The brain is the most complex organ in the body, and
it has a highly specialized structure and function. It accounts for
~2% of body weight, but consumes 20% of the metabolic reserves of
the body (1). The metabolic needs
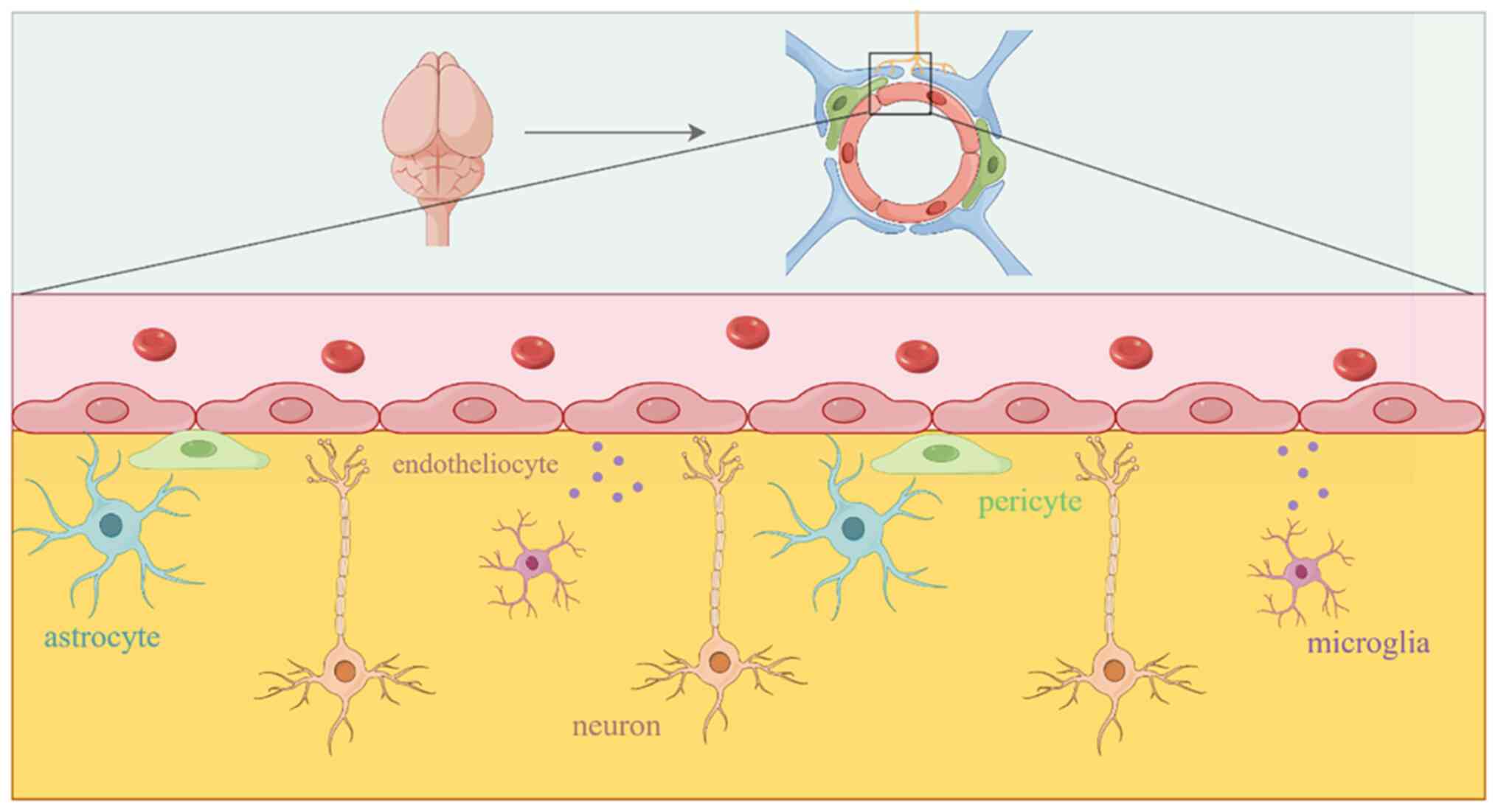
of the brain are met through blood transport, and a group of cells
from blood vessels and nerves known as the neurovascular unit (NVU)
complete the blood supply of the brain (2). The NVU is a complex component that is
composed of neurons, astrocytes, oligodendrocytes, microglia,
pericytes, and brain microvascular endothelial cells (BMECs)
(3-5)
(Fig. 1). The blood-brain barrier
(BBB) is an important NVU component (6). It is impossible to observe the
functional activity of the BBB without the NVU.
The BBB controls the movement of nutrients and
metabolites into and out of the brain parenchyma, regulates the
brain microenvironment by providing transportation of selective
substances, controls molecules and cells, and protects the normal
structure and function of the brain (7). As early as 1885, Paul Ehrlich marked
all tissues except the brain and spinal cord by injecting aniline
stains into the blood vessels (8).
Subsequently, Edwin Goldmann successfully stained the brain cells
by injecting the dye into the spinal fluid, but other tissues and
organs remained intact. This researcher then discovered a special
barrier between the central nervous system (CNS) and circulating
blood (9). Max Lewandowsky further
discovered that capillary obstruction prevented some molecules from
entering the brain and introduced the term ‘Bluthirnschranke’
(9). Until the late 19th century,
Dr. Lewandowski from Berlin officially named it the BBB (8). It has now been established that the
BBB is composed of BMECs, pericytes, astrocytes, and a basement
membrane as the barrier matrix (10). The BBB prevents toxic substances,
microorganisms, and pathogens in the blood from directly contacting
the brain tissue. This protects the brain parenchyma from
blood-borne pathogens and prevents drugs and other exogenous
compounds from entering the CNS (11,12).
Therefore, the BBB function is closely related to stroke (13), traumatic brain injury (TBI)
(14), surgical brain injury (SBI)
(15), intracerebral hemorrhage
(ICH) (16), Alzheimer's disease
(17), epilepsy (18), Huntington's disease (17), as well as other neurological
diseases.
The present review focuses on the mechanism by which
the NVU constructs the BBB. New possibilities for protecting the
integrity of the BBB are also provided.
The primary function of the nervous system (NS) is
to perceive and analyze changes in the internal and external
environment of the body to control it (19). Neurons are the basic structures and
functional units of the NS that perform the primary functional
activities of the NS (20). Neurons
are divided into cell bodies and protrusions, and the protrusions
contain dendrites and axons (21).
Axons comprise >95% of the total volume of neurons, and they
carry electrical impulses and project them to other neurons
(22). The perivascular neuron
axons include the presynaptic portion that is inserted into the
basement membrane through the astrocytic endfeet and forms a
synaptic-like transmission with vascular smooth muscle cells to
regulate high levels of neuronal activity and brain metabolic
requirements (23,24). Higher brain functions depend on the
electrophysiological signals that are released by neurons. Hence,
the BBB is needed to maintain a precise and balanced ionic
environment in the brain (23).
Neurons form before the BBB during brain development. Neuronal
cells combine with other nerve cells to construct the BBB. The BBB,
in turn, protects neurons from harmful toxins, pathogens, and
immune cells by controlling substance exchanges. Therefore, it is
suggested that BBB formation and neuronal development are
interrelated and complement each other. Specialized cells that
sense the environment have emerged in the simplest multicellular
organisms with epithelial tissue (25). These cells may have evolved into the
first sensory neurons. As sensory neurons specialize in function,
they require the support and protection of other cells. These
supporting cells may then evolve into glial cells that form a tight
barrier in the brain. This process would promote the separation of
neuroactive substances in the central and peripheral areas, helping
to establish a consistent microenvironment in the brain (25,26).
A result of BBB breakdown is cerebral edema, and
this can occur in all types of brain cells and brain parenchyma,
such as neurons, astrocytes, and endothelial cells (ECs) (38). Following brain injury, neuronal
water disintegrates cell bodies and processes, the neuronal nuclei
become sparse, the water-soluble cytoplasm disintegrates, and brain
edema becomes significantly aggravated (39). Mature nerve cells cannot
proliferate. Therefore, the specific function and viability of
nerve cells are impaired, and this can lead to BBB damage and
further affect brain function (40). This provides strong support for the
role of neurons in the BBB. Although the specific pathways of these
regulatory mechanisms require further exploration, some signaling
pathways have been shown to affect the BBB by disrupting neurons.
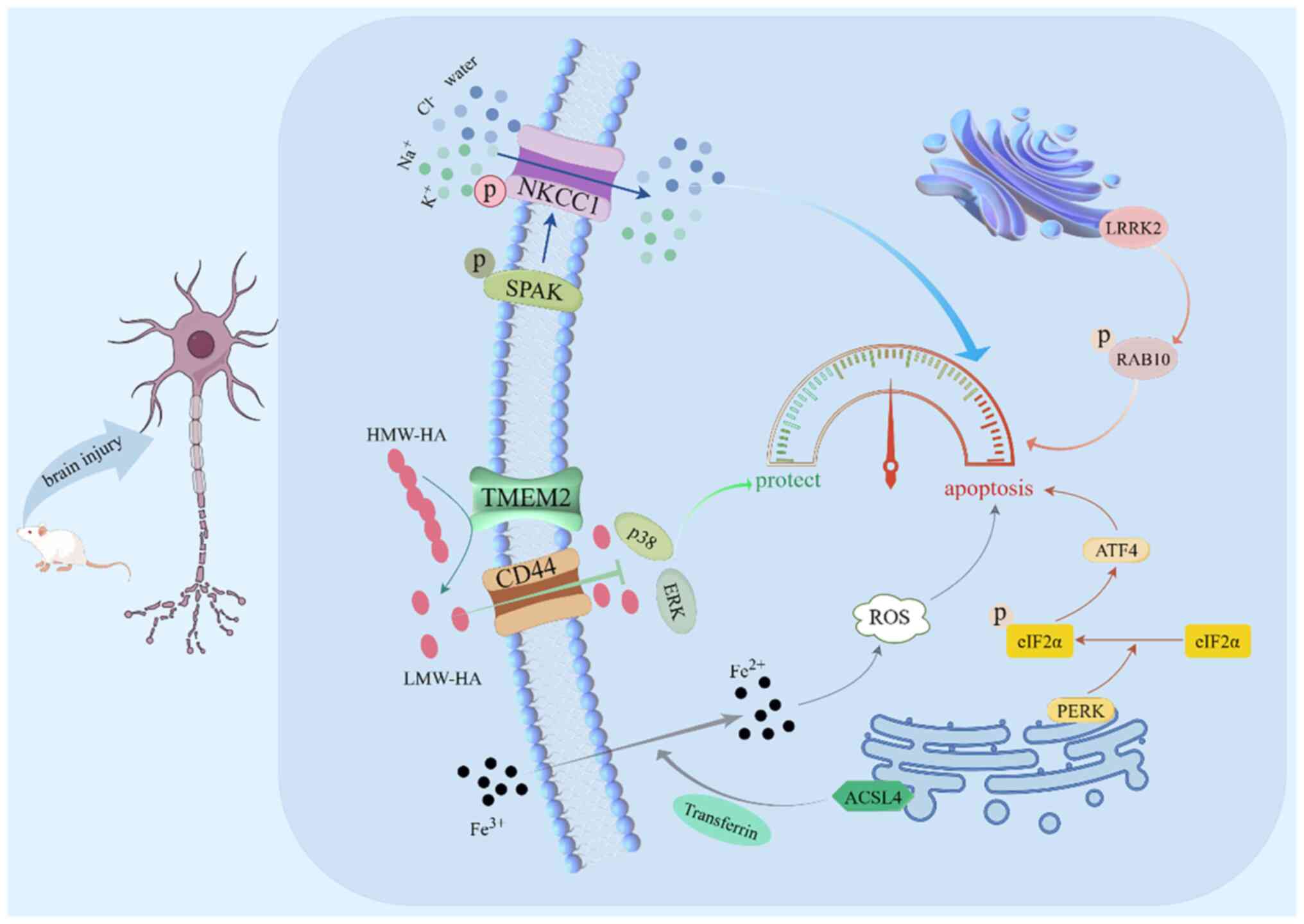
It was determined from our previous studies that intraperitoneal
injection of closantel allows bumetanide to pass through the BBB
and inhibits the phosphorylation of the STE20/SPS1-related
proline/alanine-rich protein kinase
(SPAK)/Na+-K+-Cl- cotransporter 1
(NKCC1) signaling pathway in neurons. This reduces nerve cell
apoptosis and has a protective BBB effect (41,42).
The SPAK/NKCC1 signaling pathway is activated by phosphorylation
after brain injury. Ion channels in the cell membrane open, and
Na+, K+, Cl−, and water enter the
neuronal cells and disrupt the cellular structure. A large amount
of neuronal apoptosis, BBB damage, albumin infiltration into the
brain parenchyma, and brain edema of the injured side were found to
be significantly aggravated in rats (41,42).
Endoplasmic reticulum stress (ERS) was also found to
play an important role in neuronal death. Ca2+
homeostasis of the neuronal ER is disrupted after brain cell
injury. This causes ERS that participates in the pathological
process of BBB destruction (43).
The protein kinase R-like endoplasmic reticulum kinase (PERK)
pathway is part of the unfolded protein response (UPR) during ERS.
It is activated by phosphorylation after early ICH, and it further
phosphorylates downstream eukaryotic translation initiation factor
2α (eIF2α) and upregulates activating transcription factor 4 (ATF4)
to play a pro-apoptotic role. Acyl-CoA synthetase long chain family
member 4 (ACSL4) reduces Fe3+ to Fe2+ through
transferrin and transfers it into cells. The reactive oxygen
species (ROS) reaction with Fe2+ leads to ferroptosis,
injury of nerve cells, and destruction of the barrier function,
causing secondary cerebral edema (44). Deferoxamine can reduce ERS and
protect nerve cells by interfering with Fe3+ and
inhibiting the PERK signaling pathway (45). However, transmembrane protein 2
(TMEM2) is involved in ERS through non-UPR pathways. TMEM2
decomposes the extracellular high-molecular-weight hyaluronic acid
(HMW-HA) into low-molecular-weight (LMW)-HA. The LMW-HA enters
cells through the membrane channel, via CD44, inhibiting
intracellular p38/ERK signal transduction, and alleviating ERS.
Moreover, when TMEM2 is silenced, caspase-3 is highly expressed,
and this promotes neuronal apoptosis and cerebral edema (46). In addition, it was determined in our
previous study that leucine-rich repeat kinase 2 (LRRK2) inhibition
can effectively interfere with Ras-related protein Rab-10 (RAB10)
phosphorylation, downregulate lysosomal hydrolase secretion caused
by lysosomal overload stress after SBI, and play a protective role
in BBB and nerve function (47).
Collectively, our previous studies confirmed that the normal
structure and function of neurons contribute to BBB integrity
maintenance (Fig. 2).
Neurons are considered to be components of the NVU.
Neurons can send signals to regulate the neural network when their
energy demands change, and this has direct effects on the BBB.
Neurons can rapidly transmit chemical information through synapses,
and this may be one of the reasons why neurons respond quickly
after BBB damage. In addition, ion concentration differences inside
and outside the cell will also initiate the release of membrane
potential from the neurons, thereby adjusting the brain
microenvironment according to the current demand. It is considered
that the mechanism by which neurons participate in BBB regulation
is related to their signaling function in the brain, and some new
therapeutic targets may emerge by studying this cellular
communication.
At present, it is generally considered by most, that
it is difficult to regenerate neurons in the adult CNS, and the
function of the CNS can be restored after damage because of the
compensation of other neurons around it. However, whether the BBB
can be damaged by regulating the metabolism of peripheral neurons
to play a role in repair is still unknown. The position of neurons
is crucial. However, the mechanisms that could explain the direct
molecular effects on the integrity of the BBB remain to be
determined.
Microglia have not yet been determined as direct
contributors to BBB maintenance. However, structurally, most
para-vascular microglia are located in the perivascular space and
are closely related to vascular function (48). Vascular-associated microglia
initially maintain the integrity of the BBB by expressing the tight
junction (TJ) protein, claudin-5, and make physical contact with
ECs (49). Microglia also function
through direct contact with neuronal synapses. Microglia monitor
their surroundings and continuously extend and retract their
cellular protuberance, making direct contact with neuronal synapses
at an hourly rate. This continuous and rapid monitoring is a unique
function of microglia in the brain (50). In addition, microglia can secrete a
variety of cytokines and growth factors, such as brain-derived
neurotrophic factor (BDNF), IL-10, and transforming growth factor-β
(TGF-β), to help repair local nerve tissue (51,52).
These functions of microglia play important roles in restoring
brain balance (53). Normally, the
CNS is an immunologically privileged region, and the BBB separates
the CNS from the inflammatory state to maintain brain homeostasis
(54). However, microglia release
high levels of inflammatory factors as they are CNS immune cells.
Following brain injury, they are the first to be activated among
all immune cells to destroy the BBB and participate in the
pathological process of neuroinflammation (55,56).
The inflammatory environment in the brain is an important factor in
BBB destruction. Immune cells cross the BBB and infiltrate the
brain parenchyma through paracellular and transcellular pathways,
and this leads to extensive neuroinflammation (57). It is known that neuroinflammation is
an important driver of numerous diseases and is closely related to
the BBB. Proinflammatory cytokines, such as interleukin (IL)-1β,
IL-6, and IL-18, tumor necrosis factor (TNF), chemokine ligand
(CCL)1 and CCL5, small molecule messengers (prostaglandins, NO, and
reactive oxygen species) are produced in large quantities during
neuroinflammation. Microglia are one of the main cells involved in
this process. As the key cell of brain inflammation, when its
immune function is activated, inflammatory factors are released in
large quantities. This induces aquaporin 4 (AQP4) upregulation. TJ
expression disruption in the ECs affects their functions, leading
to vascular disintegration and severe barrier dysfunction (58,59).
The damaged barrier displays more leakage, and some of the serum
proteins enter the brain to recruit microglia into the blood
vessels to phagocytize ECs and destroy the BBB (60). Moreover, serum proteins that leak
into brain tissue can bind to local cells and induce secondary
brain injury.
The aforementioned inflammatory effects of microglia
are closely related to their morphology. Microglia are activated
and polarized into different phenotypes that include M1
(pro-inflammatory effect) and M2 (anti-inflammatory effect)
(61). Cerebral edema is one of the
most common BBB injury manifestations and can be caused by neuronal
cytotoxic edema. Neuronal axons are damaged, and myelin degradation
produces a large amount of cellular debris (62). M1 microglia are capable of engulfing
these cellular debris, apoptotic neurons, and harmful substances
such as inflammatory cytokines and chemokines that are produced
during injury (63,64). However, with an increase in M1
microglia, the phagocytic function gradually decreases,
inflammatory cells are recruited, and neurotoxic substances
accumulate in the brain. This leads to neuronal death and further
destruction of the BBB (65,66).
NF-κB regulates the expression of most microglia characteristic
genes of the M1 phenotype. Peroxisome proliferator activated
receptor (PPAR)-γ in activated microglia can inhibit NF-κB
signaling to downregulate the microglia ‘M1’ and reduce the
expression levels of inducible nitric oxide synthase (iNOS), TNF,
IL-1, IL-6, and IL-23 (67-69).
In addition, NF-κB has been revealed to promote IL-4 secretion and
microglia M2 polarization, thereby improving the tube formation
function and cell connection ability of BMECs (70,71).
Research has shown that small nucleolar RNA host gene 8 activates
microglia to release inflammatory factors to damage BMECs by
targeting the sirtuin1 (SIRT1)-mediated NF-κB pathway (72). High mobility group protein B1
interacts with Toll-like receptor 4 to activate the NF-κB/NLRP3
signaling pathway through myeloid differential protein-88 and
induce the transcription of proinflammatory cytokines (66). Inhibition of this signaling pathway
induces the upregulation of M2 markers, CD206 and arginase 1, and
the downregulation of M1 marker proteins, CD86 and iNOS, resulting
in the conversion of microglia (73,74).
The Notch1/Hes1/Stat3 signaling pathway plays an important role in
microglia activation, reprogramming M1 to M2 by inhibiting NF-κB
p65 translocation (75).
Microglia-mediated inflammation is an important cause of BBB
disruption. As the most predominant proinflammatory cell in the NS,
microglia also disrupt the BBB by engulfing astrocyte endfeet
during persistent inflammation (49).
Recent studies have found that the expression of
triggering receptor expressed on myeloid cells 2 (TREM2) in
microglia is closely related to BBB destruction after brain injury
(76,77). These findings suggest that TREM2 is
closely related to the BBB, which is consistent with the results of
our study (78). Following brain
injury, TREM2 was revealed to be highly expressed in the microglia
of the injured side of the brain and negatively correlated with the
diffusion of inflammatory factors. TREM2 downregulation resulted in
upregulation of NF-κB-mediated inflammatory signals such as IL-6,
IL-1β, and TNF-α. With a large amount of nerve cell apoptosis, the
BBB was disrupted, albumin exudated into the brain parenchyma
surrounding the lesion, and the brain water content on the affected
side increased. These results indicated that TREM2 can protect
nerve cells and maintain CNS homeostasis (78).
It was determined that under pathological
conditions, microglia morphologies rapidly change to larger cell
bodies and shorter processes. This change may be due to a shift in
the role of microglia from central monitors to phagocytes. This may
have bidirectional effects after BBB disruption. Microglia help
remove cellular debris, but they also release inflammatory factors
and aggravate neuroinflammation. The benefits and harms of this
bidirectional effect of microglia on the BBB cannot be assessed. As
important nerve cells, microglia cannot be removed to conduct a
holistic study. Thus, the mechanism by which microglia restore the
function of the BBB has yet to be determined.
As the key point of brain metabolism regulation,
astrocytic endfeet are attached to blood vessels and establish a
direct interface with the vascular chamber of the NVU. They are
closely attached to the basal membrane of blood vessels and form a
boundary along the perivascular space, an important part of the BBB
(79). Astrocytes are widely
distributed in the CNS, and they fill the space outside neurons and
blood vessels and secrete neurotrophic factors that support and
nourish neurons (80). Astrocytes
have isolation and barrier functions. They can prevent neighboring
neurons from interfering with each other and have a certain
selective effect on the transport of substances, and they
participate in BBB formation (81,82).
As satellite cells of the NS, astrocytes divide the cortex and gray
matter into functional areas. In addition, a single glial cell
regulates the internal and external output, guiding nerve axon
regeneration (79,83). A single astrocyte forms higher-order
glial vascular units along capillaries that match local neural
activity and blood flow, coordinate neuronal firing thresholds, and
provide metabolic support for neurons and their synapses (84). Astrocytic endfeet extend to the
periphery of brain microvessels, control the barrier function of
BMECs through derived factors and physical contact, and regulate
the BBB bidirectionally (85).
Astrocytes can divide and proliferate, unlike
neurons. A damaged or degenerated brain primarily relies on
astrocyte proliferation to fill the tissue defect. It has been
confirmed that astrocytes can be induced to differentiate into
neurons in vitro, and this provides hope for the treatment
of a variety of neurodegenerative diseases (86). It has been observed that altered
astrocyte communication can induce neuronal apoptosis and
phagocytosis of broken neurons, and this has direct consequences
for neuropathology (87,88). However, astrocytes detect
glutamatergic and gabaergic neuronal signals and translate them
into commands to expand/contract blood vessels (89). They promote the transmission of
ions, amino acids, neurotransmitters, and other substances in the
brain by absorbing and regulating nerve molecules, and they also
establish water homeostasis in the brain (90,91).
Thus, they regulate the local microenvironment and affect the
extent of BBB damage and subsequent repair. Astrocytes can
stabilize K+ and H+ concentrations in the
extracellular fluid of the CNS during normal brain activity
(92). Water is drained from the
brain and out of the body, while neurotransmitters and ions are
recycled. When proliferating astrocytes produce scarring, the
absorption capacity for K+ and H+ is
correspondingly weakened. This results in high K+ in the
local extracellular fluid. These boundary and scar functions play
important roles in maintaining the physiological homeostasis of the
CNS, supporting nerve function and signal transduction (93,94).
Following brain injury, the morphology of astrocytes changes from
static to reactive. This morphological change affects the BBB and
induces brain pathology (95,96).
In summary, astrocytes support neuronal cells by regulating
neurofactors and preventing hemodynamic and metabolic disorders.
They create conditions for the survival and recovery of neurons,
protect the permeability integrity of the BBB, and alleviate
secondary brain injury.
There is increasing evidence that astrocytes play a
key role in BBB breakdown after brain injury. There are abundant
organic anion transporters (OAPs) in the contact interface between
the astrocytic endfeet and a blood vessel. When this contact is
broken, the density of OAPs, such as Kir4.1 and AQP4, that are
carried by the astrocytic endfeet decreases. The ion and water
imbalances cannot maintain the BBB function, cell edema increases,
the cytoskeleton is destroyed, and the brain is damaged further
(97,98). Astrocytes secrete vascular
permeability factors that enhance the BBB permeability, including
vascular endothelial growth factor (VEGF) (99), NO (100), glutamic acid 69, matrix
metalloproteinases (MMPs) (101),
and endothelin (102).
Several receptors have been found on brain ECs and
astrocytes to cause an increase in intracellular Ca2+
upon activation. Upregulation of Ca2+ regulates the
activities of neurons, astrocytes, and ECs (121,122). When Ca2+ ions are
activated, they send signals from the CNS nerve cells to
neighboring cells through the astrocytes. This releases a signal to
dilate the vascular space, and a TJ is opened (123,124). Perivascular astrocyte processes
regulate the TJs around brain ECs. This pattern of signal
transduction between neuroglia and ECs is of great significance for
the physiological and pathological regulation of the BBB by brain
ECs. Several astrocyte-derived factors have been found to disrupt
vascular permeability by decreasing the endothelial TJ protein
expression or inducing EC apoptosis. VEGFA expression is a key
driver of BBB permeability, and astrocytes drive BBB opening by
VEGFA (125). Upregulation of
Apelin-13 promotes the phosphorylation of ERK, Akt, and AQP4 in
astrocytes (126). Following the
upregulation of Apelin-13/APOE4 and NF-κB/HIF-1α signals, MMP9 and
VEGF secretions also increase. The reduction of astrocytes endfeet
that cover blood vessels and TJ damage are evidence of BBB damage
(127,128). Lee et al (129) further found that src-suppressed
C-kinase substrate reduced VEGF by decreasing Apelin-1 while
stimulating the upregulation of Ang-1. This regulates cerebral
angiogenesis and TJs between cells, thereby affecting BBB
differentiation (129). Proteins
such as astrocytic N-myc downstream regulatory gene 2 and
thrombospondin-2 also act as regulators of BBB permeability. They
play roles in BBB permeability and immune cell infiltration
regulation by affecting MMP expression (130,131).
Astrocytes are often one of the major cell types
that initiate the inflammatory cascade. Astrocyte swelling is
considered to be an early sign of cytotoxic edema. The inflammatory
environment caused by brain injury mediates astrocyte signaling,
and this is an important cause of cellular swelling (132). The degradation of the
extracellular matrix (ECM) and TJ proteins activates astrocytes,
stimulates glial cells to release inflammatory factors that
aggravate neuroinflammation, disrupts the TJs of vascular ECs, and
accelerates BBB permeability (42,133).
Collagen-IV is a major BBB basement membrane component, and its
degradation can lead to basement membrane dysfunction. Our previous
study showed that MMP-9 was secreted by astrocytes after a TBI
(133). This degraded endothelial
TJ proteins and the ECM, including occludin and collagen-IV. It was
also determined that in addition to directly destroying basement
membrane and TJ proteins, MMP-9 also affects TJs by inhibiting the
Hedgehog pathway. MMP-9 is upregulated after brain injury, and it
partially blocks the Hedgehog pathway and reduces Scube2 and Shh
expression levels in astrocytes. This causes further damage to the
BBB. Inhibition of this signaling pathway has a protective effect
on the BBB mechanism (133,134).
It is considered that the contribution of astrocytes
to the BBB is primarily due to their supporting, nutritional, and
other auxiliary effects on nerve cells. Astrocytes can secrete a
variety of neurotrophic factors to promote the repair or activation
of ECs, BMECs, and pericytes so that they provide better barrier
characteristics. Furthermore, astrocytes adhere to the wall of
microvessels, encase parts of the microvessels, and strengthen the
basement membrane. All of these functions play important roles in
maintaining BBB homeostasis. However, the role of astrocytes is
much more than this. Astrocytes are also able to release glutamate
and thus participate in electrical signaling in the NS. They have
an important physiological role in the NS, and this also provides
new insights for the treatment of complex neurological
diseases.
Astrocytes have attracted notable attention due to
their pivotal role in the NVU and have been studied as
aforementioned. BBB homeostasis imbalance has been implicated in
numerous CNS diseases. The role and mechanism of astrocytes in
maintaining BBB homeostasis, especially in the transition from
normal BBB homeostasis to imbalances of BBB homeostasis in disease
states, require further investigations.
The BBB is a selective barrier formed by ECs that
line the brain microvessels. As a key NVU regulator, BMECs are key
cells for BBB integrity (135).
BMECs are a single layer of flat ECs that line the inner surface of
cerebral blood vessels. BMECs have a more obese appearance than ECs
from other non-neural tissues (136). BMECs have some unique features,
such as the presence of numerous intercellular TJs that generate
high transendothelial impedance and delay paracellular flux. They
lack the fenestra structure of ECs and have low levels of
pinocytosis of the liquid phase material (137). In addition, BMECs possess
asymmetrically localized enzymes and vector-mediated transport
systems.
These characteristics make the connection between
two cells tighter, forming a liquid impenetrable barrier that
prevents molecules and ions from passing through the intercellular
space (37,138). The basement membrane of brain
microvessels is intact, and a few phagocytic vesicles are
distributed in the vascular lumen. In addition, the number of
mitochondria is high (139). BMEC
mitochondria account for approximately one-tenth of the cytoplasmic
volume, a proportion much higher than that of ECs in other non-BBB
tissues (140,141). The high mitochondria proportion
helps to improve the efficiency of metabolism and transport,
enhance the signal transmitted by sensing neurons, and promote the
propagation of vasodilator signals to the tiny arteries in the
brain to regulate the vasodilation function (142). BMECs can also transport oxygen and
glucose from the blood to the brain to increase its energy supply
while emitting carbon dioxide and other waste products in the
opposite direction (143,144). This is essential to maintain the
physiological function of the NVU and regulate the pH of normal
brain metabolism, and is one of the reasons for the sensitivity of
the BBB to oxidative stress (145). BMECs can form a tighter capillary
endothelium than peripheral ECs, and this aspect renders the BBB
essentially impermeable to macromolecules (135). The TJs fill the gaps between BMECs
and limit paracellular permeability by expressing TJ proteins such
as occludin, claudins, and zonula occludens. These suggest that the
TJs of BMECs are indispensable for a normal structure and function
of the BBB. The TJs connect adjacent cells and seal intercellular
spaces, and this prevents top and bottom membrane proteins from
spreading laterally (146,147). The purpose is to block the
paracellular fusion pathway of ECs to form a physical barrier.
Hydrophobic molecules are allowed to diffuse internally, whereas
hydrophilic molecules are hindered by barriers, thus maintaining
CNS homeostasis (148). Therefore,
drugs that target nerve regions or nerve cells must have the
ability to cross the BBB to achieve the desired therapeutic effect
(149). BMECs contain
transporters, such as ATP synthase-binding cassette (ABC), ABC
subfamily member 1, and solute carrier organic anion transporter
family member 1C1, as well as receptors, such as endothelial
protein C receptor, insulin receptors, transferrin receptors
(TfRs), and LDL receptor related protein 1, that can help CNS drugs
open the BBB (150). Multiple
receptors on BMECs enable cells to respond to inflammatory
mediators (151). One of the
hallmarks of vascular inflammation is the increased expression of
adhesion molecules on the BMEC membrane. Vascular inflammation can
affect the infiltration of peripheral immune cells, activate
microglia, change the shape of blood vessels, affect angiogenesis,
and ultimately destroy blood vessels (152,153).
The barrier function of BMECs is maintained through
close association with other cell types within the NVU. Direct
contact between ECs and astrocytes is necessary for BBB formation
and functioning (154). When
co-cultured with astrocytes, endothelial soluble factors showed
higher transendothelial resistance (155). Changes in ECs and astrocytes were
also found in cerebral cortex biopsy specimens from patients with
brain injuries (156). This may
have been related to EC morphological changes. The injured EC
surfaces showed longitudinal folds and invagination, the activity
was enhanced, and granulation and some multivesicular bodies were
observed in the cytoplasm. The space between ECs and astrocytes was
significantly enlarged (157). The
interaction between astrocytes and ECs involves intercellular and
intracellular communication, which is key to BBB induction and
maintenance (84,158). Research has found that autophagy
related 7 (Atg7) regulates the function of astrocyte adhesion to
the BMECs to maintain BBB homeostasis through endothelial
fibronectin (159). Atg7 affects
the phosphorylation of the cyclic adenosine
monophosphate-responsive element-binding protein by regulating
protein kinase A activity, thereby triggering endothelial
fibronectin expression. Loss of endothelial Atg7 downregulates
fibronectin expression, a component of the BBB, and reduces the
extent of astrocyte coverage of cerebral microvessels (159). Other signaling mechanisms between
ECs and the BBB have also been gradually discovered. Among them,
the VEGF/VEGF receptor (VEGFR) pathway, Notch, and Wnt pathway are
considered to be the key signaling pathways that drive the
phenotype of BMECs during BBB formation (160). The VEGF/VEGFR signaling pathway is
related to the proliferation, invasion, migration, and survival of
ECs and is involved in the activation of most mechanisms of
angiogenesis (161). The
endothelial nitric oxide synthase (eNOS)/NO pathway regulates
vascular remodeling, and inhibition of this signaling pathway
destroys VEGF. This leads to impaired angiogenesis (162). The eNOS/NO pathway in ECs is
related to BDNF. After the impairment of this pathway, BDNF is
downregulated, and this affects neurotransmitter release, thereby
impairing neuronal regeneration and leading to BBB leakage
(163). It is worth noting that
the eNOS/NO pathway in BMECs is closely aligned with
mitochondria-associated oxidative stress, and as a downstream
effector of VEGF, the reduction of NO impairs the VEGF/VEGFR
pathway (164). Wnt signaling is
also essential for CNS angiogenesis and BBB formation (165). In addition, Notch signaling is
involved in EC proliferation and survival processes (166). SIRT1 promotes angiogenesis by
promoting VEGFR-2 expression through Notch signaling (167). Taken together, BBB integrity
depends to a large extent on the interaction of BEMCs with other
cells in the NVU.
VEGF is a growth factor that induces angiogenesis
and increases vascular permeability, and it has a key role in BBB
integrity loss (168). The
blocking of VEGF was demonstrated to alleviate the degradation of
the TJ protein occludin (169).
Collagen-IV is a major BBB basement membrane component, and its
degradation can lead to dysfunction of the basement membrane; this
may be one of the causes of BBB disruption after a brain injury
(170). Our results, in a previous
study showed that in the brain tissues surrounding the TBI in rats,
VEGF expression increased, and occludin and collagen-IV expression
levels decreased. The basement membrane and TJs of the BBB were
disrupted. The TUNEL and Fluoro Jade C-positive cell levels
increased, nerve cells were apoptotic, and the BBB was impaired.
VEGF inhibition protected the BBB and reduced TBI-induced brain
edema. This effect may be achieved through MMP9 regulation of
occludin and collagen-IV (133).
As one of the major cell types constituting the BBB,
the contribution of BMECs to the BBB originates from their
structural location. BMECs form the BBB through TJs and gap
junctions that prevent the passage of macromolecular substances. Of
course, in addition to forming barriers, BMECs also participate in
a variety of neural metabolism activities and release neuroactive
substances that affect neural function. These mechanisms all serve
to further regulate the brain microenvironment and protect the
BBB.
Pericytes were discovered as early as 1873 by French
scientist Charles Benjamin Rouget, and they were originally called
‘Rouget's’ cells. They were later officially named ‘pericytes’ by
Karl Wilhelm Zimmermann (171).
Pericytes are an important component of the NVU, and they attach to
the lateral wall of the lumen of ECs in capillaries, embed into
ECs, and cover approximately one-third of the capillary wall.
Together with vascular ECs, they constitute the cellular component
of the blood vessel wall and play an important role in the BBB
(172). Within the NVU, pericytes
are located in a unique location between neurons, astrocytes, and
ECs, and their coverage is inversely proportional to the
permeability of the blood vessels (173). The cytoplasm of pericyte stellate
processes is wrapped outside the vascular basement membrane and is
primarily distributed at the TJs of ECs. ECs are surrounded by
pericytes and communicate directly with the microvascular
endothelium through physical contact and paracrine signals. The
structural substances constituting the basement membrane and the
ECM, including integrins, N-cadherin, fibronectin, and connexin,
regulate capillary permeability and participate in the perfusion
and infiltration of microcirculation (171). Pericytes have contractility
similar to smooth muscle cells and can regulate cerebral vessel
formation and maturation (174).
The cerebral angiogenesis signaling pathway is an important
regulator that maintains the normal structure of the BBB, and its
signaling pathway abnormality can lead to an aberrant BBB
structure. Studies have shown that pericytes express receptors for
vascular mediators, such as catecholamines, β-adrenoceptors,
thromboxane A2, vasoactive intestinal peptide,
endothelin-1, and vasopressin, that play crucial roles in
maintaining vascular wall stability (175,176). Peripheral cells also participate
in angiogenesis by expressing TGF-β, VEGF, Ang-1/2, angiotensin I,
and other vascular-related factors (177). Type IV collagen, polysaccharides,
and laminin synthesized by pericytes are also important for
basement membrane formation. TfR1 is one of the known markers of
the BBB, and TfR1 is downregulated in ECs lacking pericellular
contact (178). It has been shown
that capillary permeability increases after pericyte depletion.
This phenomenon supports that pericytes have an important role in
maintaining endothelial barrier function (179).
Pericytes share a basement membrane with ECs as
they can regulate microcirculatory blood flow. Direct synapse-like
contacts are formed with ECs via N-cadherin and connexin (180). The interaction between pericytes
and BMECs is essential for basement membranes and TJs and directly
affects BBB permeability. Gap junctions, focal adhesion, and nail
hole junctions are three ways in which pericytes interact with ECs
(181). Gap junctions transmit
ionic currents and small molecules between neighboring cells
(182). Focal adhesion kinase was
revealed to immobilize capillary pericytes on ECs (183). Pericytes play a key role in BBB
homeostasis by communicating with ECs through these specialized
roles (184). Daneman et al
(185) found that ECs co-cultured
with pericytes had reduced intercellular spaces and increased
transepithelial electrical resistance. Abundant TJ proteins that
included occludin and claudin 5 were present at the cell boundary,
suggesting that the pericytes enhanced the TJ function (185). ECs associated with pericytes are
more resistant to apoptosis than ECs alone and have a protective
effect on ECs. This provides new evidence that pericytes together
with ECs support BBB structural integrity. The phagocytic function
of ECs is enhanced after pericyte depletion. Vascular permeability
genes, such as Angpt2, Plvap, and LAM, were increased, and
molecular transporters were overexpressed. Some of the
macromolecules are allowed to cross the BBB to affect the brain
parenchyma (186). In addition,
the receptor, PDGFR, expressed by pericytes can respond rapidly
after brain injury, mediate pericyte migration to ECs, release
fibrinolytic enzymes, and increase BBB permeability (187). It is worth exploring that in the
absence of pericytes, embryonic brain ECs can still express some
BBB-specific molecules. This may be induced by neural progenitor
cells that then regulate the functional integrity of the BBB during
development through pericytes and adult astrocytes (188).
Pericytes are an important part of brain
capillaries that have a phagocytic function and can remove cell
debris and regulate capillary blood flow and BBB permeability.
Pericytes in different vascular beds have different frequencies and
can transform into multipotent vascular stem cells that generate
microglia in the late stage of brain injury and participate in
microglia proliferation to acquire a microglial phenotype with a
phagocytic capacity (189,190). Pericytes can also direct
astrocytic endfeet into the endothelium (191). The interaction between pericytes,
ECs, and astrocytes can regulate angiogenesis and hemodynamics and
maintain BBB integrity. This is essential for normal brain
function.
Currently, pericyte loss is considered to be a
hallmark of BBB dysfunction, and it causes a variety of secondary
brain injuries such as brain edema and brain parenchymal lesions
(192). Oxidative stress, a high
lipid and high glucose internal environment, and toxic substances
in the blood can damage pericytes. This then destroys the BBB. Aβ
is the most widely studied of the toxic substances that cause BBB
damage. Aβ binds to RAGE to increase oxidative stress, promoting Aβ
accumulation in the brain. This process causes BMEC cytotoxicity
and induces pericyte ferroptosis (193-195).
The role of pericytes in the BBB has been further
revealed with increasing research. Pericytes are located lateral to
the BMECs and control the endothelial function through direct
contact to maintain BBB stability. In addition, pericytes are
involved in physiological processes such as substance transport and
signal transmission to ensure normal brain function. Understanding
pericyte functions helps us to better understand BBB processes and
their importance in protecting the brain from harmful agents.
The normal functioning of the brain depends on the
interactions between the components of the NVU. The positive role
of these cells in CNS homeostasis is encouraging. Neurons, glial
cells, pericytes and vascular ECs constitute the BBB, which
provides a stable internal environment for the realization of nerve
function. Among them, astrocytes are closely related to
neuronutrition and repair, and it is speculated that astrocytes may
also have some benign effects on nerve cells and brain
microvascular diseases, such as the promotion of microvascular and
nerve injury regeneration to help BBB function repair. However,
further studies are required to confirm this. The focus on the
relationship between neuroinflammation and the BBB has uncovered a
notable phenomenon. Activated microglia and reactive astrocytes
communicate and cooperate with each other in the NVU to achieve
immune ‘optimization’. Brain inflammatory signals are amplified not
only by microglial activation but also by the unique anatomy of
astrocytes in the immune network (196,197). Microglia and astrocyte
interactions may become an effective and accurate therapeutic
target in the future with further technological advances. These NVU
cell-to-cell interactions are critical to regulate cerebrovascular
functions. BBB permeability alterations after acute CNS injury are
due to a loss or alteration of these cellular structures, and
understanding the structure and mechanisms of these cells is
important to control injury progression.
Various NVU components and their interactions are
essential to maintain BBB stability. The damage of the BBB is the
pathological basis of vascular cerebral edema. The destruction of
the BBB can lead to brain edema and increased intracranial
pressure, which in turn can aggravate brain edema and cause
functional and structural damage to brain tissue. If the brain
edema progresses from localized to diffuse, irreversible secondary
pathological changes are formed, and even brain death is developed.
Brian edema is often secondary to neurological diseases such as
TBI, intracranial space occupying lesions, cerebrovascular
diseases, cerebral parasitosis and some cerebral congenital
diseases. The severity of cerebral edema is related to the course
and prognosis of intracranial diseases and has important
significance. In the field of neurology, if we can better
understand the mechanism of the BBB and effectively prevent and
treat the secondary brain edema, we can achieve better results in
the treatment of intracranial diseases. The therapeutic potential
of the BBB in a variety of neurological diseases has great
potential. Hence, it is necessary to investigate the effect of the
NVU on the BBB as a whole to further reveal possible therapeutic
mechanisms.
The recovery of the barrier function is considered
to be the key to the treatment of NS diseases. An exploration of
the NVU composition and its cell-cell interactions will help to
further unlock the mechanism of the BBB and provide new insights
for the clinical treatment of NS diseases. Drug therapy should
protect the whole NVU from different angles and in various ways.
The exploration of multi-target protective agents has become one of
the important research and development directions for the treatment
of NS diseases in the future.
In conclusion, it is considered that the NVU should
be regarded as a functional whole, and the dynamic interaction
between all cells affects brain function. However, most of the
current BBB studies have only focused on a single nerve cell or
target, and few have studied a variety of nerve cells and brain
microvascular endothelium as a whole. In the future, focus will be
on the cellular structure and function of the NVU, and exploration
on how a variety of cells combine to repair the BBB after
disruption.
Not applicable.
Funding: The present review was supported by the National
Natural Science Foundation of China (grant no. 82205254), the 11th
Batch of Science and Technology Development Projects of Suzhou in
2023 (grant no. SKY2023014), the University-local Collaborative
Innovation Research Project (grant no. 20239606), the Jiangsu
Traditional Chinese Medicine Science and Technology Development
Program project (grant no. MS2023101) and the Wumen Medical Project
of Suzhou (grant no. SKYXD2022034).
BD and YG conceived and designed the manuscript.
MW, XH and YH searched the literature and wrote the manuscript. JY
created the figures. BD reviewed and revised the manuscript. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Diaz-Garcia CM, Mongeon R, Lahmann C,
Koveal D, Zucker H and Yellen G: Neuronal stimulation triggers
neuronal glycolysis and not lactate uptake. Cell Metab.
26:361–374.e4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roy CS and Sherrington CS: On the
regulation of the blood-supply of the brain. J Physiol.
11:85–158.117. 1890.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xue Q, Liu Y, Qi H, Ma Q, Xu L, Chen W,
Chen G and Xu X: A novel brain neurovascular unit model with
neurons, astrocytes and microvascular endothelial cells of rat. Int
J Biol Sci. 9:174–189. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harder DR, Zhang C and Gebremedhin D:
Astrocytes function in matching blood flow to metabolic activity.
News Physiol Sci. 17:27–31. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Simard M, Arcuino G, Takano T, Liu QS and
Nedergaard M: Signaling at the gliovascular interface. J Neurosci.
23:9254–9262. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reese TS and Karnovsky MJ: Fine structural
localization of a blood-brain barrier to exogenous peroxidase. J
Cell Biol. 34:207–217. 1967.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamamizu K, Iwasaki M, Takakubo H,
Sakamoto T, Ikuno T, Miyoshi M, Kondo T, Nakao Y, Nakagawa M, Inoue
H and Yamashita JK: In vitro modeling of blood-brain barrier with
human iPSC-derived endothelial cells, pericytes, neurons, and
astrocytes via notch signaling. Stem Cell Reports. 8:634–647.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ehrlich P: Das sauerstoff-bedürfnis des
organismus: Eine farbenanalytische studie. August Hirschwald,
1885.
|
|
9
|
Saunders NR, Dreifuss JJ, Dziegielewska
KM, Johansson PA, Habgood MD, Møllgård K and Bauer HC: The rights
and wrongs of blood-brain barrier permeability studies: A walk
through 100 years of history. Front Neurosci. 8(404)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Patel R, Page S and Al-Ahmad AJ: Isogenic
blood-brain barrier models based on patient-derived stem cells
display inter-individual differences in cell maturation and
functionality. J Neurochem. 142:74–88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Salmina AB, Kharitonova EV, Gorina YV,
Teplyashina EA, Malinovskaya NA, Khilazheva ED, Mosyagina AI,
Morgun AV, Shuvaev AN, Salmin VV, et al: Blood-brain barrier and
neurovascular unit in vitro models for studying mitochondria-driven
molecular mechanisms of neurodegeneration. Int J Mol Sci.
22(4661)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Salmina AB, Kuvacheva NV, Morgun AV,
Komleva YK, Pozhilenkova EA, Lopatina OL, Gorina YV, Taranushenko
TE and Petrova LL: Glycolysis-mediated control of blood-brain
barrier development and function. Int J Biochem Cell Biol.
64:174–184. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lv J, Hu W, Yang Z, Li T, Jiang S, Ma Z,
Chen F and Yang Y: Focusing on claudin-5: A promising candidate in
the regulation of BBB to treat ischemic stroke. Prog Neurobiol.
161:79–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu J, Lin X, Chen C, Tan H, Gao Y, Li D
and Chen G: WNK3 promotes neuronal survival after traumatic brain
injury in rats. Neuroscience. 477:76–88. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang W, Li J, Geng X, Li S, Zou Y, Li Y,
Jing C and Yu H: The reactive astrocytes after surgical brain
injury potentiates the migration, invasion, and angiogenesis of C6
glioma. World Neurosurg. 168:e595–e606. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu D, Lai N, Deng R, Liang T, Pan P, Yuan
G, Li X, Li H, Shen H, Wang Z and Chen G: Activated WNK3 induced by
intracerebral hemorrhage deteriorates brain injury maybe via
WNK3/SPAK/NKCC1 pathway. Exp Neurol. 332(113386)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Drouin-Ouellet J, Sawiak SJ, Cisbani G,
Lagacé M, Kuan WL, Saint-Pierre M, Dury RJ, Alata W, St-Amour I,
Mason SL, et al: Cerebrovascular and blood-brain barrier
impairments in Huntington's disease: Potential implications for its
pathophysiology. Ann Neurol. 78:160–177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mohi-Ud-Din R, Mir RH, Mir PA, Banday N,
Shah AJ, Sawhney G, Bhat MM, Batiha GE and Pottoo FH: Dysfunction
of ABC transporters at the surface of BBB: Potential implications
in intractable epilepsy and applications of nanotechnology enabled
drug delivery. Curr Drug Metab. 23:735–756. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ferraro S, Klugah-Brown B, Tench CR,
Bazinet V, Bore MC, Nigri A, Demichelis G, Bruzzone MG, Palermo S,
Zhao W, et al: The central autonomic system revisited-convergent
evidence for a regulatory role of the insular and midcingulate
cortex from neuroimaging meta-analyses. Neurosci Biobehav Rev.
142(104915)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bittern J, Pogodalla N, Ohm H, Brüser L,
Kottmeier R, Schirmeier S and Klämbt C: Neuron-glia interaction in
the Drosophila nervous system. Dev Neurobiol. 81:438–452.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rolls MM and Jegla TJ: Neuronal polarity:
An evolutionary perspective. J Exp Biol. 218:572–580.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Muzio MR and Cascella M: Marco Cascella
declares no relevant financial relationships with ineligible
companies. In: Histology, Axon. StatPearls, Treasure Island, FL,
2024.
|
|
23
|
Zhang D, Ruan J, Peng S, Li J, Hu X, Zhang
Y, Zhang T, Ge Y, Zhu Z, Xiao X, et al: Synaptic-like transmission
between neural axons and arteriolar smooth muscle cells drives
cerebral neurovascular coupling. Nat Neurosci. 27:232–248.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qin D and Wang J, Le A, Wang TJ, Chen X
and Wang J: Traumatic brain injury: Ultrastructural features in
neuronal ferroptosis, glial cell activation and polarization, and
blood-brain barrier breakdown. Cells. 10(1009)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schirmeier S and Klämbt C: The Drosophila
blood-brain barrier as interface between neurons and hemolymph.
Mech Dev. 138:50–55. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Miller F, Afonso PV, Gessain A and
Ceccaldi PE: Blood-brain barrier and retroviral infections.
Virulence. 3:222–229. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cataldi M: The changing landscape of
voltage-gated calcium channels in neurovascular disorders and in
neurodegenerative diseases. Curr Neuropharmacol. 11:276–297.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lauritzen M, Mathiesen C, Schaefer K and
Thomsen KJ: Neuronal inhibition and excitation, and the dichotomic
control of brain hemodynamic and oxygen responses. Neuroimage.
62:1040–1050. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cheng YT, Luna-Figueroa E, Woo J, Chen HC,
Lee ZF, Harmanci AS and Deneen B: Inhibitory input directs
astrocyte morphogenesis through glial GABA(B)R. Nature.
617:369–376. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ancatén-González C, Segura I,
Alvarado-Sánchez R, Chávez AE and Latorre R: Ca2+- and
voltage-activated K+ (BK) channels in the nervous
system: One gene, a myriad of physiological functions. Int J Mol
Sci. 24(3407)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cohen S and Greenberg ME: Communication
between the synapse and the nucleus in neuronal development,
plasticity, and disease. Annu Rev Cell Dev Biol. 24:183–209.
2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun J, Zheng Y, Chen Z and Wang Y: The
role of Na+ -K+ -ATPase in the epileptic
brain. CNS Neurosci Ther. 28:1294–1302. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jiang S, Fan F, Yang L, Chen K, Sun Z,
Zhang Y, Cairang N, Wang X and Meng X: Salidroside attenuates high
altitude hypobaric hypoxia-induced brain injury in mice via
inhibiting NF-κB/NLRP3 pathway. Eur J Pharmacol.
925(175015)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li B, Li N, Chen L, Ren S, Gao D, Geng H,
Fu J, Zhou M and Xing C: Alleviating neuroinflammation through
photothermal conjugated polymer nanoparticles by regulating
reactive oxygen species and Ca2+ signaling. ACS Appl
Mater Interfaces. 14:48416–48425. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Reddiar SB, de Veer M, Paterson BM,
Sepehrizadeh T, Wai DCC, Csoti A, Panyi G, Nicolazzo JA and Norton
RS: A biodistribution study of the radiolabeled Kv1.3-blocking
peptide DOTA-HsTX1[R14A] demonstrates brain uptake in a mouse model
of neuroinflammation. Mol Pharm. 20:255–266. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Carbajal-Contreras H, Murillo-de-Ozores
AR, Magaña-Avila G, Marquez-Salinas A, Bourqui L, Tellez-Sutterlin
M, Bahena-Lopez JP, Cortes-Arroyo E, Behn-Eschenburg SG,
Lopez-Saavedra A, et al: Arginine vasopressin regulates the renal
Na+-Cl- and
Na+-K+-Cl- cotransporters through
with-no-lysine kinase 4 and inhibitor 1 phosphorylation. Am J
Physiol Renal Physiol. 326:F285–F299. 2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Engelhardt B and Sorokin L: The
blood-brain and the blood-cerebrospinal fluid barriers: Function
and dysfunction. Semin Immunopathol. 31:497–511. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Stokum JA, Gerzanich V and Simard JM:
Molecular pathophysiology of cerebral edema. J Cereb Blood Flow
Metab. 36:513–538. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wiley CA, Bissel SJ, Lesniak A, Dixon CE,
Franks J, Beer Stolz D, Sun M, Wang G, Switzer R, Kochanek PM and
Murdoch G: Ultrastructure of diaschisis lesions after traumatic
brain injury. J Neurotrauma. 33:1866–1882. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hosokawa M and Ueno M: Aging of
blood-brain barrier and neuronal cells of eye and ear in SAM mice.
Neurobiol Aging. 20:117–123. 1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gong Y, Wu M, Gao F, Shi M, Gu H, Gao R,
Dang BQ and Chen G: Inhibition of the p-SPAK/p-NKCC1 signaling
pathway protects the blood-brain barrier and reduces neuronal
apoptosis in a rat model of surgical brain injury. Mol Med Rep.
24(717)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gong Y, Wu M, Shen J, Tang J, Li J, Xu J,
Dang B and Chen G: Inhibition of the NKCC1/NF-κB signaling pathway
decreases inflammation and improves brain edema and nerve cell
apoptosis in an SBI rat model. Front Mol Neurosci.
14(641993)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wu MY, Gao F, Tang JF, Shen JC, Gao R,
Dang BQ and Chen G: Possible mechanisms of the PERK pathway on
neuronal apoptosis in a rat model of surgical brain injury. Am J
Transl Res. 13:732–742. 2021.PubMed/NCBI
|
|
44
|
Shen J, Qian M, Wu M, Tang J, Gong Y, Li
J, Ji J and Dang B: Rosiglitazone inhibits acyl-CoA synthetase
long-chain family number 4 and improves secondary brain injury in a
rat model of surgical brain injury. Clin Exp Pharmacol Physiol.
50:927–935. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu M, Gao R, Dang B and Chen G: The blood
component iron causes neuronal apoptosis following intracerebral
hemorrhage via the PERK pathway. Front Neurol.
11(588548)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wu M, Wang C, Gong Y, Huang Y, Jiang L,
Zhang M, Gao R and Dang B: Potential mechanism of TMEM2/CD44 in
endoplasmic reticulum stress-induced neuronal apoptosis in a rat
model of traumatic brain injury. Int J Mol Med.
52(119)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li J, Wu M, Gong Y, Tang J, Shen J, Xu L,
Dang B and Chen G: Inhibition of LRRK2-Rab10 pathway improves
secondary brain injury after surgical brain injury in rats. Front
Surg. 8(749310)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Anthony IC, Ramage SN, Carnie FW, Simmonds
P and Bell JE: Does drug abuse alter microglial phenotype and cell
turnover in the context of advancing HIV infection? Neuropathol
Appl Neurobiol. 31:325–338. 2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Haruwaka K, Ikegami A, Tachibana Y, Ohno
N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, et
al: Dual microglia effects on blood brain barrier permeability
induced by systemic inflammation. Nat Commun.
10(5816)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Merlini M, Rafalski VA, Ma K, Kim KY,
Bushong EA, Rios Coronado PE, Yan Z, Mendiola AS, Sozmen EG, Ryu
JK, et al: Microglial Gi-dependent dynamics regulate
brain network hyperexcitability. Nat Neurosci. 24:19–23.
2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang J, Zhang C, Zhu J, Ding J, Chen Y and
Han X: Blood-brain barrier disruption and inflammation reaction in
mice after chronic exposure to Microcystin-LR. Sci Total Environ.
689:662–678. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ueno M, Fujita Y, Tanaka T, Nakamura Y,
Kikuta J, Ishii M and Yamashita T: Layer V cortical neurons require
microglial support for survival during postnatal development. Nat
Neurosci. 16:543–551. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Corps KN, Roth TL and McGavern DB:
Inflammation and neuroprotection in traumatic brain injury. JAMA
Neurol. 72:355–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ikegami A and Wake H: Microglial
regulation of blood brain barrier, the neuro-immunological
interface. Brain Nerve. 73:913–919. 2021.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
55
|
Planas AM: Role of immune cells migrating
to the ischemic brain. Stroke. 49:2261–2267. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Park GH, Noh H, Shao Z, Ni P, Qin Y, Liu
D, Beaudreault CP, Park JS, Abani CP, Park JM, et al: Activated
microglia cause metabolic disruptions in developmental cortical
interneurons that persist in interneurons from individuals with
schizophrenia. Nat Neurosci. 23:1352–1364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang Z, Zhang Z, Lu H, Yang Q, Wu H and
Wang J: Microglial polarization and inflammatory mediators after
intracerebral hemorrhage. Mol Neurobiol. 54:1874–1886.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Roseborough AD, Zhu Y, Zhao L, Laviolette
SR, Pasternak SH and Whitehead SN: Fibrinogen primes the microglial
NLRP3 inflammasome and propagates pro-inflammatory signaling via
extracellular vesicles: Implications for blood-brain barrier
dysfunction. Neurobiol Dis. 177(106001)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kant R, Halder SK, Fernández JA, Griffin
JH and Milner R: Activated protein C attenuates experimental
autoimmune encephalomyelitis progression by enhancing vascular
integrity and suppressing microglial activation. Front Neurosci.
14(333)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Jolivel V, Bicker F, Binamé F, Ploen R,
Keller S, Gollan R, Jurek B, Birkenstock J, Poisa-Beiro L, Bruttger
J, et al: Perivascular microglia promote blood vessel
disintegration in the ischemic penumbra. Acta Neuropathol.
129:279–295. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pan JJ, Qi L, Wang L, Liu C, Song Y,
Mamtilahun M, Hu X, Li Y, Chen X, Khan H, et al: M2 microglial
extracellular vesicles attenuated blood-brain barrier disruption
via MiR-23a-5p in cerebral ischemic mice. Aging Dis. 15:1344–1356.
2024.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Clarner T, Diederichs F, Berger K, Denecke
B, Gan L, van der Valk P, Beyer C, Amor S and Kipp M: Myelin debris
regulates inflammatory responses in an experimental demyelination
animal model and multiple sclerosis lesions. Glia. 60:1468–1480.
2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Schafer DP, Lehrman EK, Kautzman AG,
Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME,
Barres BA and Stevens B: Microglia sculpt postnatal neural circuits
in an activity and complement-dependent manner. Neuron. 74:691–705.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh
JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin
RJM and Ffrench-Constant C: M2 microglia and macrophages drive
oligodendrocyte differentiation during CNS remyelination. Nat
Neurosci. 16:1211–1218. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wang Y, Luo J and Li SY: Nano-curcumin
simultaneously protects the blood-brain barrier and reduces M1
microglial activation during cerebral ischemia-reperfusion injury.
ACS Appl Mater Interfaces. 11:3763–3770. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X and
Fu J: Curcumin inhibits LPS-induced neuroinflammation by promoting
microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2
cells. Mol Immunol. 116:29–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Demirdağ F, Yavuzer S, Cengiz M, Yavuzer
H, Kara Z, Ayvacı A, Avcı S, Yürüyen M, Uzun H, Altıparmak MR, et
al: The role of NF-κB, PPAR-α, and PPAR-γ in older adults with
metabolic syndrome. Horm Metab Res. 55:733–740. 2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Li YF, Ren X, Zhang L, Wang YH and Chen T:
Microglial polarization in TBI: Signaling pathways and influencing
pharmaceuticals. Front Aging Neurosci. 14(901117)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Takuathung MN, Potikanond S, Sookkhee S,
Mungkornasawakul P, Jearanaikulvanich T, Chinda K, Wikan N and
Nimlamool W: Anti-psoriatic and anti-inflammatory effects of
Kaempferia parviflora in keratinocytes and macrophage cells. Biomed
Pharmacother. 143(112229)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ruan Z, Zhang D, Huang R, Sun W, Hou L,
Zhao J and Wang Q: Microglial activation damages dopaminergic
neurons through MMP-2/-9-mediated increase of blood-brain barrier
permeability in a Parkinson's disease mouse model. Int J Mol Sci.
23(2793)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Guo Y, Dai W, Zheng Y, Qiao W, Chen W,
Peng L, Zhou H, Zhao T, Liu H, Zheng F and Sun P: Mechanism and
regulation of microglia polarization in intracerebral hemorrhage.
Molecules. 27(7080)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tian J, Liu Y, Wang Z, Zhang S, Yang Y,
Zhu Y and Yang C: LncRNA Snhg8 attenuates microglial inflammation
response and blood-brain barrier damage in ischemic stroke through
regulating miR-425-5p mediated SIRT1/NF-κB signaling. J Biochem Mol
Toxicol. 35(e22724)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Liao Y, Hu J, Guo C, Wen A, Wen L, Hou Q,
Weng Y, Wang J, Ding Y and Yang J: Acteoside alleviates blood-brain
barrier damage induced by ischemic stroke through inhibiting
microglia HMGB1/TLR4/NLRP3 signaling. Biochem Pharmacol.
220(115968)2024.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chen G, Hou Y, Li X, Pan R and Zhao D:
Sepsis-induced acute lung injury in young rats is relieved by
calycosin through inactivating the HMGB1/MyD88/NF-κB pathway and
NLRP3 inflammasome. Int Immunopharmacol. 96(107623)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Yin N, Zhao Y, Liu C, Yang Y, Wang ZH, Yu
W, Zhang K, Zhang Z, Liu J, Zhang Y and Shi J: Engineered
nanoerythrocytes alleviate central nervous system inflammation by
regulating the polarization of inflammatory microglia. Adv Mater.
34(e2201322)2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Chang H, Ma J, Feng K, Feng N, Wang X, Sun
J, Guo T, Wei Y, Xu Y, Wang H, et al: Elevated blood and
cerebrospinal fluid biomarkers of microglial activation and
blood-brain barrier disruption in anti-NMDA receptor encephalitis.
J Neuroinflammation. 20(172)2023.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yan J, Zhang Y, Wang L, Li Z, Tang S, Wang
Y, Gu N, Sun X and Li L: TREM2 activation alleviates neural damage
via Akt/CREB/BDNF signalling after traumatic brain injury in mice.
J Neuroinflammation. 19(289)2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Shi M, Gong Y, Wu M, Gu H, Yu J, Gao F,
Ren Z, Qian M, Dang B and Chen G: Downregulation of TREM2/NF-кB
signaling may damage the blood-brain barrier and aggravate neuronal
apoptosis in experimental rats with surgically injured brain. Brain
Res Bull. 183:116–126. 2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Sofroniew MV: Astrocyte barriers to
neurotoxic inflammation. Nat Rev Neurosci. 16:249–263.
2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Cheng X, Wang J, Sun X, Shao L, Guo Z and
Li Y: Morphological and functional alterations of astrocytes
responding to traumatic brain injury. J Integr Neurosci.
18:203–215. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lauranzano E, Rasile M and Matteoli M:
Integrating primary astrocytes in a microfluidic model of the
blood-brain barrier. Methods Mol Biol. 2492:225–240.
2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yosef N, Xi Y and McCarty JH: Isolation
and transcriptional characterization of mouse perivascular
astrocytes. PLoS One. 15(e0240035)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Huang J, Ding J, Wang X, Gu C, He Y, Li Y,
Fan H, Xie Q, Qi X, Wang Z and Qiu P: Transfer of neuron-derived
α-synuclein to astrocytes induces neuroinflammation and blood-brain
barrier damage after methamphetamine exposure: Involving the
regulation of nuclear receptor-associated protein 1. Brain Behav
Immun. 106:247–261. 2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Abbott NJ, Rönnbäck L and Hansson E:
Astrocyte-endothelial interactions at the blood-brain barrier. Nat
Rev Neurosci. 7:41–53. 2006.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Weber CM, Moiz B, Zic SM, Alpizar Vargas
V, Li A and Clyne AM: Induced pluripotent stem cell-derived cells
model brain microvascular endothelial cell glucose metabolism.
Fluids Barriers CNS. 19(98)2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Wu Z, Parry M, Hou XY, Liu MH, Wang H,
Cain R, Pei ZF, Chen YC, Guo ZY, Abhijeet S and Chen G: Gene
therapy conversion of striatal astrocytes into GABAergic neurons in
mouse models of Huntington's disease. Nat Commun.
11(1105)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Bezzi P, Domercq M, Brambilla L, Galli R,
Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J
and Volterra A: CXCR4-activated astrocyte glutamate release via
TNFalpha: Amplification by microglia triggers neurotoxicity. Nat
Neurosci. 4:702–710. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
88
|
Nedergaard M, Ransom B and Goldman SA: New
roles for astrocytes: Redefining the functional architecture of the
brain. Trends Neurosci. 26:523–530. 2003.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Simard M and Nedergaard M: The
neurobiology of glia in the context of water and ion homeostasis.
Neuroscience. 129:877–896. 2004.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Karve IP, Taylor JM and Crack PJ: The
contribution of astrocytes and microglia to traumatic brain injury.
Br J Pharmacol. 173:692–702. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Willis EF, MacDonald KPA, Nguyen QH,
Garrido AL, Gillespie ER, Harley SBR, Bartlett PF, Schroder WA,
Yates AG, Anthony DC, et al: Repopulating microglia promote brain
repair in an IL-6-dependent manner. Cell. 180:833–846.e16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Lee H and Koh JY: Roles for H+
/K+ -ATPase and zinc transporter 3 in cAMP-mediated
lysosomal acidification in bafilomycin A1-treated astrocytes. Glia.
69:1110–1125. 2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Chen Y and Swanson RA: Astrocytes and
brain injury. J Cereb Blood Flow Metab. 23:137–149. 2003.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Burda JE and Sofroniew MV: Reactive
gliosis and the multicellular response to CNS damage and disease.
Neuron. 81:229–248. 2014.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Lee EJ, Hung YC and Lee MY: Early
alterations in cerebral hemodynamics, brain metabolism, and
blood-brain barrier permeability in experimental intracerebral
hemorrhage. J Neurosurg. 91:1013–1019. 1999.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lee EJ, Chio CC, Chang CH and Chen HH:
Prognostic significance of altered cerebral blood flow velocity in
acute head trauma. J Formos Med Assoc. 96:5–12. 1997.PubMed/NCBI
|
|
97
|
Lien CF, Mohanta SK, Frontczak-Baniewicz
M, Swinny JD, Zablocka B and Górecki DC: Absence of glial
α-dystrobrevin causes abnormalities of the blood-brain barrier and
progressive brain edema. J Biol Chem. 287:41374–41385.
2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wolburg H, Noell S, Wolburg-Buchholz K,
Mack A and Fallier-Becker P: Agrin, aquaporin-4, and astrocyte
polarity as an important feature of the blood-brain barrier.
Neuroscientist. 15:180–193. 2009.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kim I, Moon SO, Park SK, Chae SW and Koh
GY: Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to
endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin
expression. Circ Res. 89:477–479. 2001.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Liu B and Neufeld AH: Expression of nitric
oxide synthase-2 (NOS-2) in reactive astrocytes of the human
glaucomatous optic nerve head. Glia. 30:178–186. 2000.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Jiang L, Pan CL, Wang CY, Liu BQ, Han Y,
Hu L, Liu L, Yang Y, Qu JW and Liu WT: Selective suppression of the
JNK-MMP2/9 signal pathway by tetramethylpyrazine attenuates
neuropathic pain in rats. J Neuroinflammation.
14(174)2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Lu L, Hogan-Cann AD, Globa AK, Lu P, Nagy
JI, Bamji SX and Anderson CM: Astrocytes drive cortical
vasodilatory signaling by activating endothelial NMDA receptors. J
Cereb Blood Flow Metab. 39:481–496. 2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Mizee MR, Nijland PG, van der Pol SMA,
Drexhage JAR, van Het Hof B, Mebius R, van der Valk P, van Horssen
J, Reijerkerk A and de Vries HE: Astrocyte-derived retinoic acid: A
novel regulator of blood-brain barrier function in multiple
sclerosis. Acta Neuropathol. 128:691–703. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Li Y, Xia Y, Wang Y, Mao L, Gao Y, He Q,
Huang M, Chen S and Hu B: Sonic hedgehog (Shh) regulates the
expression of angiogenic growth factors in oxygen-glucose-deprived
astrocytes by mediating the nuclear receptor NR2F2. Mol Neurobiol.
47:967–975. 2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Zacharek A, Chen J, Cui X, Li A, Li Y,
Roberts C, Feng Y, Gao Q and Chopp M: Angiopoietin1/Tie2 and
VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and
vascular stabilization after stroke. J Cereb Blood Flow Metab.
27:1684–1691. 2007.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Chen M, Ba H, Lu C, Dai J and Sun J: Glial
cell line-derived neurotrophic factor (GDNF) promotes angiogenesis
through the demethylation of the fibromodulin (FMOD) promoter in
glioblastoma. Med Sci Monit. 24:6137–6143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Rodriguez-Perez AI, Borrajo A, Diaz-Ruiz
C, Garrido-Gil P and Labandeira-Garcia JL: Crosstalk between
insulin-like growth factor-1 and angiotensin-II in dopaminergic
neurons and glial cells: Role in neuroinflammation and aging.
Oncotarget. 7:30049–30067. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Cao F, Jiang Y, Wu Y, Zhong J, Liu J, Qin
X, Chen L, Vitek MP, Li F, Xu L and Sun X: Apolipoprotein E-mimetic
COG1410 reduces acute vasogenic edema following traumatic brain
injury. J Neurotrauma. 33:175–182. 2016.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Leybaert L: Neurobarrier coupling in the
brain: A partner of neurovascular and neurometabolic coupling? J
Cereb Blood Flow Metab. 25:2–16. 2005.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Chen Z, Kelly JR, Morales JE, Sun RC, De
A, Burkin DJ and McCarty JH: The alpha7 integrin subunit in
astrocytes promotes endothelial blood-brain barrier integrity.
Development. 150(dev201356)2023.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Mayer U, Saher GFässler R, Bornemann A,
Echtermeyer F, von der Mark H, Miosge N, Pöschl E and von der Mark
K: Absence of integrin alpha 7 causes a novel form of muscular
dystrophy. Nat Genet. 17:318–323. 1997.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Mielenz D, Hapke S, Pöschl E, von Der Mark
H and von Der Mark K: The integrin alpha 7 cytoplasmic domain
regulates cell migration, lamellipodia formation, and p130CAS/Crk
coupling. J Biol Chem. 276:13417–13426. 2001.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Kim H, Leng K, Park J, Sorets AG, Kim S,
Shostak A, Embalabala RJ, Mlouk K, Katdare KA, Rose IVL, et al:
Reactive astrocytes transduce inflammation in a blood-brain barrier
model through a TNF-STAT3 signaling axis and secretion of alpha
1-antichymotrypsin. Nat Commun. 13(6581)2022.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Lee LL, Aung HH, Wilson DW, Anderson SE,
Rutledge JC and Rutkowsky JM: Triglyceride-rich lipoprotein
lipolysis products increase blood-brain barrier transfer
coefficient and induce astrocyte lipid droplets and cell stress. Am
J Physiol Cell Physiol. 312:C500–C516. 2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Malik VA, Zajicek F, Mittmann LA, Klaus J,
Unterseer S, Rajkumar S, Pütz B, Deussing JM, Neumann ID, Rupprecht
R and Di Benedetto B: GDF15 promotes simultaneous astrocyte
remodeling and tight junction strengthening at the blood-brain
barrier. J Neurosci Res. 98:1433–1456. 2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Shimizu F, Sano Y, Tominaga O, Maeda T,
Abe MA and Kanda T: Advanced glycation end-products disrupt the
blood-brain barrier by stimulating the release of transforming
growth factor-β by pericytes and vascular endothelial growth factor
and matrix metalloproteinase-2 by endothelial cells in vitro.
Neurobiol Aging. 34:1902–1912. 2013.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Senatorov VV Jr, Friedman AR, Milikovsky
DZ, Ofer J, Saar-Ashkenazy R, Charbash A, Jahan N, Chin G, Mihaly
E, Lin JM, et al: Blood-brain barrier dysfunction in aging induces
hyperactivation of TGFβ signaling and chronic yet reversible neural
dysfunction. Sci Transl Med. 11(eaaw8283)2019.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Guo Y, Dong L, Gong A, Zhang J, Jing L,
Ding T, Li PA and Zhang JZ: Damage to the blood-brain barrier and
activation of neuroinflammation by focal cerebral ischemia under
hyperglycemic condition. Int J Mol Med. 48(142)2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Wang QS, Ding HG, Chen SL, Liu XQ, Deng
YY, Jiang WQ, Li Y, Huang LQ, Han YL, Wen MY, et al: Hypertonic
saline mediates the NLRP3/IL-1β signaling axis in microglia to
alleviate ischemic blood-brain barrier permeability by
downregulating astrocyte-derived VEGF in rats. CNS Neurosci Ther.
26:1045–1057. 2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
You L, Yu PP, Dong T, Guo W, Chang S,
Zheng B, Ci Y, Wang F, Yu P, Gao G and Chang YZ: Astrocyte-derived
hepcidin controls iron traffic at the blood-brain-barrier via
regulating ferroportin 1 of microvascular endothelial cells. Cell
Death Dis. 13(667)2022.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Pasti L, Volterra A, Pozzan T and
Carmignoto G: Intracellular calcium oscillations in astrocytes: A
highly plastic, bidirectional form of communication between neurons
and astrocytes in situ. J Neurosci. 17:7817–7830. 1997.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Huber JD, Egleton RD and Davis TP:
Molecular physiology and pathophysiology of tight junctions in the
blood-brain barrier. Trends Neurosci. 24:719–725. 2001.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Cotrina ML, Lin JH, Alves-Rodrigues A, Liu
S, Li J, Azmi-Ghadimi H, Kang J, Naus CC and Nedergaard M:
Connexins regulate calcium signaling by controlling ATP release.
Proc Natl Acad Sci USA. 95:15735–15740. 1998.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Sneyd J, Charles AC and Sanderson MJ: A
model for the propagation of intercellular calcium waves. Am J
Physiol. 266:C293–C302. 1994.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Chapouly C, Tadesse Argaw A, Horng S,
Castro K, Zhang J, Asp L, Loo H, Laitman BM, Mariani JN, Straus
Farber R, et al: Astrocytic TYMP and VEGFA drive blood-brain
barrier opening in inflammatory central nervous system lesions.
Brain. 138:1548–1567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Chu H, Yang X, Huang C, Gao Z, Tang Y and
Dong Q: Apelin-13 protects against ischemic blood-brain barrier
damage through the effects of aquaporin-4. Cerebrovasc Dis.
44:10–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Jackson RJ, Meltzer JC, Nguyen H, Commins
C, Bennett RE, Hudry E and Hyman BT: APOE4 derived from astrocytes
leads to blood-brain barrier impairment. Brain. 145:3582–3593.
2022.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Qin X, Wang J, Chen S, Liu G, Wu C, Lv Q,
He X, Bai X, Huang W and Liao H: Astrocytic p75NTR
expression provoked by ischemic stroke exacerbates the blood-brain
barrier disruption. Glia. 70:892–912. 2022.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Lee SW, Kim WJ, Choi YK, Song HS, Son MJ,
Gelman IH, Kim YJ and Kim KW: SSeCKS regulates angiogenesis and
tight junction formation in blood-brain barrier. Nat Med.
9:900–906. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
130
|
Takarada-Iemata M, Yoshikawa A, Ta HM,
Okitani N, Nishiuchi T, Aida Y, Kamide T, Hattori T, Ishii H,
Tamatani T, et al: N-myc downstream-regulated gene 2 protects
blood-brain barrier integrity following cerebral ischemia. Glia.
66:1432–1446. 2018.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Tian W, Sawyer A, Kocaoglu FB and
Kyriakides TR: Astrocyte-derived thrombospondin-2 is critical for
the repair of the blood-brain barrier. Am J Pathol. 179:860–868.
2011.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Jayakumar AR, Tong XY, Ruiz-Cordero R,
Bregy A, Bethea JR, Bramlett HM and Norenberg MD: Activation of
NF-κB mediates astrocyte swelling and brain edema in traumatic
brain injury. J Neurotrauma. 31:1249–1257. 2014.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Wu M, Gong Y, Jiang L, Zhang M, Gu H, Shen
H and Dang B: VEGF regulates the blood-brain barrier through MMP-9
in a rat model of traumatic brain injury. Exp Ther Med.
24(728)2022.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Wu MY, Gao F, Yang XM, Qin X, Chen GZ, Li
D, Dang BQ and Chen G: Matrix metalloproteinase-9 regulates the
blood brain barrier via the hedgehog pathway in a rat model of
traumatic brain injury. Brain Res. 1727(146553)2020.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Persidsky Y, Ramirez SH, Haorah J and
Kanmogne GD: Blood-brain barrier: Structural components and
function under physiologic and pathologic conditions. J Neuroimmune
Pharmacol. 1:223–236. 2006.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Kassan M, Kwon Y, Munkhsaikhan U, Sahyoun
AM, Ishrat T, Galán M, Gonzalez AA, Abidi AH, Kassan A and
Ait-Aissa K: Protective role of short-chain fatty acids against
Ang-II-induced mitochondrial dysfunction in brain endothelial
cells: A potential role of heme oxygenase 2. Antioxidants (Basel).
12(160)2023.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Wang YI, Abaci HE and Shuler ML:
Microfluidic blood-brain barrier model provides in vivo-like
barrier properties for drug permeability screening. Biotechnol
Bioeng. 114:184–194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Min XL, Zou H, Yan J, Lyu Q, He X and
Shang FF: Stress conditions induced circRNAs profile of
extracellular vesicles in brain microvascular endothelial cells.
Metab Brain Dis. 37:1977–1987. 2022.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Furtado D, Björnmalm M, Ayton S, Bush AI,
Kempe K and Caruso F: Overcoming the blood-brain barrier: The role
of nanomaterials in treating neurological diseases. Adv Mater.
30(e1801362)2018.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Grammas P, Martinez J and Miller B:
Cerebral microvascular endothelium and the pathogenesis of
neurodegenerative diseases. Expert Rev Mol Med.
13(e19)2011.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Oldendorf WH, Cornford ME and Brown WJ:
The large apparent work capability of the blood-brain barrier: A
study of the mitochondrial content of capillary endothelial cells
in brain and other tissues of the rat. Ann Neurol. 1:409–417.
1977.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Grutzendler J and Nedergaard M: Cellular
control of brain capillary blood flow: In vivo imaging veritas.
Trends Neurosci. 42:528–536. 2019.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Raut S, Patel R and Al-Ahmad AJ: Presence
of a mutation in PSEN1 or PSEN2 gene is associated with an impaired
brain endothelial cell phenotype in vitro. Fluids Barriers CNS.
18(3)2021.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Lisk C, McCord J, Bose S, Sullivan T,
Loomis Z, Nozik-Grayck E, Schroeder T, Hamilton K and Irwin DC:
Nrf2 activation: A potential strategy for the prevention of acute
mountain sickness. Free Radic Biol Med. 63:264–273. 2013.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Nicolicht-Amorim P, Delgado-Garcia LM,
Nakamura TKE, Courbassier NR, Mosini AC and Porcionatto MA: Simple
and efficient protocol to isolate and culture brain microvascular
endothelial cells from newborn mice. Front Cell Neurosci.
16(949412)2022.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Sawada N: Tight junction-related human
diseases. Pathol Int. 63:1–12. 2013.PubMed/NCBI View Article : Google Scholar
|
|
147
|
González-Mariscal L, Posadas Y, Miranda J,
Uc PY, Ortega-Olvera JM and Hernández S: Strategies that target
tight junctions for enhanced drug delivery. Curr Pharm Des.
22:5313–5346. 2016.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Keaney J and Campbell M: The dynamic
blood-brain barrier. FEBS J. 282:4067–4079. 2015.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Kumar R, Sharma A and Tiwari RK: Can we
predict blood brain barrier permeability of ligands using
computational approaches? Interdiscip Sci. 5:95–101.
2013.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Saxena D, Sharma A, Siddiqui MH and Kumar
R: Blood brain barrier permeability prediction using machine
learning techniques: An update. Curr Pharm Biotechnol.
20:1163–1171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Song D, Jiang X, Liu Y, Sun Y, Cao S and
Zhang Z: Asiaticoside attenuates cell growth inhibition and
apoptosis induced by Aβ1-42 via inhibiting the
TLR4/NF-κB signaling pathway in human brain microvascular
endothelial cells. Front Pharmacol. 9(28)2018.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Uraoka M, Ikeda K, Kurimoto-Nakano R,
Nakagawa Y, Koide M, Akakabe Y, Kitamura Y, Ueyama T, Matoba S,
Yamada H, et al: Loss of bcl-2 during the senescence exacerbates
the impaired angiogenic functions in endothelial cells by
deteriorating the mitochondrial redox state. Hypertension.
58:254–263. 2011.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Rajeev V, Fann DY, Dinh QN, Kim HA, De
Silva TM, Lai MKP, Chen CL, Drummond GR, Sobey CG and Arumugam TV:
Pathophysiology of blood brain barrier dysfunction during chronic
cerebral hypoperfusion in vascular cognitive impairment.
Theranostics. 12:1639–1658. 2022.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Sweeney MD, Sagare AP and Zlokovic BV:
Blood-brain barrier breakdown in Alzheimer disease and other
neurodegenerative disorders. Nat Rev Neurol. 14:133–150.
2018.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Bernard-Patrzynski F, Lécuyer MA, Puscas
I, Boukhatem I, Charabati M, Bourbonnière L, Ramassamy C, Leclair
G, Prat A and Roullin VG: Isolation of endothelial cells, pericytes
and astrocytes from mouse brain. PLoS One.
14(e0226302)2019.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Vajtr D, Benada O, Kukacka J, Průša R,
Houstava L, Ťoupalík P and Kizek R: Correlation of ultrastructural
changes of endothelial cells and astrocytes occurring during blood
brain barrier damage after traumatic brain injury with biochemical
markers of BBB leakage and inflammatory response. Physiol Res.
58:263–268. 2009.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Yao X, Uchida K, Papadopoulos MC, Zador Z,
Manley GT and Verkman AS: Mildly reduced brain swelling and
improved neurological outcome in aquaporin-4 knockout mice
following controlled cortical impact brain injury. J Neurotrauma.
32:1458–1464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Abbott NJ: Astrocyte-endothelial
interactions and blood-brain barrier permeability. J Anat.
200:629–638. 2002.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Liu H, Wei JY, Li Y, Ban M, Sun Q, Wang
HJ, Zhao D, Tong PG, Wang L, Wang KJ, et al: Endothelial depletion
of Atg7 triggers astrocyte-microvascular disassociation at
blood-brain barrier. J Cell Biol. 222(e202103098)2023.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Wang Y, Wu J, Wang J, He L, Lai H, Zhang
T, Wang X and Li W: Mitochondrial oxidative stress in brain
microvascular endothelial cells: Triggering blood-brain barrier
disruption. Mitochondrion. 69:71–82. 2023.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Sun P, Zhang K, Hassan SH, Zhang X, Tang
X, Pu H, Stetler RA, Chen J and Yin KJ: Endothelium-targeted
deletion of microRNA-15a/16-1 promotes poststroke angiogenesis and
improves long-term neurological recovery. Circ Res. 126:1040–1057.
2020.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Ren C, Li N, Li S, Han R, Huang Q, Hu J,
Jin K and Ji X: Limb ischemic conditioning improved cognitive
deficits via eNOS-dependent augmentation of angiogenesis after
chronic cerebral hypoperfusion in rats. Aging Dis. 9:869–879.
2018.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Zhu HY, Hong FF and Yang SL: The roles of
nitric oxide synthase/nitric oxide pathway in the pathology of
vascular dementia and related therapeutic approaches. Int J Mol
Sci. 22(4540)2021.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Ungvari Z, Tarantini S, Kiss T, Wren JD,
Giles CB, Griffin CT, Murfee WL, Pacher P and Csiszar A:
Endothelial dysfunction and angiogenesis impairment in the ageing
vasculature. Nat Rev Cardiol. 15:555–565. 2018.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Hübner K, Cabochette P, Diéguez-Hurtado R,
Wiesner C, Wakayama Y, Grassme KS, Hubert M, Guenther S, Belting
HG, Affolter M, et al: Wnt/β-catenin signaling regulates
VE-cadherin-mediated anastomosis of brain capillaries by
counteracting S1pr1 signaling. Nat Commun. 9(4860)2018.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Swaminathan B, Youn SW, Naiche LA, Du J,
Villa SR, Metz JB, Feng H, Zhang C, Kopan R, Sims PA and Kitajewski
JK: Endothelial Notch signaling directly regulates the small GTPase
RND1 to facilitate Notch suppression of endothelial migration. Sci
Rep. 12(1655)2022.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Zhu T, Xie WJ, Wang L, Jin XB, Meng XB,
Sun GB and Sun XB: Notoginsenoside R1 activates the
NAMPT-NAD+-SIRT1 cascade to promote postischemic
angiogenesis by modulating Notch signaling. Biomed Pharmacother.
140(111693)2021.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Ma C, Zhou J, Xu X, Wang L, Qin S, Hu C,
Nie L and Tu Y: The construction of a radiation-induced brain
injury model and preliminary study on the effect of human
recombinant endostatin in treating radiation-induced brain injury.
Med Sci Monit. 25:9392–9401. 2019.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Deng Z, Zhou L, Wang Y, Liao S, Huang Y,
Shan Y, Tan S, Zeng Q, Peng L, Huang H and Lu Z: Astrocyte-derived
VEGF increases cerebral microvascular permeability under high salt
conditions. Aging (Albany NY). 12:11781–11793. 2020.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Lee WH, Warrington JP, Sonntag WE and Lee
YW: Irradiation alters MMP-2/TIMP-2 system and collagen type IV
degradation in brain. Int J Radiat Oncol Biol Phys. 82:1559–1566.
2012.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Kisler K, Nelson AR, Rege SV, Ramanathan
A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, et al:
Pericyte degeneration leads to neurovascular uncoupling and limits
oxygen supply to brain. Nat Neurosci. 20:406–416. 2017.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Kur J, Newman EA and Chan-Ling T: Cellular
and physiological mechanisms underlying blood flow regulation in
the retina and choroid in health and disease. Prog Retin Eye Res.
31:377–406. 2012.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Mae MA, He L, Nordling S, Vazquez-Liebanas
E, Nahar K, Jung B, Li X, Tan BC, Chin Foo J, Cazenave-Gassiot A,
et al: Single-cell analysis of blood-brain barrier response to
pericyte loss. Circ Res. 128:e46–e62. 2021.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Prager O, Kamintsky L, Hasam-Henderson LA,
Schoknecht K, Wuntke V, Papageorgiou I, Swolinsky J, Muoio V,
Bar-Klein G, Vazana U, et al: Seizure-induced microvascular injury
is associated with impaired neurovascular coupling and blood-brain
barrier dysfunction. Epilepsia. 60:322–336. 2019.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Stefanska A, Kenyon C, Christian HC,
Buckley C, Shaw I, Mullins JJ and Péault B: Human kidney pericytes
produce renin. Kidney Int. 90:1251–1261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Korte N, James G, You H, Hirunpattarasilp
C, Christie I, Sethi H and Attwell D: Noradrenaline released from
locus coeruleus axons contracts cerebral capillary pericytes via
α2 adrenergic receptors. J Cereb Blood Flow Metab.
43:1142–1152. 2023.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Huang H: Pericyte-endothelial interactions
in the retinal microvasculature. Int J Mol Sci.
21(7413)2020.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Dehouck MP, Tachikawa M, Hoshi Y, Omori K,
Maurage CA, Strecker G, Dehouck L, Boucau MC, Uchida Y, Gosselet F,
et al: Quantitative targeted absolute proteomics for better
characterization of an in vitro human blood-brain barrier model
derived from hematopoietic stem cells. Cells.
11(3963)2022.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Winkler EA, Sengillo JD, Bell RD, Wang J
and Zlokovic BV: Blood-spinal cord barrier pericyte reductions
contribute to increased capillary permeability. J Cereb Blood Flow
Metab. 32:1841–1852. 2012.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Armulik A, Genové G and Betsholtz C:
Pericytes: Developmental, physiological, and pathological
perspectives, problems, and promises. Dev Cell. 21:193–215.
2011.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Figueira I, Galego S, Custódio-Santos T,
Vicente R, Molnár K, Haskó J, Malhó R, Videira M, Wilhelm I,
Krizbai I and Brito MA: Picturing breast cancer brain metastasis
development to unravel molecular players and cellular crosstalk.
Cancers (Basel). 13(910)2021.PubMed/NCBI View Article : Google Scholar
|
|
182
|
Alarcon-Martinez L, Villafranca-Baughman
D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, Prat A, Drapeau
P and Di Polo A: Interpericyte tunnelling nanotubes regulate
neurovascular coupling. Nature. 585:91–95. 2020.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Lechertier T, Reynolds LE, Kim H, Pedrosa
AR, Gómez-Escudero J, Muñoz-Félix JM, Batista S, Dukinfield M,
Demircioglu F, Wong PP, et al: Pericyte FAK negatively regulates
Gas6/Axl signalling to suppress tumour angiogenesis and tumour
growth. Nat Commun. 11(2810)2020.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Wu Y, Fu J, Huang Y, Duan R, Zhang W, Wang
C, Wang S, Hu X, Zhao H, Wang L, et al: Biology and function of
pericytes in the vascular microcirculation. Animal Model Exp Med.
6:337–345. 2023.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Daneman R, Zhou L, Kebede AA and Barres
BA: Pericytes are required for blood-brain barrier integrity during
embryogenesis. Nature. 468:562–566. 2010.PubMed/NCBI View Article : Google Scholar
|
|
186
|
Bell RD, Winkler EA, Sagare AP, Singh I,
LaRue B, Deane R and Zlokovic BV: Pericytes control key
neurovascular functions and neuronal phenotype in the adult brain
and during brain aging. Neuron. 68:409–427. 2010.PubMed/NCBI View Article : Google Scholar
|
|
187
|
Abramsson A, Kurup S, Busse M, Yamada S,
Lindblom P, Schallmeiner E, Stenzel D, Sauvaget D, Ledin J,
Ringvall M, et al: Defective N-sulfation of heparan sulfate
proteoglycans limits PDGF-BB binding and pericyte recruitment in
vascular development. Genes Dev. 21:316–331. 2007.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Weidenfeller C, Svendsen CN and Shusta EV:
Differentiating embryonic neural progenitor cells induce
blood-brain barrier properties. J Neurochem. 101:555–565.
2007.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Huang Y, Chen S, Luo Y and Han Z:
Crosstalk between inflammation and the BBB in stroke. Curr
Neuropharmacol. 18:1227–1236. 2020.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Thurgur H and Pinteaux E: Microglia in the
neurovascular unit: Blood-brain barrier-microglia interactions
after central nervous system disorders. Neuroscience. 405:55–67.
2019.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Armulik A, Genové G, Mäe M, Nisancioglu
MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter
K, et al: Pericytes regulate the blood-brain barrier. Nature.
468:557–561. 2010.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Hellström M, Kalén M, Lindahl P, Abramsson
A and Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of
vascular smooth muscle cells and pericytes during embryonic blood
vessel formation in the mouse. Development. 126:3047–3055.
1999.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Li J, Li M, Ge Y, Chen J, Ma J, Wang C,
Sun M, Wang L, Yao S and Yao C: β-amyloid protein induces
mitophagy-dependent ferroptosis through the CD36/PINK/PARKIN
pathway leading to blood-brain barrier destruction in Alzheimer's
disease. Cell Biosci. 12(69)2022.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Ma Q, Zhao Z, Sagare AP, Wu Y, Wang M,
Owens NC, Verghese PB, Herz J, Holtzman DM and Zlokovic BV:
Blood-brain barrier-associated pericytes internalize and clear
aggregated amyloid-β42 by LRP1-dependent apolipoprotein E
isoform-specific mechanism. Mol Neurodegener. 13(57)2018.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Bongarzone S, Savickas V, Luzi F and Gee
AD: Targeting the receptor for advanced glycation endproducts
(RAGE): A medicinal chemistry perspective. J Med Chem.
60:7213–7232. 2017.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Bell-Temin H, Culver-Cochran AE, Chaput D,
Carlson CM, Kuehl M, Burkhardt BR, Bickford PC, Liu B and Stevens
SM Jr: Novel molecular insights into classical and alternative
activation states of microglia as revealed by stable isotope
labeling by amino acids in cell culture (SILAC)-based proteomics.
Mol Cell Proteomics. 14:3173–3184. 2015.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Becerra-Calixto A and Cardona-Gómez GP:
The role of astrocytes in neuroprotection after brain stroke:
Potential in cell therapy. Front Mol Neurosci.
10(88)2017.PubMed/NCBI View Article : Google Scholar
|