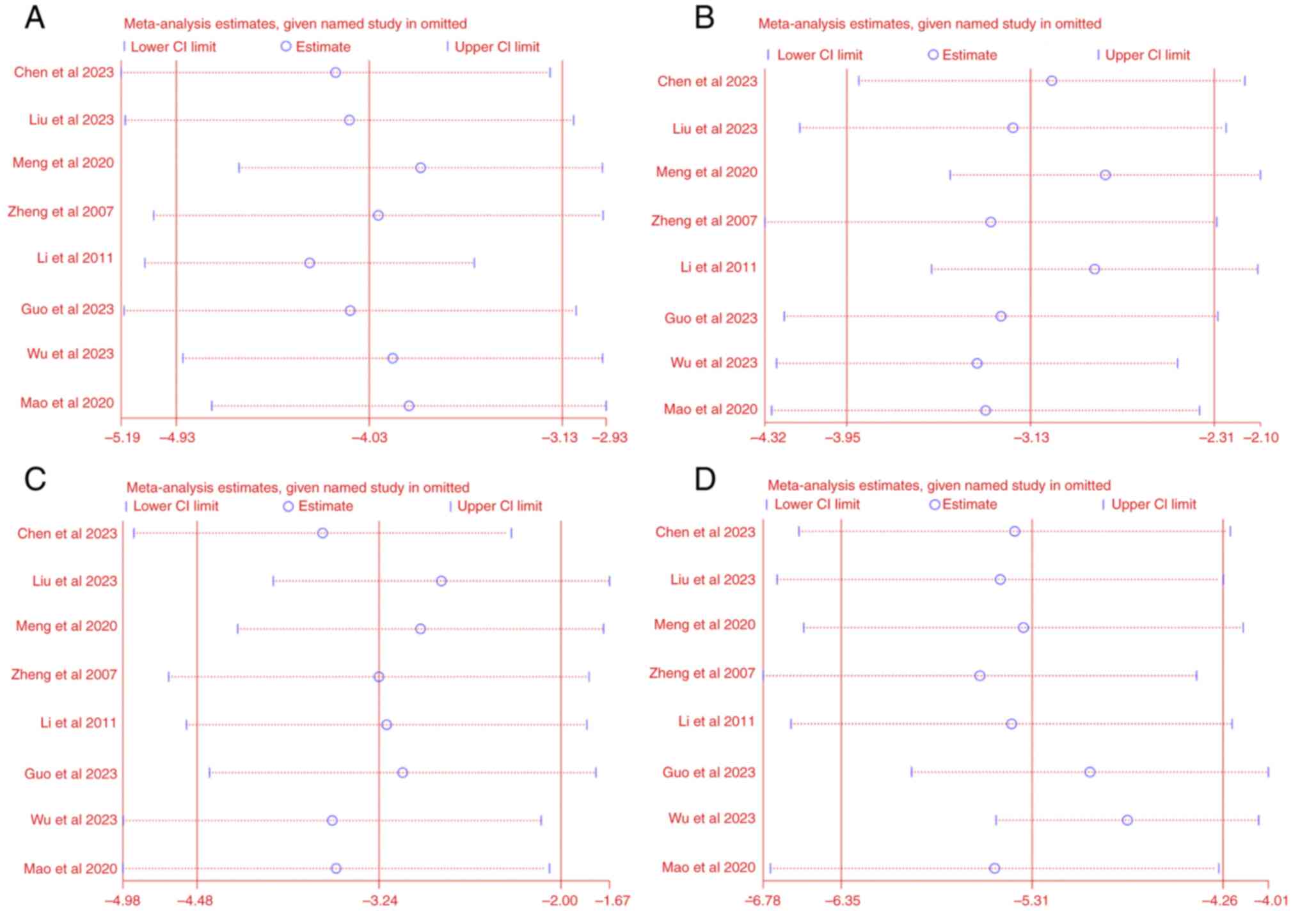
The sensitivity of FBG, BUN, Cr and Upro was
subsequently analyzed. After sequentially excluding each study from
the meta-analysis, no significant difference was observed between
the pre-sensitivity and post-pooled effects of FBG, BUN, Cr and
Upro (Fig. 11). Due to the high
heterogeneity of each study, FBG, Cr, BUN, Upro and other
indicators associated with animal species, the type of DN modeling
method, treatment time and APS dose were evaluated. These analyses
revealed that the DN modeling method, animal species and APS dose
may have provided the source of heterogeneity in FBG and BUN,
whereas the DN modeling method and animal species may have provided
the source of heterogeneity in Upro and Cr.
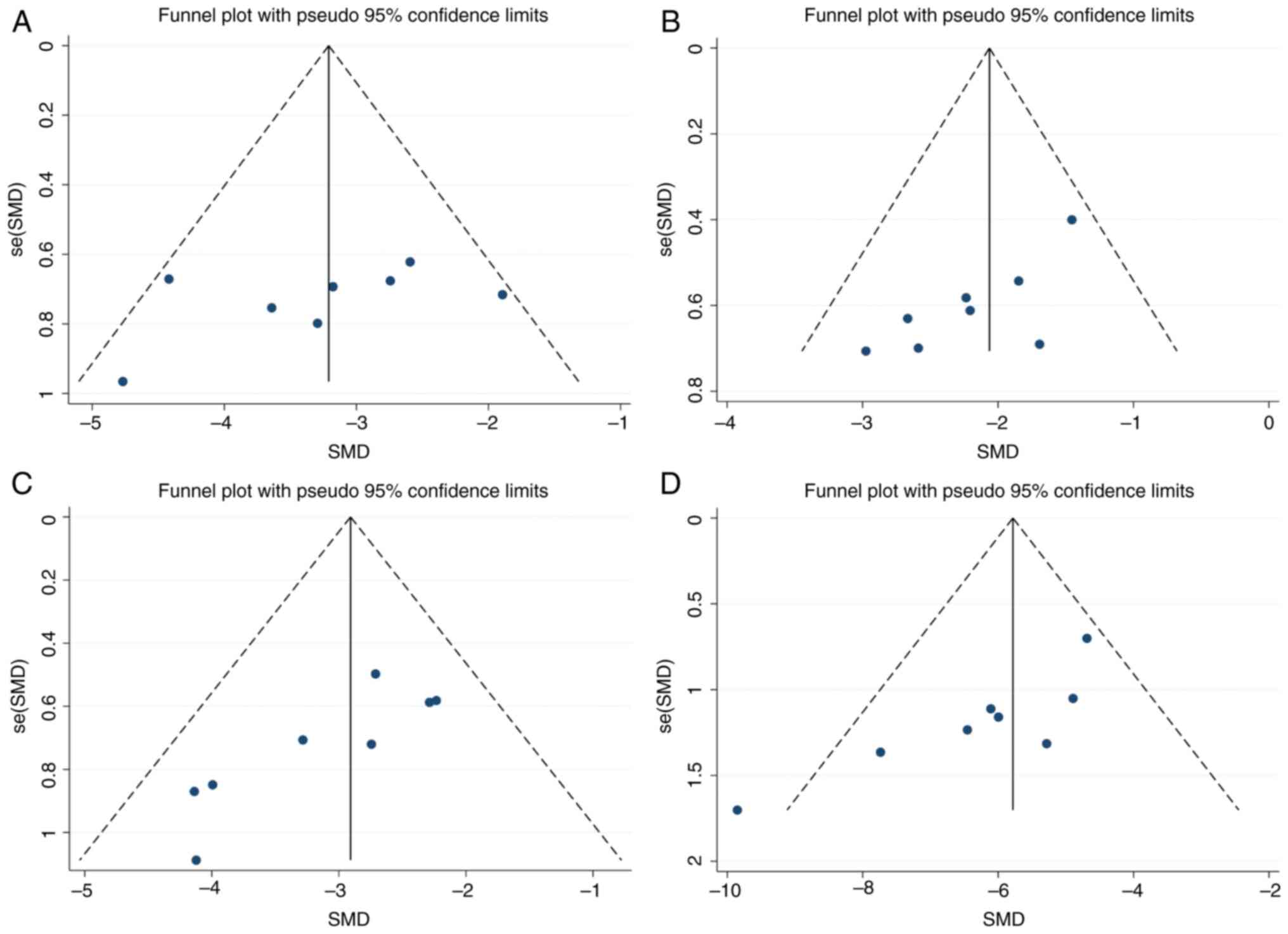
Publication bias
The results obtained showed that the publication
bias of FBG, Cr, BUN and Upro was statistically significant
(P<0.05). The findings also revealed that data from the missing
studies did not change the size of the overall combined effect
size. The publication bias details are shown in Fig. 12.
Discussion
Effectiveness summary of APS treatment for
DN. The present systematic review and meta-analysis of 8
preclinical studies revealed that APS plays a beneficial role in DN
treatment, particularly in improving glucose intolerance and
alleviating pathological renal damage. Since HbA1c is influenced by
multiple factors such as diet, exercise and different antidiabetic
medications in patients, including it would impede isolating and
accurately assessing APS's independent impact on kidney damage. The
selected indicators (renal function: BUN, Cr, Upro, KI;
inflammatory: TNF-α, IL 6; endocrine: FBG; risk factors: TG, TC)
were chosen due to their mention in all included literature,
facilitating subsequent data analysis. The analyzed data showed
that APS treatment improves these indicators and reduces the risk
factors. Subgroup analysis indicated that study heterogeneity may
arise from animal species, DN modeling protocols and APS dose.
Moreover, sensitivity analysis suggests that any publication bias
of BUN, FBG, Cr and Upro does not affect result stability.
Furthermore, the highest dose group was selected for meta-analysis
as it is more likely to manifest the maximal therapeutic effect,
clarifying the drug's upper limit of efficacy in treating diabetic
kidney damage. Future study will explore other doses, conducting a
detailed dose-response analysis for a comprehensive understanding
of the optimal dosing strategy.
Summary of the potential mechanisms of
APS treatment of DN
The analysis of the included studies demonstrated
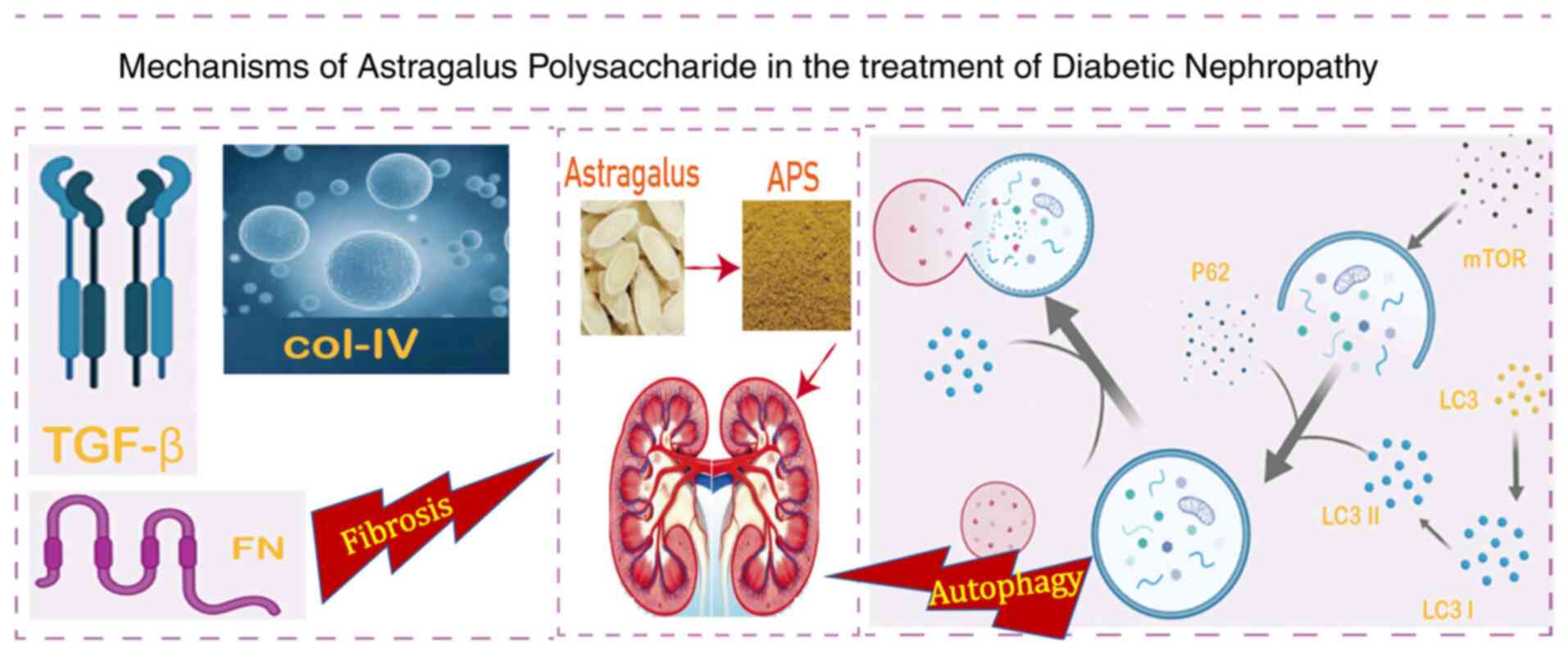
the potential primary mechanisms of APS treatment on DN (Fig. 13).
Anti-fibrotic and autophagy promotion. Renal
interstitial fibrosis is the eventual result of all progressive
chronic kidney diseases that lead to end-stage renal disease
(35). APS treatment is known to
promote autophagy via reducing the protein levels of p62 and
mammalian target of rapamycin, while promoting the
microtubule-associated protein light chain 3 I/II ratio.
Furthermore, APS has been shown to reduce fibrosis by
downregulating the expression levels of fibronectin, collagen IV
and transforming growth factor-β (TGF-β) (18). The activation of the advanced
glycation end products pathway in advanced diabetes mellitus has
been identified to be closely associated with the occurrence of DN
(36), and activation of AGE
receptor can induce activation of the PI3K-Akt, TGF-β/Smad and
NF-κB signaling pathways, among others (37). APS was also shown to reduce the
expression of the inflammatory factors IL-1β, IL-6 and monocyte
chemoattractant protein 1 in DN rats, to inhibit the activity of
the Toll-like receptor 4 (TLR4)/NF-κB pathway, and to significantly
alleviate kidney injury (21). The
PI3K-Akt signaling pathway is involved in multiple cellular
processes, including cell proliferation, migration, adhesion and
survival (38). APS treatment also
has been revealed to decrease the levels of serum inflammatory
factors, and to increase the extent of apoptosis of glomerular
cells in DN rats, which is associated with inhibition of the
PI3K/Akt signaling pathway (32).
Ski-related protein N functions as a primary nuclear
transcriptional suppressor in the TGF-β/Smad pathway (39). APS treatment has also been shown to
influence the TGF-β/Smad signaling pathway, leading to the
protection of renal function (19).
Activation of aldose reductase can trigger the activation of
protein kinase C, mitogen-activated protein kinase, and other
signaling pathways, resulting in the overexpression of TGF-β, TNF-α
and other cytokines, leading to a variety of pathophysiological
chain reactions associated with DN (40).
The relief of inflammation. The chemokines
and cytokines that participate in inflammatory processes have been
shown to be closely associated with the occurrence and development
of DN (41). NF-κB is a downstream
effector of the TLR4 signaling pathway that mediates various
inflammatory processes (42). APS
has been demonstrated to ameliorate DN renal injury through
inhibiting the TLR4/NF-κB signaling pathway, and thereby
alleviating the inflammatory response. In addition, APS treatment
was also found to increase the expression of IκB mRNA and decrease
the level of NF-κB mRNA in the renal cortex (20).
The relief of oxidative stress. Oxidative
stress is one of the most important factors in the occurrence and
development of DN (43).
AMP-activated protein kinase (AMPK), as a metabolic regulator and
energy sensor, has an important role in the entire
pathophysiological process of DN (44). It has been reported that AMPK can
affect the expression and phosphorylation process of peroxisome
proliferator-activated receptor gamma coactivator 1α, thereby
influencing the production of cell energy (45). APS treatment has been revealed to
downregulate the AMPK/SIRT1/FOXO1 signaling pathway in DN rats, and
to activate autophagy, inhibit oxidative stress and alleviate
kidney injury (30).
Limitations and considerations. In general,
demonstrating the effects of therapeutic drugs through performing
animal experiments is an important requirement in the preclinical
research and development of new drugs. However, problems with
preclinical animal studies may arise from a high risk of
experimental bias and the low reproducibility of results. These
lead to low success rates for the development of safe and effective
drugs in clinical trials (46).
Along these lines, the systematic evaluation of the present study
has the following shortcomings. First, only 8 high-quality studies
were identified and screened, which may inevitably lead to a bias.
Secondly, the data extracted from certain of these articles were
obtained indirectly through data extraction software, which may
have led to measurement bias. Thirdly, the high heterogeneity of
the FBG, BUN, Cr, Upro and KI values cannot be ignored in spite of
the subgroup analysis. This heterogeneity may have caused the
calculated results to differ from the actual values. The current
experimental conclusions are mainly based on male animals, and the
lack of female mice is a limitation, thus their applicability to
females remains uncertain until further studies with female
subjects are conducted. While suggesting APS could improve DN
potentially via relieving inflammatory responses and attenuating
the TLR4/NF-κB signaling pathway, it is acknowledged that basing
the current findings on only male mice is a limitation as sex
differences may influence these mechanisms, warranting future
studies with female mice for a more comprehensive
understanding.
In conclusion, the data obtained to date have
suggested that treatment with APS leads to improvements in renal
function and proteinuria in DN animal models, and that this has a
role in reducing the burden of renal dysfunction secondary to
diabetes. According to our data, the protective mechanisms of APS
on DN may be associated with improving the inflammatory status,
fibrosis degree and oxidative stress status of DN model animals. In
order to more accurately evaluate the DN efficacy and safety of
APS, however, larger and more long-term studies of higher quality
are required to confirm these findings prior to clinical
application.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Chongqing
Medical Scientific Research project (Joint project of Chongqing
Health Commission and Science and Technology Bureau) of China
(grant no. 2025ZYYB004)
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YH curated data and wrote the original draft. YH and
SL confirm the authenticity of all the raw data. WJZ, ZCY, QW, SL
and MMQ performed literature review, data analyses and manuscript
revision. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun H, Saeedi P, Karuranga S, Pinkepank M,
Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et
al: IDF diabetes atlas: Global, regional and country-level diabetes
prevalence estimates for 2021 and projections for 2045. Diabetes
Res Clin Pract. 183(109119)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Papatheodorou K, Papanas N, Banach M,
Papazoglou D and Edmonds M: Complications of diabetes 2016. J
Diabetes Res. 2016(6989453)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lotfy M, Adeghate J, Kalasz H, Singh J and
Adeghate E: Chronic complications of diabetes mellitus: A mini
review. Curr Diabetes Rev. 13:3–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mariye Zemicheal T, Bahrey Tadesse D,
Tasew Atalay H, Teklay Weldesamuel G, Gebremichael GB, Tesfay HN
and Haile TG: Determinants of diabetic nephropathy among diabetic
patients in general public hospitals of tigray, ethiopia, 2018/19.
Int J Endocrinol. 2020(6396483)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nagib AM, Elsayed Matter Y, Gheith OA,
Refaie AF, Othman NF and Al-Otaibi T: diabetic nephropathy
following posttransplant diabetes mellitus. Exp Clin Transplant.
17:138–146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Koye DN, Magliano DJ, Nelson RG and Pavkov
ME: The global epidemiology of diabetes and kidney disease. Adv
Chronic Kidney Dis. 25:121–132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rao Kondapally Seshasai S, Kaptoge S,
Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal
KJ, Gillum RF, Holme I, et al: Diabetes mellitus, fasting glucose,
and risk of cause-specific death. N Engl J Med. 364:829–841.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamout H, Lazich I and Bakris GL: Blood
pressure, hypertension, RAAS blockade, and drug therapy in diabetic
kidney disease. Adv Chronic Kidney Dis. 21:281–286. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Doulton TW: ACE inhibitor-angiotensin
receptor blocker combinations: A clinician's perspective. Mini Rev
Med Chem. 6:491–497. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Forclaz A, Maillard M, Nussberger J,
Brunner HR and Burnier M: Angiotensin II receptor blockade: Is
there truly a benefit of adding an ACE inhibitor? Hypertension.
41:31–36. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shen MR, He Y and Shi SM: Development of
chromatographic technologies for the quality control of traditional
Chinese medicine in the Chinese pharmacopoeia. J Pharm Anal.
11:155–162. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Song Y, Yang J, Bai WL and Ji WY:
Antitumor and immunoregulatory effects of Astragalus on
nasopharyngeal carcinoma in vivo and in vitro. Phytother Res.
25:909–915. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Song J, Li J, Zheng SR, Jin Y and Huang Y:
Anti-inflammatory and immunoregulatory effects of Yupingfeng powder
on chronic bronchitis rats. Chin J Integr Med. 19:353–359.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qin Q, Niu J, Wang Z, Xu W, Qiao Z and Gu
Y: Astragalus embranaceus extract activates immune response
in macrophages via heparanase. Molecules. 17:7232–7240.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jung Y, Jerng U and Lee S: A systematic
review of anticancer effects of radix astragali. Chin J Integr Med.
22:225–236. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jin M, Zhao K, Huang Q and Shang P:
Structural features and biological activities of the
polysaccharides from Astragalus membranaceus. Int J Biol
Macromol. 64:257–266. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen Z, Liang H, Yan X, Liang Q, Bai Z,
Xie T, Dai J, Zhao X and Xiao Y: Astragalus polysaccharide
promotes autophagy and alleviates diabetic nephropathy by targeting
the lncRNA Gm41268/PRLR pathway. Ren Fail.
45(2284211)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Meng X, Wei M, Wang D, Qu X, Zhang K,
Zhang N and Li X: Astragalus polysaccharides protect renal
function and affect the TGF-β/Smad signaling pathway in
streptozotocin-induced diabetic rats. J Int Med Res.
48(300060520903612)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang YW, Wu CY and Cheng JT: Merit of
Astragalus polysaccharide in the improvement of early
diabetic nephropathy with an effect on mRNA expressions of
NF-kappaB and IkappaB in renal cortex of streptozotoxin-induced
diabetic rats. J Ethnopharmacol. 114:387–392. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guo M, Gao J, Jiang L and Dai Y:
Astragalus polysaccharide ameliorates renal inflammatory
responses in a diabetic nephropathy by suppressing the TLR4/NF-κB
pathway. Drug Des Devel Ther. 17:2107–2118. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Siddaway AP, Wood AM and Hedges LV: How to
do a systematic review: A best practice guide for conducting and
reporting narrative reviews, meta-analyses, and meta-syntheses.
Annu Rev Psychol. 70:747–770. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Levy N: The use of animal as models:
Ethical considerations. Int J Stroke. 7:440–442. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ioannidis JP, Greenland S, Hlatky MA,
Khoury MJ, Macleod MR, Moher D, Schulz KF and Tibshirani R:
Increasing value and reducing waste in research design, conduct,
and analysis. Lancet. 383:166–175. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Sandercock G: The standard error/standard
deviation mix-up: Potential impacts on meta-analyses in sports
medicine. Sports Med. 54:1723–1732. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hooijmans CR, Rovers MM, de Vries RB,
Leenaars M, Ritskes-Hoitinga M and Langendam MW: SYRCLE's risk of
bias tool for animal studies. BMC Med Res Methodol.
14(43)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wong MCS, Chan CH, Lin J, Huang JLW, Huang
J, Fang Y, Cheung WWL, Yu CP, Wong JCT, Tse G, et al: Lower
relative contribution of positive family history to colorectal
cancer risk with increasing age: A systematic review and
meta-analysis of 9.28 million individuals. Am J Gastroenterol.
113:1819–1827. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mao SM, Li CD, Li JJ and Kang B: Effect of
Astragalus polysaccharide on expression of aquaporin-2 in
kidney of diabetic rats and its protective effect on kidney. Chin J
Geriatr. 30:2301–2303. 2010.(In Chinese).
|
|
30
|
Liu XF, Feng J, Wang SJ and Zuo H: Effect
and mechanism of Astragalus polysaccharide on oxidative
stress and autophagy in diabetic nephropathy rats. J Shanxi Med
Univ. 54:343–351. 2023.(In Chinese). doi:
10.13753/j.issn.1007-6611.2023.03.010.
|
|
31
|
Li ZJ and Zhang Y, Liu YM, Lu HY, Li YL
and Zhang Y: Effects of Astragalus polysaccharide on the
expression of nephrin and podocin in podocytes of rats with early
diabetic nephropathy. Chin J Pathophysiol. 27:1772–1776. 2011.(In
Chinese). doi: 10.3969/j.issn.1000-4718.2011.09.021.
|
|
32
|
Wu D, Zhang QH, He LQ and Yang SS:
Astragalus polysaccharide regulates PI3K/AKT pathway to
improve renal injury in diabetic rats. Biotechnology. 1–7. 2023.(In
Chinese). https://link.cnki.net/urlid/23.1319.Q.20240306.1042.004.
|
|
33
|
Feng H, Wu T, Zhou Q, Li H, Liu T, Ma X
and Yue R: Protective effect and possible mechanisms of artemisinin
and its derivatives for diabetic nephropathy: A systematic review
and meta-analysis in animal models. Oxid Med Cell Longev.
2022(5401760)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Z, Zhao H, Ge D, Wang S and Qi B:
β-casomorphin-7 ameliorates sepsis-induced acute kidney injury by
targeting NF-κB pathway. Med Sci Monit. 25:121–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livingston MJ, Ding HF, Huang S, Hill JA,
Yin XM and Dong Z: Persistent activation of autophagy in kidney
tubular cells promotes renal interstitial fibrosis during
unilateral ureteral obstruction. Autophagy. 12:976–998.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kay AM, Simpson CL and Stewart JA Jr: The
role of AGE/RAGE signaling in diabetes-mediated vascular
calcification. J Diabetes Res. 2016(6809703)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sanajou D, Ghorbani Haghjo A, Argani H and
Aslani S: AGE-RAGE axis blockade in diabetic nephropathy: Current
status and future directions. Eur J Pharmacol. 833:158–164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bai C, Sun Y, Pan X, Yang J, Li X, Wu A,
Qin D, Cao S, Zou W and Wu J: Antitumor effects of trimethylellagic
acid isolated from sanguisorba officinalis L. on colorectal cancer
via angiogenesis inhibition and apoptosis induction. Front
Pharmacol. 10(1646)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sarker KP, Wilson SM and Bonni S: SnoN is
a cell type-specific mediator of transforming growth factor-beta
responses. J Biol Chem. 280:13037–13046. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Price SA, Agthong S, Middlemas AB and
Tomlinson DR: Mitogen-activated protein kinase p38 mediates reduced
nerve conduction velocity in experimental diabetic neuropathy:
Interactions with aldose reductase. Diabetes. 53:1851–1856.
2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rivero A, Mora C, Muros M, García J,
Herrera H and Navarro-González JF: Pathogenic perspectives for the
role of inflammation in diabetic nephropathy. Clin Sci (Lond).
116:479–492. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Navarro-González JF, Mora-Fernández C,
Muros de Fuentes M and García-Pérez J: Inflammatory molecules and
pathways in the pathogenesis of diabetic nephropathy. Nat Rev
Nephrol. 7:327–340. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu L, Zheng T, Wang F, Wang N, Song Y, Li
M, Li L, Jiang J and Zhao W: Pro12Ala polymorphism in the PPARG
gene contributes to the development of diabetic nephropathy in
Chinese type 2 diabetic patients. Diabetes Care. 33:144–149.
2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Dugan LL, You YH, Ali SS, Diamond-Stanic
M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G,
et al: AMPK dysregulation promotes diabetes-related reduction of
superoxide and mitochondrial function. J Clin Invest.
123:4888–4899. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jäger S, Handschin C, St-Pierre J and
Spiegelman BM: AMP-activated protein kinase (AMPK) action in
skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl
Acad Sci USA. 104:12017–12022. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Perrin S: Preclinical research: Make mouse
studies work. Nature. 507:423–425. 2014.PubMed/NCBI View Article : Google Scholar
|