1. Introduction
Fructose, a naturally occurring monosaccharide and
isomer of glucose, is commonly found in fruit and honey. Unlike
glucose, which is metabolized primarily to produce ATP, fructose
follows a different metabolic pathway, being processed primarily in
the liver to produce elevated serum levels of glucose,
triglycerides and total cholesterol (1). Owing to its low cost and potent
sweetening capacity, fructose has become a widely used sweetener in
beverages globally, especially following the development of
cost-effective synthesis methods in the 1970s. This advancement has
resulted in a significant increase in both fructose production and
consumption (2-4).
It has been found that a diet high in fructose can
result in metabolic disorders, which in turn can trigger
pathologies such as obesity, cardiovascular diseases, type 2
diabetes and cognitive dysfunction. Although these conditions
usually manifest during adulthood, it has been suggested that they
can also appear due to an adverse intrauterine environment
(5,6). Developmental programming where
maternal nutrition influences the health of offspring suggests that
excessive fructose consumption during gestation and lactation
affects neonatal development, causing metabolic diseases and
cognitive impairment. Studies in animal models, especially in
rodents, show that a maternal diet rich in fructose (MDRF) during
pregnancy and lactation can negatively affect brain function,
reduce cognitive abilities, cause inflammatory responses and
provoke multiple metabolic disorders in the offspring (4).
Murine models are highly suitable for investigating
the effects of high fructose intake during pregnancy and lactation.
Fructose consumption is known to promote lipogenesis and elevate
triglyceride levels, which can lead to hepatic insulin
resistance-an effect similar to that observed in humans (2,7).
Studying these models allows researchers to improve understanding
of the potential consequences in humans and develop effective
prevention and intervention strategies (5,6,8). While
recent and comprehensive studies have examined the effects of
fructose consumption during pregnancy in both humans and animal
models, each focuses on a specific mechanism, such as mitochondrial
dysfunction (9), epigenetic
modifications (10), fertility
issues (11), or cognitive
impairment (12). The present
review is novel in that it integrates these diverse perspectives,
exploring the cellular, molecular and functional effects of
fructose consumption in a comprehensive manner. In the present
review, the available literature on the effect of fructose
consumption during gestation and lactation on offspring development
using murine models was discussed.
2. Methodology
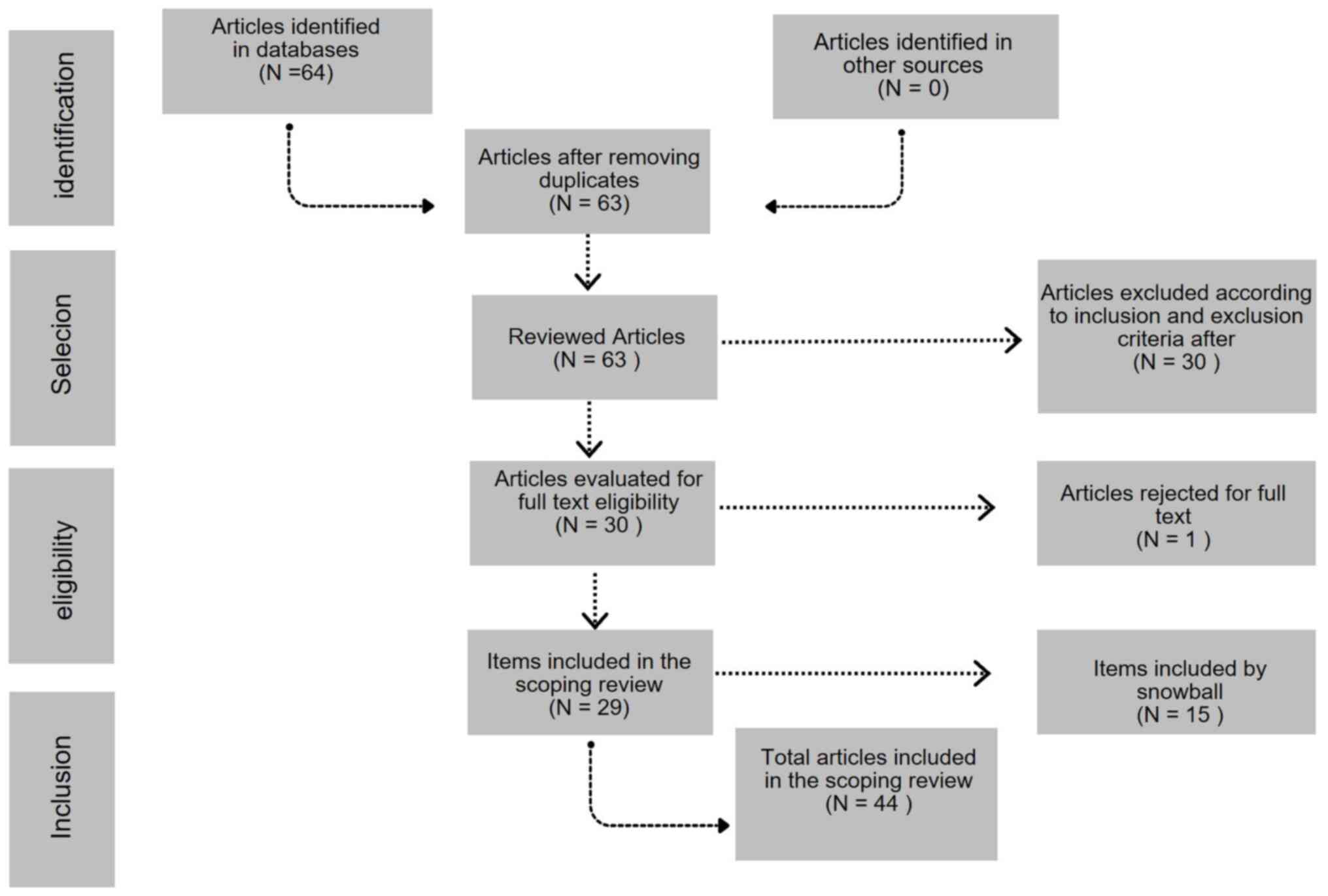
PRISMA guidelines were followed in the present
review. A search was performed using the ScienceDirect (https://www.sciencedirect.com/), PubMed
(https://pubmed.ncbi.nlm.nih.gov/),
Google Scholar (https://scholar.google.com/), Oxford Academic
(https://academic.oup.com/) and Scopus
(https://www.scopus.com/home.uri)
databases. The MeSH (Medical Subject Headings) terms ‘pregnancy’,
‘Rats’, ‘Mice’, ‘Fructose’ and ‘Brain’ were combined to form the
search matrix ‘(pregnancy) AND ((Rats) OR (Mice)) AND (Fructose)
AND (Brain)’. A total of 64 articles were found using the different
databases.
Inclusion criteria. Studies included in the
present review fulfil the following criteria: fructose had to be
used as animal feed and administered during gestation +/-
lactation.
Exclusion criteria. Studies in which fructose
was administered only during lactation were excluded, as well as
those where the term ‘fructose’ appeared only as part of a
component or term of the experimental model or in the frame of
references.
A total of 64 studies were retrieved from the
databases. After applying the inclusion and exclusion criteria, 29
articles were retained. This included 28 research articles and 1
review article. Studies involved only the use of animal models,
specifically rats and mice, articles published in 1993(1), 2011(1), 2014(2), 2015(1), 2016(3), 2017(1), 2018(4), 2019(2), 2020(5), 2021(3), 2022(2), 2023(3)
and 2024(1) were obtained. A total
of 3 articles were taken into account by snowball capture about
experimental animal models: ‘Metabolic effects of fructose’,
‘Dietary fructose-induced gut dysbiosis promotes mouse hippocampal
neuroinflammation: A benefit of short-chain fatty acids’, and ‘A
high-fructose diet induces hippocampal insulin resistance and
exacerbates memory deficits in male Sprague-Dawley rats’, this to
expand on the concept of some key terms in the development of this
review, and 12 articles per snowball about preclinical studies that
contrast studies in animal models; finishing with a total of 44
studies included as shown in Fig.
1.
3. Discussion
Mitochondrial function,
neuroinflammation and its relation to oxidative stress
Neuroinflammation, the inflammation of the nervous
system, involves the activation of microglia and astrocytes,
release of cytokines and chemokines, production of reactive oxygen
species (ROS) and often the infiltration of peripheral leukocytes
into the central nervous system (4). A diet rich in processed sugars and
fats has been reported to be associated with neuroinflammatory
effects (13). Fructose consumption
has been shown to increase the expression of proinflammatory
cytokines in some brain regions, interfering with the function of
some tissues. In addition, due to oxidative stress and brain
mitochondrial dysfunction, fructose consumption during gestation
and lactation may program susceptibility to cardio-metabolic
disease in females and fetal hypertension by affecting sympathetic
nervous system activity in the rostral ventrolateral medulla
(14). It has been found that
maternal and post-weaning exposure to high-fructose corn syrup can
increase the expression of proinflammatory proteins in the
hippocampus of the offspring, suggesting a synergistic effect on
neuroinflammation and the development of brain dysfunction
(5).
These findings indicate that fructose not only
induces neuroinflammation but also brain mitochondrial dysfunction
and a sex-dependent response to fructose and the involvement of
mechanisms such as DNA methylation in the generation of oxidative
stress in offspring as supported by several studies (5,6,8,14-20).
Fructose consumption interferes with key molecular systems required
for mitochondrial biogenesis, particularly the peroxisome
proliferator-activated receptor gamma coactivator-1 alpha and
Cytochrome c oxidase subunit II. This disruption suggests that
mitochondrial dysfunction may be an early consequence of fructose
exposure in the brain (21).
On the other hand, diet during gestation and early
life can have lasting effects on offspring, influencing brain and
metabolic function in adulthood by modifying the capacities of
astrocytes (6). Multiple studies
have shown that fructose exposure during gestation affects
mitochondrial function in offspring, which may trigger metabolic
changes associated with brain aging (5,6,9,14,19,22).
Furthermore, the relationship between MDRF and mitochondrial
dysfunction in the hippocampus could contribute to neuropsychiatric
disorders and cognitive dysfunction. This also affects glycolysis
and oxidative phosphorylation in astrocyte mitochondria,
highlighting the importance of metabolic regulatory mechanisms in
brain development. These findings underscore the complex interplay
between maternal diet, brain function and hippocampal
vulnerability, highlighting the need for further research of these
processes and to improve understanding of their implications in
long-life health (5,6,14,19,22).
In this context, fructose reprograms cellular metabolic pathways to
favor glutaminolysis and oxidative metabolism, which are required
to support increased inflammatory cytokine production in both
lipopolysaccharide (LPS)-treated human monocytes and mouse
macrophages. A fructose-dependent increase in mechanistic target of
rapamycin complex 1 activity drives the translation of
pro-inflammatory cytokines in response to LPS. LPS-stimulated
monocytes treated with fructose rely heavily on oxidative
metabolism and have reduced flexibility in response to both
glycolytic and mitochondrial inhibition, suggesting glycolysis and
oxidative metabolism are inextricably coupled in these cells. The
physiological implications of fructose exposure are demonstrated in
a model of LPS-induced systemic inflammation, with mice exposed to
fructose having increased levels of circulating IL-1β after the LPS
challenge (23). Recently,
associations have been reported between nutritional deficiencies,
particularly excessive sugar consumption, and an increased risk of
pre-eclampsia, potentially mediated by oxidative stress,
inflammation, maternal endothelial dysfunction and blood pressure
dysregulation in its pathophysiology (24). Additionally, preclinical models have
been widely used to establish a causal relationship between
mitochondrial dysfunction, oxidative stress and memory deficits.
Studies have indicated that cognitive impairment, induced by
various conditions, including pharmacological, genetic, toxic and
nutritional factors, is associated with reduced complex I activity,
decreased mitochondrial membrane potential, elevated ROS
production, lower antioxidant enzyme expression and increased lipid
peroxidation (25).
Sustained consumption of high amounts of fructose
also affects mothers by generating oxidative stress that can cause
DNA damage. A study conducted in Brazil by Magenis et al
(18) investigated whether fructose
consumption during pregnancy affects genomic stability in female
mice. The results showed more significant DNA damage in females
receiving fructose during gestation and lactation. These results
agree with previous studies in the literature stating that maternal
fructose consumption could have adverse effects on both mother and
offspring (18). The effects of
high fructose intake on several physiological and molecular
processes associated with oxidative stress in murine models are
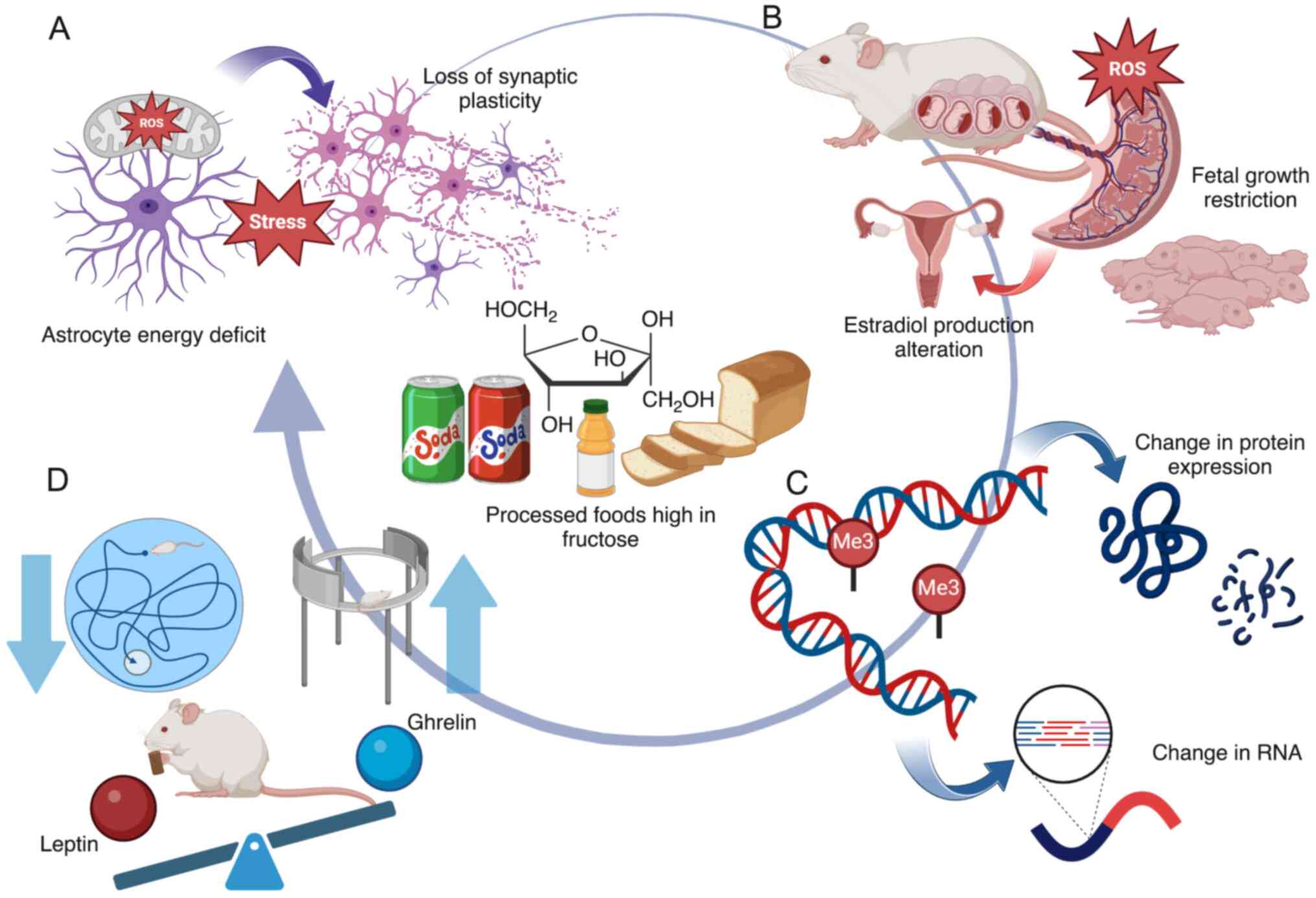
summarized in Fig. 2A and Table I.
 | Table ISummary of the main findings of the
articles included in the scoping review. |
Table I
Summary of the main findings of the
articles included in the scoping review.
| First author/s,
year | Main findings | (Refs.) |
|---|
| Mitochondrial
function, neuroinflammation and its relation to oxidative
stress |
|---|
| Spagnuolo et
al, 2020 | Excess fructose was
linked to obesity, dyslipidemia, and insulin resistance. The diet
also promoted neuroinflammation, oxidative stress, mitochondrial
dysfunction, and alterations in insulin signaling. | (4) |
| Chao et al,
2020 | The maternal
fructose rich diet during pregnancy and lactation caused fetal
programming in rats, inducing deficient expression of receptor
proteins, tissue oxidative stress, sympathetic activation, and
nutrient-sensing signals are closely related to adult hypertension
of fetal origin. | (14) |
| Yamazaki et
al, 2023 | Maternal and
post-weaning consumption of a diet consisting of 20% fructose had a
negative synergistic effect on the expression of pro-inflammatory
cytokines in the hippocampus. In the postnatal, higher expression
of tumor necrosis factor alpha was observed in the group with
prenatal and postnatal high-fructose corn syrup intake, suggesting
a synergistic detrimental impact. | (5) |
| Wu et al,
2019 | The fructose rich
diet (60%) suppressed glycolytic capacity, mitochondrial
respiration and electron transport chain. It also affected
mitochondrial DNA copies and mitochondrial transcription factors
affecting brain metabolism. | (6) |
| Mizuno et
al, 2021 | The study suggests
that a diet of 20% fructose for dams may decrease catalase (Cat)
transcript levels in the offspring's hippocampus by altering DNA
methylation, resulting in higher levels of oxidative stress. | (8) |
| Smith et al,
2022 | Epigenetic
modifications in mitochondrial genes are proposed as a potential
contributing factor to these alterations, although their precise
role remains to be fully elucidated. | (9) |
| Mortensen et
al, 2014 | Chronic maternal
consumption of a diet with 485 g of fructose through pregnancy and
lactation affects brain metabolism, cognitive function in adults
and lower body weight in the offspring after weaning. | (15) |
| Liu et al,
2020 | A maternal diet
with 60% fructose diet triggered neuroinflammation in the
hippocampus of 3-month-old female offspring. Pioglitazone treatment
reversed these effects, suggesting a potential therapeutic use to
treat the hippocampal impairment induced by a chronic fructose
consumption. | (16) |
| Chao et al,
2022 | β-biotic
supplementation in young offspring may protect against the
development of hypertension in adulthood in those exposed to a
maternal diet 60% rich in fructose. The β-biotic helped to reduce
oxidative stress and neuroinflammation. | (17) |
| Magenis et
al, 2020 | Chronic consumption
of fructose (10 and 20%) during pregnancy and lactation causes DNA
damage and negatively impacts intake, body weight, lipid profile
and fasting blood glucose. | (18) |
| Yamada et
al, 2019 | Maternal exposure
to a 20% fructose diet in rats induced detrimental effects on
mitochondrial physiology and oxidative stress in offspring through
epigenetic mechanisms. | (19) |
| Bukhari et
al, 2018 | Coexistence of
inflammation derived from a maternal high-fructose diet (60%) and
neonatal treatment with lipopolysaccharides affected anxiety-like
behaviors and proinflammatory cytokine levels at multiple life
stages of rodents. | (22) |
| Wang et al,
2022 | FOXP1 syndrome is
associated with neurological deficits. In Foxp1 +/- mice,
mitochondrial dysfunction and oxidative stress are observed, which
could explain the cognitive and motor impairment. | (25) |
| Kinshella et
al, 2022 | Foods high in added
sugar, such as sugary drinks, were associated with increased risk
of pre-eclampsia incidence. | (24) |
| Jones et al,
2021 | Dietary fructose
reprograms monocyte and macrophage metabolism, increasing
inflammation through mechanistic target of rapamycin complex 1 and
reducing metabolic flexibility. In mice, its consumption
intensifies the systemic inflammatory response. | (23) |
| Jiménez- Maldonado
et al, 2018 | The high
consumption of fructose during a week had no effect over the
liver/body weight ratio, weight gain, glucose tolerance and insulin
sensitivity, but I could reduce several aspects of hippocampal
plasticity. | (21) |
| Reproductive health
and placental and intrauterine effect of maternal fructose
consumption |
| Vickers et
al, 2011 | Female fetuses,
unlike male fetuses, had smaller placentas, with high levels of
leptin, fructose and glucose. Maternal exposure to a diet with 20%
of caloric intake from fructose differentially affects placental,
fetal and neonatal development in females. | (26) |
| Liu et al,
2021 | High fructose
consumption (10%) during pregnancy in rats caused placental
insufficiency and fetal growth restriction, with reduced fetal and
placental weight, increased oxidative stress and altered gene
expression. | (3) |
| Kearns and
Reynolds, 2024 | The impact of
non-nutritive sweetener consumption in cognitives and animal models
suggests that it affects maternal metabolic health and offspring
development. This review examines maternal sweetener intake and its
effects on fertility and maternal health. | (11) |
| Koski et al,
1993 | A maternal diet low
in carbohydrates with adequate energy intake reduced fetal brain
weight and affected composition, and altered neurotransmitter
synthesis. Glucose and fructose presented similar results across
different distributions. | (29) |
| Munetsuna et
al, 2018 | Offspring of dams
fed fructose (20%) showed reduced steroidogenesis, lower estradiol,
and downregulation of estrogen and progesterone receptors,
indicating altered ovarian physiology. | (30) |
| Clayton et
al, 2015 | Maternal fructose
intake alters fatty acid metabolism in mothers and offspring in an
age- and sex-specific manner, affecting biological clock genes. In
addition, it could impair the hepatic immune response by
suppressing the inflammasome in mothers and male neonates. | (27) |
| Epigenetic and
transcriptomic modifications in the offspring |
| Zou et al,
2023 | A maternal
high-fructose diet (13 and 40%) altered hippocampal lncRNAs and
target genes in offspring, which affected brain development and
increased dopaminergic receptor expression, leading to anxious
behaviors and suggesting a link between lncRNAs and emotional
regulation. | (32) |
| Zou et al,
2023 | Maternal fructose
to diets supplied with 13 and 40% fructose impaired conditioning
and associative memory, caused low levels of synaptic proteins, and
alterations in gene edition mechanisms. | (31) |
| Wu et al,
2024 | A maternal diet
high in fructose (60%) impaired hippocampal memory, caused
microbiota dysbiosis, and reduced butyrate levels in the offspring.
These effects were partly reversed by fructo- oligosaccharides and
butyrate, which restored protein expression, bioenergetics, and
mitochondrial function. | (33) |
| Zou et al,
2022 | Maternal intake of
diets with 13 and 40% fructose contents affected learning and
memory by inhibiting a pathway responsible for regulating processes
such as tissue regeneration and differentiation of stem cells and
cell proliferation. The diet also altered the expression of genes,
which can cause changes in the development of the nervous
system. | (35) |
| Chao et al,
2016 | This study used
next-generation sequencing to analyze transcriptome expression in
male offspring exposed to a maternal high-fructose (60%) diet,
revealing long-lasting changes in gene expression and metabolic
syndrome phenotypes in adulthood. | (34) |
| Mukai et al,
2014 | Excessive maternal
fructose intake (10%) during gestation altered the expression of
cellular energy sensors in the liver, hypothalamus and
glucose-6-phosphatase activity, especially in female offspring,
suggesting long-lasting metabolic effects and an increased risk of
metabolic syndrome. | (36) |
| Koo et al,
2021 | Offspring exposed
to diets supplied with 20% fructose through maternal consumption
had higher body weight, fatty liver, poorer glucose tolerance, and
elevated levels of serum markers and blood pressure. | (1) |
| Ohashi et
al, 2015 | Maternal fructose
intake (20%) increased mRNA levels of steroidogenic enzymes in the
hippocampus of offspring, potentially affecting
neuro-steroidogenesis and neuron survival. | (37) |
| Wu et al,
2016 | Offspring of
mothers fed high fructose (60%) showed impaired spatial learning
and memory, reduced hippocampal BDNF, and increased nuclear histone
deacetylase 4; however, post- weaning environmental stimulation for
four weeks reversed these cognitive deficits and molecular
changes. | (39) |
| Mizuno et
al, 2017 | Maternal
consumption of a diet supplied with 20% fructose differentially
affected the expression of steroidogenesis-related genes in the
hippocampus of offspring with different effects through pregnancy
and lactation. | (38) |
| Yamazaki et
al, 2018 | Excessive and early
maternal exposure to fructose (20%) generated an increase in DNA
methylation in the BDNF promoter region, which persisted until the
rat's maturity and was related to hippocampal dysfunction. | (2) |
| Bokor et al,
2024 | The study analyzes
how maternal fructose intake during gestation and lactation can
influence DNA methylation in offspring, affecting their metabolism
and risk of obesity, highlighting the role of epigenetic changes in
fetal programming. | (10) |
| Brain
characterization and cognitive function |
| Saad et al,
2016 | The study found
that exposure to fructose during pregnancy differentially affected
the brain development of offspring: in males there was a reduction
in the size of brain structures and an increase in neuronal cells,
and in females only an increase in neuronal cells was observed.
These findings suggest that maternal metabolic dysregulation
induced by fructose (10%) may alter fetal brain development. | (42) |
| Gillespie et
al, 2024 | Maternal diet rich
in fructose alters the expression of lncRNAs and their target genes
in the hippocampus of the offspring, affecting key physiological
functions, especially those related to brain development. A
relationship was found between these changes in lncRNAs and
anxiety-like behaviors in the offspring, suggesting an impact on
emotional regulation and neurodevelopment. | (12) |
| Kisioglu and
Nergiz-Unal, 2020 | High-fructose diets
led to increased body fat intake and altered appetite regulation
with lower leptin, higher ghrelin, and higher cluster of
differentiation 36 levels, suggesting that maternal fructose
contributes to obesity programming. | (28) |
| Erbas et al,
2018 | Significant
differences appeared in behavioral tests, histological alterations
in the hippocampus and differences in brain biochemical markers
between controls and fructose-exposed groups, suggesting a link
between maternal metabolic stress due to prolonged intake of a diet
with 30% fructose and neurological development disorders in
offspring. | (43) |
| Rivell and Mattson,
2019 | It suggests that
interventions that improve metabolic health may ameliorate
developmental neuronal network abnormalities and consequent
behavioral manifestations in autism spectrum disorders. | (44) |
| Crichton et
al, 2016 | Frequent
consumption of sugary soft drinks is associated with poorer
cognitive performance, especially in individuals with type 2
diabetes, while diet soft drinks showed no such effect. Additional
studies are needed to understand the underlying mechanisms. | (40) |
| Ye et al,
2011 | Greater intakes of
total sugars, added sugars and sugar-sweetened beverages, but not
of sugar- sweetened solid foods, were significantly associated with
lower Mini-Mental State Examination scores, higher sugar intake
appears to be associated with lower cognitive function, but
longitudinal studies are needed to clarify the direction of
causality. | (41) |
Reproductive health, placental and
intrauterine effect of maternal fructose consumption
Maternal nutrition during early life can
significantly affect the postnatal phenotype of offspring.
Undernutrition and hypercaloric maternal overnutrition can create
an adverse environment, leading to a fundamental impact on fetal
development and subsequent disease in adulthood, highlighting the
importance of nutritional programming (26).
The placenta is an active intermediary between the
maternal blood circulation and the fetus, thus playing a crucial
role in fetal protection and nutritional programming (3,11). Liu
et al (3) suggested that
maternal consumption of fructose during pregnancy and lactation may
lead to adverse outcomes in offspring, including insulin
resistance, fatty liver, adipose tissue dysfunction, reduced
adiponectin levels, dyslipidemia and alterations in endocrine
function. Liu et al (3)
found that pregnant rats consuming 10% fructose had a low
fetus/placenta weight ratio, which is representative of the
placental insufficiency observed in the fructose group; the same
pregnant rats developed placentas with a weight of 0.53±0.12 g,
whereas that control rats had placentas weighing of 0.74±0.08 g.
These findings indicate that the placenta responds to alterations
in maternal nutritional status and plays a crucial role in
programming the fetal environment in utero through adaptive
modifications in its structure and function. Reduced placental
weight and a low fetus-to-placenta ratio may contribute to the
asymmetric fetal growth restriction (AFGR) observed in the fructose
group. Additionally, a significant decrease in maternal serum
placental growth factor (PLGF), accompanied by an increase in
soluble fms-like tyrosine kinase-1 (sFlt-1) concentrations and an
elevated sFlt-1/PLGF ratio in the fructose group has been reported,
further emphasizing placental insufficiency (3).
Fructose can be transported across the placenta and
be present in fetal circulation, resulting in increased uric acid
(UA) synthesis in both the liver and placenta. It is proposed that
placentally produced UA may mediate the effects of fructose by
promoting endothelial dysfunction and inefficient placentation.
This dual role of UA is attributed to its ability to function as an
extracellular antioxidant while inducing oxidative stress within
cells (3). This oxidative stress
could be the general underlying mechanism linking altered placental
function to fetal programming since overproduction of ROS can lead
to massive cellular damage, changing the course of pregnancy and
generating a cascading effect leading to the genesis of in
utero programming of adult diseases (3).
Vickers et al (26) observed that maternal fructose intake
significantly elevated circulating plasma fructose and leptin
levels in female fetuses, and reduced female placental weights-an
effect absent in male fetuses. Based on these findings, they
hypothesized a sex-specific effect on placental fructose
sensitivity and/or transfer. This effect may be mediated by
specific fructose transporters such as glucose transporter 5 or
through alterations in placental growth and function influenced by
fructose's impact on growth factors such as insulin-like growth
factor and placental transporters. This hypothesis is feasible
since other early-life influences have been shown to have
sex-specific effects on the placenta, leading to changes in
placental vascularity and growth (26).
The differential effects of fructose on sex are
linked to the gene expression of various metabolic enzymes. Studies
indicate that in male neonates, key enzymes involved in hepatic
beta-oxidation, such as carnitine palmitoyl-transferase 1A and
acetyl-coA acetyltransferase, are suppressed following fructose
exposure. This suppression is associated with increased mRNA levels
of sterol regulatory element-binding protein 1c, suggesting that
hepatic fatty acid oxidation is diminished in favor of lipogenesis.
By contrast, a distinct response is observed in females,
characterized by an increase in mRNA levels of the gene encoding
adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK).
AMPK, a central metabolic regulator, enhances cellular energy
levels by inhibiting anabolic pathways, such as fatty acid
synthesis, while stimulating catabolic pathways that promote energy
production, including fatty acid oxidation and glucose transport
(27).
Other studies have pointed out that high fructose
consumption during pregnancy can trigger oxidative stress in the
placenta, generating AFGR and contributing to early pregnancy
failure and fetal malformations due to placental insufficiency and
elevated UA synthesis, which promotes endothelial dysfunction and
placental insufficiency, increasing the risk of disease in the
offspring (3,26).
A study by Vickers et al (26), which measured the impact of maternal
fructose consumption on the placenta, fetal development and
offspring growth, showed that this diet significantly increases
circulating plasma fructose and leptin levels. This
appetite-related peptide helps manage satiety through the
hypothalamic system, which could induce decreased appetite. This
change was accompanied by a decrease in placental weight of 41±9 mg
(26,28). However, these changes were only
observed in female offspring; the researchers noted no changes in
placental weight in males, an increase in leptin, or an increase in
leptin concentration observed in the mothers. Several studies
(3,26,29)
have identified adverse placental and intrauterine effects. This
could be related to the transfer of leptin to the placenta or the
alteration of placental leptin production in female fetuses exposed
to fructose (26).
On the other hand, a study by Munetsuna et al
(30) revealed that chronic
fructose consumption during pregnancy and lactation has a
transgenerational effect on offspring. Maternal fructose
consumption impacts ovarian estradiol synthesis in the offspring.
The impact of maternal fructose on ovarian estradiol synthesis in
the offspring was evaluated by performing a quantitative PCR. It
was found that some steroidogenic enzymes, such as estradiol, were
attenuated in fructose-exposed offspring. These findings suggest
that excessive maternal fructose consumption might cause
dysregulated ovarian function in the offspring throughout their
lives. It was suggested that fructose-induced endoplasmic reticulum
(ER) stress might represent one of the mechanisms by which
estradiol synthesis is altered in the offspring. Based on this, it
was hypothesized that the offspring may ingest fructose through
fetal circulation and lactation, providing an environment in which
there is abundant fructose in utero, which could produce
fructose-induced ER stress in the offspring, leading to attenuated
transcription of steroidogenic genes (30).
Although a fructose-rich diet may adversely affect
offspring health and development in murine models, fructose is a
valuable energy substrate. It has been observed that changes in
carbohydrate levels in the maternal diet during pregnancy can
affect glycogen levels in fetal brains and brain function.
Additionally, it has been reported that the absence of glucose and
fructose in the maternal diet can result in lower fetal brain
weight than carbohydrate-rich diets. Correlations between fetal
brain glycogen and amniotic fluid glucose levels demonstrate that
low-carbohydrate diets reduce amniotic fluid glucose in pregnant
rats, leading to deficits in the weight of specific organs after
fructose deprivation (29). These
findings indicate that carbohydrate deprivation in the maternal
diet may negatively affect fetal brain development. While fructose
is a common component of daily foods, excessive intake,
particularly during pregnancy, can harm intrauterine health and
offspring development. However, eliminating sugars from the diet
may also pose health risks, highlighting the importance of careful
dietary management during pregnancy (Fig. 2B and Table I).
Epigenetic and transcriptomic
modifications in the offspring
The harmful effects of a maternal fructose-rich diet
on biological processes relevant to brain development at the tissue
level have been discussed in the previous section. This section
examines the molecular changes induced by fructose-rich diets and
their impact at the organo-genic level. Zou et al (31) found that chronic fructose
consumption can influence numerous biological processes related to
brain development by altering transcriptional expression and
disrupting essential RNA editing processes, leading to adverse
effects on the nervous system of offspring (31,32).
Wu et al (6) further
highlighted that such diets impair synaptic plasticity, hippocampal
neurogenesis and spatial memory. Moreover, neuro-steroids are
particularly sensitive to maternal fructose intake. This suggests
that maternal malnutrition during pregnancy and early organ
development stages can result in fetal programming, altering the
structure and function of the offspring's systems-a concept known
as Barker's hypothesis (5). These
findings emphasize the need to understand the molecular effects of
high-fructose diets on brain and metabolic development in offspring
(6).
After sequencing whole RNA using the Full-Length
Nanopore RNA Sequencing method, researchers aimed to predict long
non-coding RNA (lncRNA) chains, which, despite not encoding
proteins, can influence metabolic processes, such as neuroreceptor
signaling (as indicated by Zou et al (31,32).
Additionally, lncRNAs regulate gene expression in pathways involved
in embryonic development. MDRF has been shown to not only alter
lncRNA expression but also affect messenger RNA sequences, poly-A
chains and splice sites, potentially predisposing individuals to
diseases such as non-alcoholic fatty liver disease or cancer
(31,32). Furthermore, other studies suggest
that altered DNA methylation following fructose ingestion may
explain changes in gene expression, adding another layer to fetal
programming mechanisms that shape offspring phenotype (23,32,33).
Supporting this, techniques such as western blotting,
immunofluorescence and gene silencing have revealed that MDRF
during gestation suppresses plasma butyrate levels and hippocampal
G-protein-coupled receptor 43 expression in offspring (Wu et
al, 2024). Butyrate, which plays a crucial role in epigenetic
regulation and mitochondrial biogenesis, is affected by the
upregulation of histone deacetylase 4 (HDAC4) in hippocampal
astrocytes following MDRF during gestation. These changes suggest
transgenerational epigenetic effects on brain function (33).
Chao et al (34) found that maternal diet containing
13-40% fructose negatively impacted offspring's learning and memory
abilities compared with a control group. Their study identified 369
differentially expressed transcripts (DETs) in the offspring of
mothers consuming a 13% fructose diet and 501 DETs in those exposed
to a 40% fructose diet. The effect of a MDRF on transcriptome
expression in the brain, heart, kidney and urinary bladder of male
offspring of Sprague-Dawley rats have been reported using
next-generation sequencing technology (34). Chao et al (34) identified ErbB receptor feedback
inhibitor 1 and connective tissue growth factor as the only two
differentially expressed genes (DEGs) in the analyzed organs.
According to the researchers, these genes may contribute to
metabolic dysfunction in adulthood, supporting the hypothesis of
prenatal metabolic programming. These results highlight the
importance of future epigenetic research to determine whether these
changes are mediated by DNA methylation or histone modifications,
further reinforcing the hypothesis that maternal diet has long-term
transgenerational effects.
These findings suggest that maternal fructose intake
during gestation and lactation can impair learning and memory in
offspring and alter brain function at the transcriptome level
(35). Zou et al (35,30)
investigated DETs and DEGs by constructing cDNA libraries from RNA
extracted from the hippocampus of MDRF-exposed rats. The extracted
RNA was sequenced, and the transcriptome analysis was conducted
using Counts Per Million to identify differences in DETs and DEGs
among groups. Their results revealed significant transcriptomic
differences between rats fed a fructose-rich diet and those on chow
and water-based diets. Some of the DET-associated functions
included terms related to ‘neuron migration’ and ‘brain
development’ (35). Similarly,
Mukai et al (36)
demonstrated that fructose consumption during pregnancy may exert
sex-specific effects on offspring. Their research indicated that
excessive fructose intake during pregnancy modulated hepatic and
hypothalamic AMPK, signalling pathways affecting carbohydrate
metabolism in female offspring postnatally but not in male
offspring.
Koo et al (1)
found that high maternal fructose intake during pregnancy and
lactation is associated with reduced fructokinase mRNA levels,
hepatic lipid accumulation and increased expression of lipogenesis.
However, these effects could involve interactions between RNA
expression and sex hormones. Immunoblotting analysis demonstrated
reduced expression and subsequent phosphorylation of proteins that
act as energy sensors in female offspring exposed to fructose. The
same study found differences in basal gene expression between
female and male offspring after a maternal fructose diet, with a
higher expression of pro-oxidant genes in female offspring than
male offspring exposed to fructose. On the other hand, males
exposed to fructose showed a higher expression level of genes
responsible for synthesizing sodium transporter proteins than
female offspring whose dams had a fructose-rich diet, suggesting an
interaction with sex hormones (1).
Maternal diets rich in fructose can impact multiple
biological processes and functions through fetal programming,
influencing brain development and altering the transcriptional
expression of several genes involved in fructose metabolism,
glycolysis/gluconeogenesis (10).
These changes are associated with insulin resistance, impaired
glucose tolerance, disruptions in fatty acid metabolism and altered
insulin signalling. Notably, some of the physiological changes
favor pathways related to ‘glutamatergic synapses’ and ‘long-term
depression’ (31,32,35-37).
Additionally, DNA methylation in the promoter region of the
brain-derived neurotrophic factor (BDNF) gene, along with fructose
intake, has been linked to persistent cognitive deficits in areas
such as memory and learning. Furthermore, fructose consumption
affects the expression of specific BDNF exons in the hippocampus of
offspring, which may contribute to cognitive impairments (2).
Other studies did not compare the effects on male
and female counterparts but provided different insights. Zou et
al (32,30) examined RNA sequences and, using the
Kyoto Encyclopedia of Genes and Genomes and Gene Ontology
databases, concluded that maternal high fructose intake might alter
gene expression related to fetal brain development, affecting
neuronal signalling and synaptic plasticity. Meanwhile, other
studies focused on different exposure periods. Mizuno et al
(38) evaluated the impact of
fructose consumption during gestation or lactation on the
transcriptional regulation of neuro-steroidogenic enzymes, which
are crucial for neuronal protection and synapse formation. They
found that the expression of the peripheral benzodiazepine receptor
was downregulated even when rats consumed fructose only during
lactation. By contrast, fructose intake during gestation increased
the expression of cytochrome P450 (11β)-2. They concluded that
maternal fructose intake during gestation or lactation
differentially affects the offspring's expression of hippocampal
neuro-steroidogenic enzymes (38).
Previous findings indicate that epigenetic
modifications can occur indirectly; maternal fructose consumption
during gestation and lactation reduces butyrate synthesis, a fatty
acid that promotes epigenetic remodeling by inhibiting proteins
crucial for mitochondrial biogenesis in the hippocampus. Wu et
al (39) investigated this
phenomenon using chromatin immunoprecipitation assays, reverse
transcription-quantitative PCR, western blotting,
immunofluorescence and ELISA, first to evaluate the presence and
expression of HDAC4 and BDNF, then to evaluate the binding of HDAC4
to BDNF promoters in the hippocampus. Their results showed that a
maternal high-fructose diet alters hippocampal epigenetics in
offspring by increasing nuclear accumulation of HDAC4, which
represses BDNF transcription, a key molecule for learning and
memory. These findings suggest transgenerational modulation due to
chronic fructose consumption (2,33,39).
Interestingly, environmental stimulation has been
reported to improve cognitive function in rats that developed
cognitive impairment due to chronic fructose consumption during
gestation. Wu et al (39)
describe how providing toys and nesting material to these rats -
and replacing them weekly-helped restore their cognitive
function.
Several studies have shown that a high-fructose diet
during gestation can lead to molecular changes, including altered
gene expression and epigenetic modifications, which may impair
synaptic plasticity, hippocampal neurogenesis and spatial memory
(35,16). These findings underscore the
importance of understanding how maternal fructose consumption can
influence brain and metabolic development in offspring, with
effects potentially varying by sex and impacting processes
including carbohydrate metabolism, neuro-steroid regulation and
enzymatic signalling pathways. Moreover, research suggests that
environmental stimulation might help mitigate some cognitive
deficits caused by maternal fructose exposure. Overall, the
evidence highlights the need for further investigation into the
complex relationships between maternal diet, fetal programming and
offspring's long-term health and brain function, providing insights
that could guide strategies to improve prenatal nutrition and
support healthy brain development (Fig.
2C and Table I).
Brain characterization and cognitive
function
Maternal nutrition during fetal and early postnatal
development is critical in determining the risk of diseases that
manifest later in the offspring's life. Increasing evidence
suggests that reduced cognitive performance in offspring may be
linked to maternal dietary intake and, in utero,
environmental factors (12). These
diseases are considered to develop through epigenetic mechanisms, a
process referred to as developmental programming (33,38,40).
According to some studies, MDRF during gestation and
lactation affects offspring learning and memory (35,38).
Offspring of mothers exposed to fructose showed delays in finding
the platform in the aquatic Morris maze, possibly due to oxidative
stress in the hippocampus that triggers cognitive dysfunction. In
addition, housing with toys and nesting material was observed to
improve learning and memory in fructose-exposed hatchlings,
highlighting the positive influence of environmental stimulation on
neurogenesis. Other studies also revealed that latency time
increased in the offspring of mothers with higher fructose
consumption, resulting in longer training times and navigational
disorientation, suggesting that MDRF may impact offspring cognitive
development (35,38). Although studies in humans are
limited, some evidence suggests an association between fructose and
memory impairment. To assess cognitive status in a population with
habitual sugar intake, Ye et al (41) conducted a study among middle-aged
and older Puerto Ricans without diabetes, utilizing the Mini-Mental
State Examination (MMSE). This tool assesses orientation,
attention, memory, language and visuospatial skills. Their analysis
revealed that sucrose and fructose were the sugars most consumed by
this population. Notably, higher fructose intake was significantly
associated with cognitive impairment, as indicated by lower MMSE
scores (41).
It has been observed that gestational diabetes in
rodents can lead to excessive activation of microglia in brain
regions associated with memory and learning, resulting in anxious
behaviors and increased sensitivity to stress in adulthood. Bukhari
et al (22) proposed an
experimental model that combines immune alterations and gestational
diabetes using a 60% maternal diet-induced rat model of diabetes
(MDRF) along with neonatal inflammation induced by LPS. This
combination led to an abnormal glucocorticoid response, which has
been linked to neuropsychiatric disorders such as anxiety,
depression and schizophrenia. Furthermore, neonatal LPS exposure
treatment combined with an MDRF diet resulted in elevated IL-1β
levels, indicating prolonged inflammation in postnatal rats.
Anxious behaviors persisted into the juvenile stage, regardless of
sex, highlighting the detrimental impact of gestational diabetes on
behavior and suggesting long-term effects on quality of life.
Additionally, studies (12,40,41)
have reported that populations with high soft drink consumption
exhibit poorer cognitive performance across multiple domains,
including global cognition composite score, working memory,
scanning and tracking abilities, executive function and MMSE
scores. Given that fructose constitutes 55-60% of the total sugar
content in popular soft drinks, evidence strongly suggests that
fructose is a critical factor in triggering cognitive impairment
(40).
Fetal programming, which influences behaviour, may
exacerbate genetic predispositions to autism (42). High fructose intake during pregnancy
has been shown to alter brain regions associated with autism
spectrum disorder (ASD). Research indicates that dysregulated
nutrition, including high fructose consumption during gestation and
lactation, is linked to neurobehavioral disorders such as ASD,
anxiety, learning deficits and even changes in eating behaviors
(31,32,28,43).
These findings underscore the importance of considering nutritional
dysregulation and other factors in understanding the potential
impact of fructose consumption on the development of
neuropsychiatric diseases (31,32,28,43).
Over the past 4 decades, the prevalence of ASD has
risen significantly. This has been associated with excessive
dietary energy intake, particularly from fructose, accompanied by a
rise in metabolic syndrome, which includes obesity, insulin
resistance and hyperlipidemia. Research indicates that children
born to mothers who are obese and/or diabetic face a heightened
risk of developing ASD. Sedentary lifestyles and the consumption of
large amounts of high-fructose corn syrup are considered to
contribute to numerous cases of obesity and diabetes. Emerging
evidence supporting a cause-and-effect relationship between
metabolic syndrome and ASD underscores the need to improve
education of parents on the effects of obesity on the mental health
of their children. Medical communities, relevant government
agencies, and private foundations have a responsibility to
disseminate the implications of research on metabolic syndrome and
ASD to the public (44).
Kisioglu and Nergiz-Unal (28) found that added sugars, such as
fructose, contribute to obesity by altering appetite-related
peptides that regulate the hypothalamic feeding system,
specifically ghrelin and leptin. When these peptides become
dysregulated, it can lead to dysfunctional hypothalamic signalling,
resulting in increased hunger, excessive food intake and enhanced
lipogenesis. Their research showed that fructose stimulates
appetite by lowering circulating levels of leptin and reducing the
suppression of ghrelin, which influences eating behavior and
contributes to obesity (28).
The reviewed studies consistently demonstrate that
exposure to MDRF during gestation and lactation significantly
changes offspring's brain development and cognitive function.
Therefore, it is essential to investigate the underlying mechanisms
further to develop preventive and therapeutic strategies that can
mitigate the adverse effects of fructose exposure during early life
(Fig. 2D and Table I).
4. Conclusions
The findings from these studies suggest that chronic
maternal fructose consumption during gestation and lactation may
negatively impact offspring health by affecting mechanisms related
to the regulation of food intake, motivation, learning, memory, and
reproductive health. In experimental models, these adverse effects
are associated with neuroinflammation, metabolic dysfunction,
oxidative stress and transcriptional and epigenetic changes
impairing cognitive and physical health (Table I). Additionally, these studies
highlight the significant influence of maternal nutrition on
offspring, emphasizing the potential transgenerational effects of
fructose, which is consistent with Barker's hypothesis. They also
underscore the complexity of factors involved in organogenesis and
postnatal growth. Several studies demonstrate that identical
external conditions can result in vastly different outcomes
depending on factors such as sex or genetic predisposition,
indicating that each case must be considered individually, even
though malnutrition remains a primary factor.
While the present literature review provides
valuable insights into the effects of maternal fructose
consumption, some limitations must be acknowledged. Over 80% of the
studies analyzed have been conducted in murine models rather than
other animal models. This is primarily due to ethical
considerations, greater experimental control, shorter reproductive
cycles, and easier access to fetal and postnatal tissues, rendering
murine models more suitable for research. However, despite their
usefulness, murine models do not fully replicate human pregnancy.
Differences in placental function, metabolism and lifespan may
limit the direct applicability of findings to humans. Existing
evidence suggests that excessive fructose intake during pregnancy
may negatively impact fetal development and offspring health.
Therefore, further human studies are necessary to confirm or
challenge the mechanisms proposed in experimental animal
models.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Corporacion
Universitaria Remington (grant no. 4000000421).
Availability of data and materials
Not applicable.
Authors' contributions
ALUA, CDMU, JDGC and JAGV wrote the manuscript. JDGC
edited the manuscript. JAGV conceived the idea of the study. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koo S, Kim M, Cho HM and Kim I: Maternal
high-fructose intake during pregnancy and lactation induces
metabolic syndrome in adult offspring. Nutr Res Pract. 15:160–172.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yamazaki M, Yamada H, Munetsuna E,
Ishikawa H, Mizuno G, Mukuda T, Mouri A, Nabeshima T, Saito K,
Suzuki K, et al: Excess maternal fructose consumption impairs
hippocampal function in offspring via epigenetic modification of
BDNF promoter. FASEB J. 32:2549–2562. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu S, Zhang H, Yan B, Zhao H, Wang Y, Gao
T and Liang H: Maternal high-fructose consumption provokes
placental oxidative stress resulting in asymmetrical fetal growth
restriction in rats. J Clin Biochem Nutr. 69:68–76. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Spagnuolo MS, Iossa S and Cigliano L:
Sweet but bitter: Focus on fructose impact on brain function in
rodent models. Nutrients. 13(1)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yamazaki M, Yamada H, Munetsuna E, Ando Y,
Kageyama I, Sadamoto N, Nouchi Y, Teshigawara A, Mizuno G, Ishikawa
H, et al: Interaction between prenatal and postnatal exposure to
high-fructose corn syrup increases gene expression of Tnfa in
hippocampus of offspring. J Nutr Sci Vitaminol (Tokyo). 69:237–242.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu CW, Hung CY, Hirase H, Tain YL, Lee WC,
Chan JYH, Fu MH, Chen LW, Liu WC, Liang CK, et al: Pioglitazone
reversed the fructose-programmed astrocytic glycolysis and
oxidative phosphorylation of female rat offspring. Am J Physiol
Endocrinol Metab. 316:E622–E634. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lê KA and Tappy L: Metabolic effects of
fructose. Curr Opin Clin Nutr Metab Care. 9:469–475.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mizuno G, Munetsuna E, Yamada H, Yamazaki
M, Ando Y, Hattori Y, Kageyama I, Teshigawara A, Nouchi Y, Fujii R,
et al: Maternal fructose consumption downregulates hippocampal
catalase expression via DNA methylation in rat offspring. Nutr Res.
92:40–48. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Smith EVL, Dyson RM, Weth FR, Berry MJ and
Gray C: Maternal fructose intake, programmed mitochondrial function
and predisposition to adult disease. Int J Mol Sci.
23(12215)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bokor S, Csölle I, Felső R, Vass RA, Ertl
T and Molnár D: Dietary nutrients during gestation cause obesity
and related metabolic changes by altering DNA methylation in the
offspring. Front Endocrinol (Lausanne). 15(1287255)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kearns ML and Reynolds CM: The impact of
non-nutritive sweeteners on fertility, maternal and child health
outcomes: A review of human and animal studies. Proc Nutr Soc.
83:280–292. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gillespie KM, White MJ, Kemps E, Moore H,
Dymond A and Bartlett SE: The impact of free and added sugars on
cognitive function: A systematic review and meta-analysis.
Nutrients. 16(75)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li JM, Yu R, Zhang LP, Wen SY, Wang SJ,
Zhang XY, Xu Q and Kong LD: Dietary fructose-induced gut dysbiosis
promotes mouse hippocampal neuroinflammation: A benefit of
short-chain fatty acids. Microbiome. 7(98)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chao YM, Wu KLH, Tsai PC, Tain YL, Leu S,
Lee WC and Chan JYH: Anomalous AMPK-regulated angiotensin
AT1R expression and SIRT1-mediated mitochondrial
biogenesis at RVLM in hypertension programming of offspring to
maternal high fructose exposure. J Biomed Sci.
27(68)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mortensen OH, Larsen LH, Ørstrup LK,
Hansen LH, Grunnet N and Quistorff B: Developmental programming by
high fructose decreases phosphorylation efficiency in aging
offspring brain mitochondria, correlating with enhanced UCP5
expression. J Cereb Blood Flow Metab. 34:1205–1211. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu WC, Wu CW, Fu MH, Tain YL, Liang CK,
Hung CY, Chen IC, Lee YC, Wu CY and Wu KLH: Maternal high
fructose-induced hippocampal neuroinflammation in the adult female
offspring via PPARγ-NF-κB signaling. J Nutr Biochem.
81(108378)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chao YM, Tain YL, Lee WC, Wu KLH, Yu HR
and Chan JYH: Protection by -biotics against hypertension
programmed by maternal high fructose diet: Rectification of
dysregulated expression of short-chain fatty acid receptors in the
hypothalamic paraventricular nucleus of adult offspring. Nutrients.
14(4306)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Magenis ML, Damiani AP, De Marcos PS, De
Pieri E, De Souza E, Vilela TC and De Andrade VM: Fructose
consumption during pregnancy and lactation causes DNA damage and
biochemical changes in female mice. Mutagenesis. 35:179–187.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yamada H, Munetsuna E, Yamazaki M, Mizuno
G, Sadamoto N, Ando Y, Fujii R, Shiogama K, Ishikawa H, Suzuki K,
et al: Maternal fructose-induced oxidative stress occurs via Tfam
and Ucp5 epigenetic regulation in offspring hippocampi. FASEB J.
33:11431–11442. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu HW, Ren LF, Zhou X and Han DW: A
high-fructose diet induces hippocampal insulin resistance and
exacerbates memory deficits in male sprague-dawley rats. Nutr
Neurosci. 18:323–328. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jiménez-Maldonado A, Ying Z, Byun HR and
Gomez-Pinilla F: Short-term fructose ingestion affects the brain
independently from establishment of metabolic syndrome. Biochim
Biophys Acta Mol Basis Dis. 1864:24–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bukhari SHF, Clark OE and Williamson LL:
Maternal high fructose diet and neonatal immune challenge alter
offspring anxiety-like behavior and inflammation across the
lifespan. Life Sci. 197:114–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jones N, Blagih J, Zani F, Rees A, Hill
DG, Jenkins BJ, Bull CJ, Moreira D, Bantan AIM, Cronin JG, et al:
Fructose reprogrammes glutamine-dependent oxidative metabolism to
support LPS-induced inflammation. Nat Commun.
12(1209)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kinshella MLW, Omar S, Scherbinsky K,
Vidler M, Magee LA, von Dadelszen P, Moore SE and Elango R: PRECISE
Conceptual Framework Working Group. Maternal nutritional risk
factors for pre-eclampsia incidence: Findings from a narrative
scoping review. Reprod Health. 19(188)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang J, Fröhlich H, Torres FB, Silva RL,
Poschet G, Agarwal A and Rappold GA: Mitochondrial dysfunction and
oxidative stress contribute to cognitive and motor impairment in
FOXP1 syndrome. Proc Natl Acad Sci USA.
119(e2112852119)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vickers MH, Clayton ZE, Yap C and Sloboda
DM: Maternal fructose intake during pregnancy and lactation alters
placental growth and leads to sex-specific changes in fetal and
neonatal endocrine function. Endocrinology. 152:1378–1387.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Clayton ZE, Vickers MH, Bernal A, Yap C
and Sloboda DM: Early life exposure to fructose alters maternal,
fetal and neonatal hepatic gene expression and leads to
sex-dependent changes in lipid metabolism in rat offspring. PLoS
One. 10(e0141962)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kisioglu B and Nergiz-Unal R: Potential
effect of maternal dietary sucrose or fructose syrup on CD36,
leptin, and ghrelin-mediated fetal programming of obesity. Nutr
Neurosci. 23:210–220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Koski KG, Lanoue S and Young SN:
Restriction of maternal dietary carbohydrate decreases fetal brain
indoles and glycogen in rats. J Nutr. 123:42–51. 1993.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Munetsuna E, Yamada H, Yamazaki M, Ando Y,
Mizuno G, Ota T, Hattori Y, Sadamoto N, Suzuki K, Ishikawa H, et
al: Maternal fructose intake disturbs ovarian estradiol synthesis
in rats. Life Sci. 202:117–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zou Y, Guo Q, Chang Y, Zhong Y, Cheng L
and Wei W: Alternative splicing affects synapses in the hippocampus
of offspring after maternal fructose exposure during gestation and
lactation. Chem Biol Interact. 379(110518)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zou Y, Guo Q, Chang Y, Zhong Y, Cheng L
and Wei W: Effects of maternal high-fructose diet on long
non-coding RNAs and anxiety-like behaviors in offspring. Int J Mol
Sci. 24(4460)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu KLH, Liu WC, Wu CW, Fu MH, Huang HM,
Tain YL, Liang CK, Hung CY, Chen IC, Hung P, et al: Butyrate
reduction and HDAC4 increase underlie maternal high
fructose-induced metabolic dysfunction in hippocampal astrocytes in
female rats. J Nutr Biochem. 126(109571)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chao YM, Tain YL, Leu S, Wu KL, Lee WC and
Chan JY: Developmental programming of the metabolic syndrome:
Next-gener-ation sequencing analysis of transcriptome expression in
a rat model of maternal high fructose intake. Sheng Li Xue Bao.
68:557–567. 2016.PubMed/NCBI
|
|
35
|
Zou Y, Guo Q, Chang Y, Jia L, Zhai L, Bai
Y, Sun Q and Wei W: Learning and memory impairment and
transcriptomic profile in hippocampus of offspring after maternal
fructose exposure during gestation and lactation. Food Chem
Toxicol. 169(113394)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mukai Y, Ozaki H, Serita Y and Sato S:
Maternal fructose intake during pregnancy modulates hepatic and
hypothalamic AMP-activated protein kinase signalling in a
sex-specific manner in offspring. Clin Exp Pharmacol Physiol.
41:331–337. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ohashi K, Ando Y, Munetsuna E, Yamada H,
Yamazaki M, Nagura A, Taromaru N, Ishikawa H, Suzuki K and
Teradaira R: Maternal fructose consumption alters messenger RNA
expression of hippocampal StAR, PBR, P450(11β), 11β-HSD, and
17β-HSD in rat offspring. Nutr Res. 35:259–264. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mizuno G, Munetsuna E, Yamada H, Ando Y,
Yamazaki M, Murase Y, Kondo K, Ishikawa H, Teradaira R, Suzuki K
and Ohashi K: Fructose intake during gestation and lactation
differentially affects the expression of hippocampal
neurosteroidogenic enzymes in rat offspring. Endocr Res. 42:71–77.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu KL, Wu CW, Tain YL, Huang LT, Chao YM,
Hung CY, Wu JC, Chen SR, Tsai PC and Chan JY: Environmental
stimulation rescues maternal high fructose intake-impaired learning
and memory in female offspring: Its correlation with redistribution
of histone deacetylase 4. Neurobiol Learn Mem. 130:105–117.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Crichton GE, Elias MF and Torres RV:
Sugar-sweetened soft drinks are associated with poorer cognitive
function in individuals with type 2 diabetes: The maine-syracuse
longitudinal study. Br J Nutr. 115:1397–1405. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ye X, Gao X, Scott T and Tucker KL:
Habitual sugar intake and cognitive function among middle-aged and
older Puerto Ricans without diabetes. Br J Nutr. 106:1423–1432.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Saad AF, Alshehri W, Lei J, Kechichian TB,
Gamble P, Alhejaily N, Shabi Y, Saade GR, Costantine MM and Burd I:
Maternal fructose consumption disrupts brain development of
offspring in a murine model of autism spectrum disorder. Am J
Perinatol. 33:1357–1364. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Erbas O, Erdogan MA, Khalilnezhad A,
Gürkan FT, Yiğittürk G, Meral A and Taskiran D: Neurobehavioral
effects of long-term maternal fructose intake in rat offspring. Int
J Dev Neurosci. 69:68–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rivell A and Mattson MP: Intergenerational
metabolic syndrome and neuronal network hyperexcitability in
autism. Trends Neurosci. 42:709–726. 2019.PubMed/NCBI View Article : Google Scholar
|