Introduction
Myelin oligodendrocyte glycoprotein-associated
disease (MOGAD) is a rare autoimmune inflammatory demyelinating
disorder, distinct from multiple sclerosis (MS) and aquaporin-4
(AQP4)-seropositive neuromyelitis optica spectrum disorder (NMOSD).
MOGAD can affect individuals of any age, with the highest incidence
in children, and an equal distribution between males and females
(1). The incidence of MOGAD ranges
from 1.6 to 3.4 cases per million individuals per year, with an
estimated prevalence of 20 cases per million (2). Clinical manifestations are diverse and
include optic neuritis, transverse myelitis, acute disseminated
encephalomyelitis, and encephalitis predominantly involving the
cortex, brainstem and cerebellum (2). Approximately one-half of the patients
experience a monophasic disease course, but relapses are also
possible. Serum immunoglobulin G (IgG) antibodies against MOG, a
protein expressed on the surface of central nervous system (CNS)
myelin sheaths, are considered the hallmark of the disease. A
prodromal infectious disease is considered to trigger an autoimmune
response against the CNS through mechanisms such as molecular
mimicry or epitope spreading (1).
The gold-standard treatment of acute phase attacks is based on
high-dose glucοcοrtiсοiԁѕ. Plasma exchange or intravenous immune
globulin are suggested for refractory cases or incomplete
responses. Immunosuppressive therapy is typically reserved for
patients with relapsing disease (3). Autoimmune diseases are recognized as a
risk factor for cerebral venous thrombosis (CVT), which is an
uncommon cause of stroke (4,5). To
date, few cases of CVT have been reported in the context of MS
(6), and only three cases in
association with MOGAD (6,7). The present study reports a case of CVT
occurring in an adult with MOGAD.
Case report
A healthy, 37-year-old man who was a non-smoker
presented to the Neurology Unit of ‘Bianchi-Melacrino-Morelli’
Hospital (Reggio Calabria, Italy) with a 2-week history of
asthenia, gait difficulties, numbness of the lower limbs and
urinary urgency. There was no history of recent vaccination, fever
or systemic infections. Neurological examination revealed sensory
ataxia, brisk tendon reflexes in all four limbs and hypoesthesia
extending from the thoracic region (corresponding to the C5 sensory
level) to the lower limbs. An urgent spinal magnetic resonance
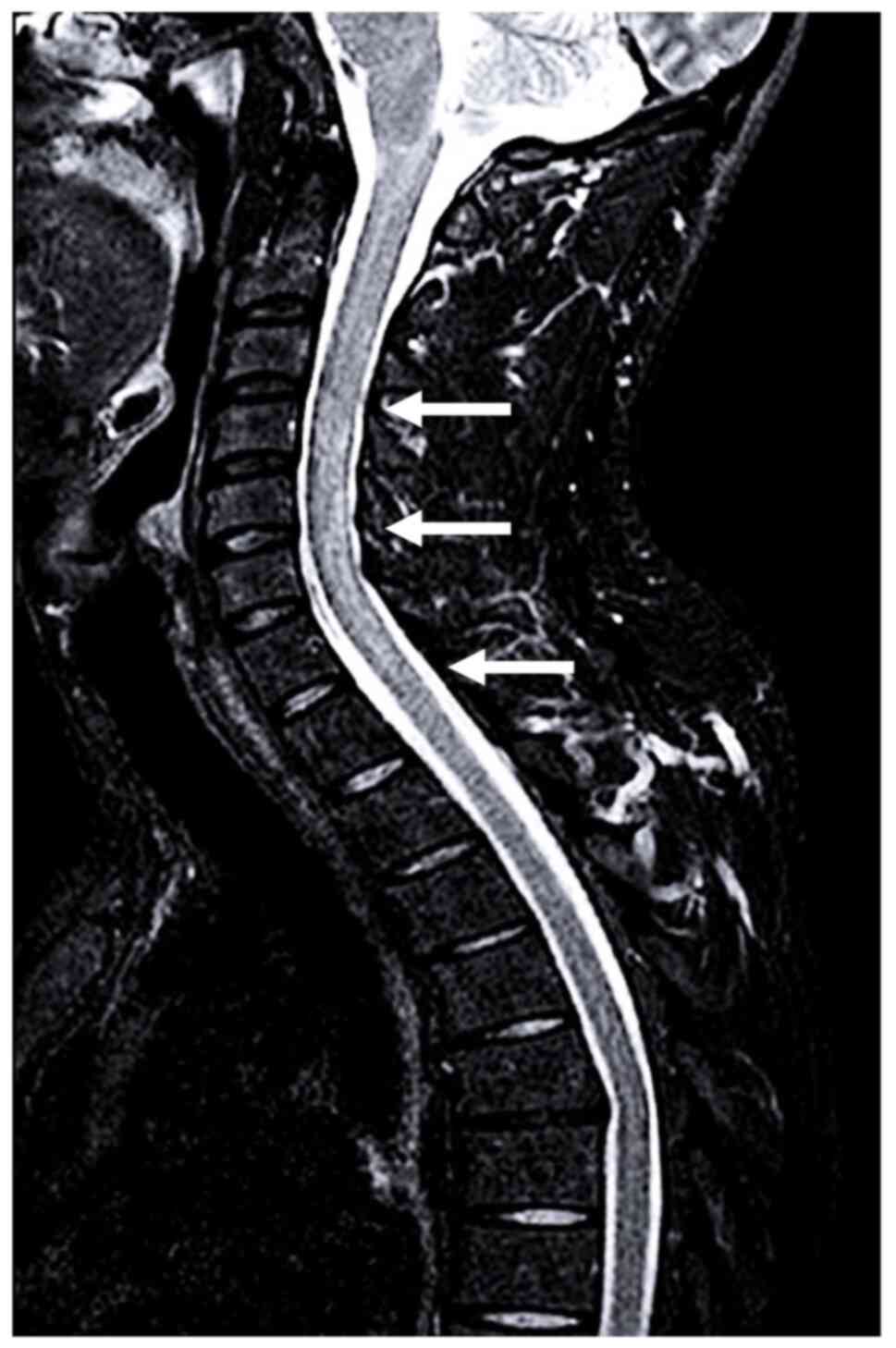
imaging (MRI) scan (Fig. 1) showed
a T2- and short-TI inversion recovery-hyperintense, continuous cord
lesion from C2 to T1, without contrast enhancement (CE), consistent
with longitudinally extensive myelitis (LETM). Additional multiple
smaller dorsal cord hyperintensities showed CE, whereas brain and
orbital MRI findings were normal. Blood tests revealed mild
neutrophilic leukocytosis. A broad infectious and autoimmune
work-up, including a COVID-19 test and antinuclear antibodies, was
unremarkable. Cerebrospinal fluid (CSF) analysis of a specimen from
a lumbar puncture (LP) revealed elevated protein levels (100 mg/dl;
normal value, <43 mg/dl), with normal leukocyte count, CSF/serum
albumin ratio and glucose levels. The CSF-Film Array assay was
performed by the Department of Pathology of
‘Bianchi-Melacrino-Morelli’ Hospital and was negative for common
infectious agents. No intrathecal-restricted synthesis of IgG
oligoclonal bands was detected in the CSF. Serum anti-MOG
antibodies, tested by the Department of Pathology of
‘Bianchi-Melacrino-Morelli’ Hospital using a fixed cell-based
assay, were positive at a titer of 1:100, whereas antibodies
against AQP4 in both CSF and serum were absent.
A diagnosis of MOGAD was made according to the
International MOGAD Panel criteria (1), based on a core clinical demyelinating
event (myelitis) with supporting MRI features of LETM, positive
MOG-IgG antibodies and seronegative AQP4-IgG, after excluding
alternative diagnoses. The patient underwent high-dose intravenous
methylprednisolone therapy (1,000 mg per day for 5 days), followed
by oral prednisone (50 mg daily). The patient fully recovered and
was discharged after 10 days with a prescribed tapering of
prednisone over 1 month. However, at 3 days post-discharge, the
patient returned to the Emergency Department of
‘Bianchi-Melacrino-Morelli’ Hospital due to a focal to bilateral
epileptic seizure, preceded by 2 days of continuous right
frontotemporal headaches. Neurological examination revealed mild
left hemiparesis and drowsiness, with a National Institutes of
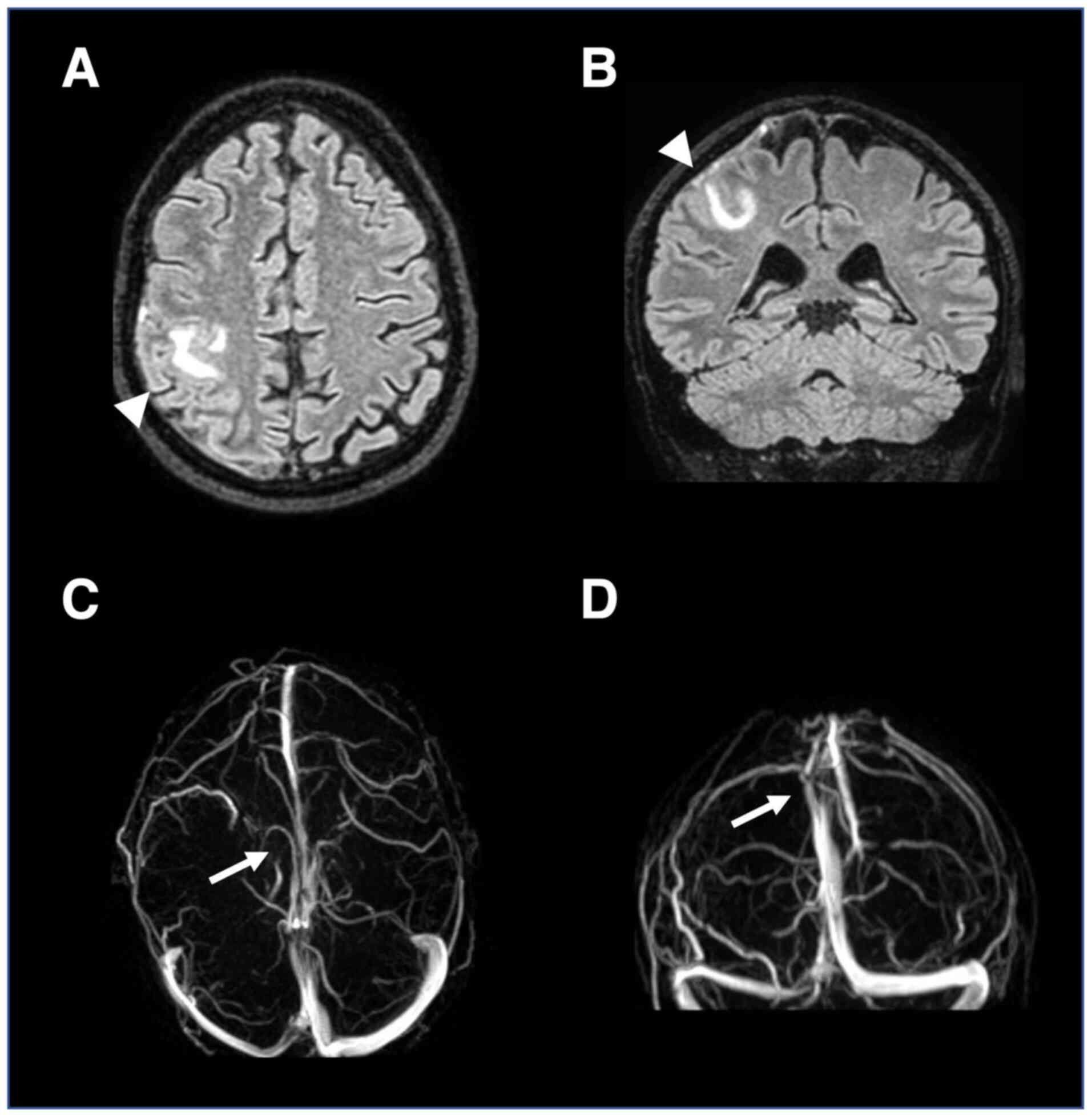
Health Stroke Scale (NIHSS) score of 4(7). An urgent brain computed tomography
scan showed a right cortical parietal hypodense lesion. Brain MRI
revealed a fluid-attenuated inversion recovery-hyperintense right
frontoparietal lesion, and an MRI angiogram showed absence of flow
in the right superficial cortical veins and superior sagittal
sinus, confirming CVT (Fig. 2).
Blood work-up for thrombophilia, including
antinuclear antibodies, factor V Leiden and prothrombin mutation,
and deficiencies in antithrombin, protein C and protein S, was
unremarkable. Tests for COVID-19 and serum autoantibodies
associated with antiphospholipid syndrome, systemic lupus
erythematosus and Behçet's disease were also negative. Paroxysmal
nocturnal hemoglobinuria was excluded. The patient was treated with
subcutaneous low molecular weight heparin (4,000 IU,
subcutaneously, twice daily) for 10 days and lacosamide (200 mg per
day for 3 months) to prevent seizure recurrence. The patient
achieved a complete recovery within 30 days and was discharged with
50 mg/day prednisone, which was slowly tapered and discontinued
after 3 months. The patient's clinical condition remained stable
over the following year, with unchanged MRI results at the 6- and
12-month follow-ups.
Discussion
The present report describes the rare co-occurrence
of CVT in a patient diagnosed with MOGAD. The patient developed CVT
in the context of an acute MOGAD attack shortly after undergoing a
LP and receiving intravenous corticosteroids. Although the
coincidental coexistence of MOGAD and CVT in the same patient
should be considered, exploring the possible association is of
interest due to the inflammatory nature of both conditions. The
association between CVT and demyelinating syndromes (DS) has been
infrequently reported in the literature.
A review of the literature was performed using the
terms ‘MOGAD’ OR ‘Myelin oligodendrocyte glycoprotein-associated
disease’ OR ‘Multiple Sclerosis’ OR ‘MS’ OR ‘anti-MOG’ OR
‘neuromyelitis optica spectrum disorder’ OR ‘NMOSD’ OR
‘demyelinating disorder’, in various combinations with ‘cerebral
venous thrombosis’ OR ‘cerebral venous sinus thrombosis’, in PubMed
(https://pubmed.ncbi.nlm.nih.gov/) and
Google Scholar (https://scholar.google.com/) up to October 2024.
Primary studies, case reports and case series were included, and
English was the only language selected. A total of 19 studies were
identified, nearly all of which were case reports, reporting a
total of 31 patients with CVT associated with MS (26 patients),
NMOSD (2 patients) or MOGAD (3 patients) (Table I) (6-25).
Only 9 patients exhibited the most common risk factors for CVT
(oral contraceptives in 8 cases and inherited prothrombotic
disorders in 1 case) (6,10,12,13,18,19,21).
In ~84% of cases, procedures such as LP and high-dose
corticosteroid administration were reported, highlighting their
role as predisposing factors for CVT development (6,19,21,12).
 | Table IReview of the literature for reported
cerebral venous thrombosis cases in patients with demyelinating
disorders. |
Table I
Review of the literature for reported
cerebral venous thrombosis cases in patients with demyelinating
disorders.
| First author,
year | Study design | Patients, n | Sex | Age, years | DS type | Risk factors for
CVT | CVT symptoms | CVT outcome | (Refs.) |
|---|
| Malanga and Gangemi,
1994 | Case report | 1 | F | 35 | MS | Oral
contraceptive | Seizure | Recovery | (10) |
| Al Bunyan and
Ogunniyi, 1997 | Case report | 1 | F | 32 | MS | Bed confinement | Asymptomatic | Recovery | (11) |
| Aidi et al,
1999 | Case report | 2 | F | 30, 36 | MS | Oral contraceptive
(n=1), LP (n=2), high-dose i.v. corticosteroid (n=2) | Headache,
diplopia | Recovery | (12) |
| Albucher et
al, 1999 | Case series | 3 | 2 F, 1 M | 28, 45, 38 | MS | Oral contraceptive
(n=1), LP (n=2), high-dose i.v. corticosteroid (n=3) | Headache, seizure,
focal motor deficit | Recovery | (13) |
| Städler et al,
2000 | Case report | 2 | n.a. | n.a. | MS | LP, high-dose i.v.
corticosteroid (n=2) | n.a. | Death (n=1), recovery
(n=1) | (14) |
| Gunal et al,
2002 | Case report | 1 | F | 39 | MS | LP, high-dose i.v.
corticosteroid | Headache, seizures,
hemianopia | Recovery | (15) |
| Mouraux et al,
2002 | Case report | 1 | F | 35 | MS | LP | Headache, seizures,
hemiparesis | Recovery | (16) |
| Kadayifçilar et
al, 2003 | Case report | 1 | F | 44 | MS | Oral contraceptive,
high-dose i.v. corticosteroid | Headache, hemianopia
and hemiparesis | n.a. | (17) |
| Stolz et al,
2003 | Observational
study | 6 | n.a. | n.a. | MS (4), NMOSD
(2) | High-dose i.v.
corticosteroid (n=6), LP (n=3) oral contraceptive (n=3), smoking
(n=4) | Headaches (5),
seizures (4), focal motor deficits (3) | n.a. | (18) |
| Vandenberghe et
al, 2003 | Case series | 3 | 2 F, 1 M | 40, 23, 49 | MS | No risk factor (n=2);
oral contraceptive (n=2), high-dose i.v. corticosteroid (n=2), LP
(n=2) | Seizure, headache,
focal motor deficit | n.a. | (6) |
| Maurelli et
al, 2005 | Case report | 1 | F | 48 | MS | LP, high-dose i.v.
corticosteroid | Headache,
tetraparesis, coma, seizure | Recovery | (19) |
| Kalanie et
al, 2011 | Prospective
study | 2 | F | 35, 39 | MS | High-dose i.v.
corticosteroid (n=2) | n.a. | n.a. | (20) |
| Presicci et
al, 2013 | Case report | 1 | F | 13 | MS | Thrombocytosis,
thrombophilia, LP, high-dose i.v. corticosteroid | Headache,
seizure | Improvement | (21) |
| Gazioglu et
al, 2013 | Case report | 1 | F | 32 | MS | High-dose i.v.
corticosteroid | Headache | Recovery | (22) |
| Soto-Insuga et
al, 2016 | Case report | 1 | F | 6 | MOGAD | Infection
(otitis) | Headache,
diplopia | Recovery | (8) |
| Gasparini et
al, 2020 | Case report | 1 | M | 40 | MS | Alemtuzumab | Headache | Recovery | (23) |
| Fontana et
al, 2021 | Case report | 1 | M | 11 | MOGAD | LP, high-dose i.v.
corticosteroid | Headache, facial
tics | Recovery | (9) |
| Zhu et al,
2023 | Case report | 1 | F | 19 | MS | High-dose i.v.
corticosteroid | Headache | Improvement | (24) |
| Virupakshaiah et
al, 2024 | Case report | 1 | M | 35 | MOGAD | High-dose i.v.
corticosteroid | Focal motor
deficit, speech distur- bance, impaired consciousness | Improvement | (25) |
Dural puncture is a rare but well-known risk factor
for CVT. While the exact mechanism remains unclear, it is likely
associated with intracranial hypotension, leading to venous
dilation, displacement of intracranial vascular structures, stasis
and congestion, thereby promoting a thrombotic process (12). A potential procoagulant effect of
high-dose steroids in CVT development has also been suggested. This
is primarily attributable to vascular endothelial cell injury and a
hypercoagulable state, characterized by increased prothrombotic
factors and impaired fibrinolytic activity, similar to what is
observed in Cushing's syndrome (22,24,12).
Accordingly, the administration of high-dose intravenous
methylprednisolone and LP might have contributed to CVT development
in the present case. However, the occurrence of CVT is reported in
only a small percentage of individuals exposed to LP or
corticosteroids, suggesting that additional risk factors are likely
involved.
Moreover, cases of DS-associated CVT occurring
without corticosteroid exposure or other recognized risk factors
have been described (6,23). Given the shared inflammatory origin
of both conditions, a thrombotic-inflammatory pathogenic mechanism
between MOGAD and CVT in susceptible individuals is plausible
(8,24). The autoimmune and inflammatory
pathways implicated in DS may play a role in CVT pathogenesis
(5,6,12). The
interplay between inflammation, demyelination and coagulation,
however, remains poorly understood. The presence of lymphocytic
infiltration surrounding veins in DS supports evidence of vascular
inflammation (6). Immune-mediated
and inflammatory processes in DS may lead to endothelial
dysfunction. Cytokines released during inflammation, such as
IL-6(25), could activate the
clotting cascade, increasing the likelihood of CVT. Additionally,
the severe and often rapid progression of MOGAD could result in
brain swelling or lesions that compress adjacent venous sinuses,
impairing blood flow and predisposing to thrombosis (25). Vascular damage, coupled with
inflammation-induced hypercoagulability, may contribute to CVT
development (5).
Despite these observations, the pathogenic
association between these two conditions remains largely unknown.
To date, there is insufficient evidence to confirm that MOGAD leads
to CVT, and a fortuitous association cannot be excluded. Although
CVT is rare in DS, the present case underscores the importance of
closely monitoring patients for new or worsening headaches and the
onset of epileptic seizures. Additionally, patients with MOGAD and
identified pro-thrombotic conditions may benefit from careful
observation and preventive measures, such as adequate hydration, to
reduce CVT risk.
Only 1 year of follow-up was performed in the
present study, which limits our understanding of long-term health
in patients with MOGAD after CVT, as well as the assessment of
disease progression and management strategies.
In conclusion, the current study presents a rare
case of MOGAD with CVT in an adult patient. To date, the causal
association between MOGAD and CVT lacks compelling evidence.
Corticosteroid therapy and LP represent potential mediators or
confounding factors, but the possible role of anti-MOG antibodies
and the inflammatory pathways occurring in DS should be better
ascertained in this uncommon condition. Further studies on
pathophysiological mechanisms are required to establish whether a
direct causal association exists.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by #NEXTGENERATIONEU and funded
by the Ministry of University and Research, National Recovery and
Resilience Plan, project MNESYS (project no. PE0000006)-A
Multiscale integrated approach to the study of the nervous system
in health and disease (DN. 1553; 11.10.2022).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
OM and AP were responsible for study concept and
design. Data curation, collection, analysis and interpretation was
performed by OM, AP, RC, DT, AB and EF. OM, AP and SG drafted the
manuscript. UA and SG advised on patient treatment, revised the
manuscript critically for important intellectual content and gave
the final approval of the version to be published. OM and AP
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was performed following the ethical
standards laid down in the 1964 Declaration of Helsinki and its
later amendments. Written informed consent was obtained from the
patient.
Patient consent for publication
The patient provided written informed consent
concerning the publication of the case report and accompanying
patient diagnostic images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banwell B, Bennett JL, Marignier R, Kim
HJ, Brilot F, Flanagan EP, Ramanathan S, Waters P, Tenembaum S,
Graves JS, et al: Diagnosis of myelin oligodendrocyte glycoprotein
antibody-associated disease: International MOGAD Panel proposed
criteria. Lancet Neurol. 22:268–282. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al-Ani A, Chen JJ and Costello F: Myelin
oligodendrocyte glycoprotein antibody-associated disease (MOGAD):
Current understanding and challenges. J Neurol. 270:4132–4150.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Häußler V, Trebst C, Engels D, Pellkofer
H, Havla J, Duchow A, Schindler P, Schwake C, Pakeerathan T,
Fischer K, et al: Real-world multicentre cohort study on choices
and effectiveness of immunotherapies in NMOSD and MOGAD. J Neurol
Neurosurg Psychiatry: jnnp-2024-334764, 2024.
|
|
4
|
Silvis SM, de Sousa DA, Ferro JM and
Coutinho JM: Cerebral venous thrombosis. Nat Rev Neurol.
13:555–565. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Koudriavtseva T, Renna R, Plantone D and
Mainero C: Demyelinating and thrombotic diseases of the central
nervous system: Common pathogenic and triggering factors. Front
Neurol. 6(63)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vandenberghe N, Debouverie M, Anxionnat R,
Clavelou P, Bouly S and Weber M: Cerebral venous thrombosis in four
patients with multiple sclerosis. Eur J Neurol. 10:63–66.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kwah LK and Diong J: National institutes
of health stroke scale (NIHSS). J Physiother. 60(61)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Soto-Insuga V, Salvador TA, Martinez LC,
Losada del Pozo R, Moreno MR, Gonzalez CO and Catalanet JR:
Anti-myelin oligodendrocyte glycoprotein autoantibodies in optic
neuritis and venous sinus thrombosis in a girl. J Pediatr Neurol
Med. 1:1–3. 2016.
|
|
9
|
Fontana A, Greco F, Smilari P, Praticò AD,
Fiumara A, Ruggieri M and Pavone P: Anti-MOG antibody syndrome and
cerebral sinovenous thrombosis: A Cause-effect hypothesis. J
Pediatric Neurol. 19:127–131. 2021.
|
|
10
|
Malanga GA and Gangemi E: Intracranial
venous thrombosis in a patient with multiple sclerosis. A case
report and review of contraceptive alternatives in patients with
disabilities. Am J Phys Med Rehabil. 73:283–285. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al Bunyan M and Ogunniyi A: Incidental
cerebral venous thrombosis in a patient with multiple sclerosis. J
Neurol Sci. 149:191–194. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Aidi S, Chaunu MP, Biousse V and Bousser
MG: Changing pattern of headache pointing to cerebral venous
thrombosis after lumbar puncture and intravenous high-dose
corticosteroids. Headache. 39:559–564. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Albucher JF, Vuillemin-Azaïs C, Manelfe C,
Clanet M, Guiraud-Chaumeil B and Chollet F: Cerebral
thrombophlebitis in three patients with probable multiple
sclerosis. Role of lumbar puncture or intravenous corticosteroid
treatment. Cerebrovasc Dis. 9:298–303. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Städler C, Vuadens P, Dewarrat A, Janzer
R, Uske A and Bogousslavsky J: Cerebral venous thrombosis after
lumbar puncture and intravenous steroids in two patients with
multiple sclerosis. Rev Neurol (Paris). 156:155–159.
2000.PubMed/NCBI
|
|
15
|
Gunal DI, Afsar N, Tuncer N and Aktan S: A
case of multiple sclerosis with cerebral venous thrombosis: The
role of lumbar puncture and High-dose steroids. Eur Neurol.
47:57–58. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mouraux A, Gille M, Dorban S and Peeters
A: Cortical venous thrombosis after lumbar puncture. J Neurol.
249:1313–1315. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kadayifçilar S, Gedik S, Eldem B, Balaban
H and Kansu T: Panuveitis associated with multiple sclerosis
complicated by cerebral venous thrombosis. Ocul Immunol Inflamm.
12:153–157. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stolz E, Klötzsch C, Schlachetzki F and
Rahimi A: High-dose corticosteroid treatment is associated with an
increased risk of developing cerebral venous thrombosis. Eur
Neurol. 49:247–248. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maurelli M, Bergamaschi R, Candeloro E,
Todeschini A and Micieli G: Cerebral venous thrombosis and
demyelinating diseases: Report of a case in a clinically isolated
syndrome suggestive of multiple sclerosis onset and review of the
literature. Mult Scler. 11:242–244. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kalanie H, Harandi AA, Alidaei S, Heidari
D, Shahbeigi S and Ghorbani M: Venous thrombosis in multiple
sclerosis patients after High-dose intravenous methylprednisolone:
The preventive effect of enoxaparin. Thrombosis.
2011(785459)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Presicci A, Garofoli V, Simone M, Campa
MG, Lamanna AL and Margari L: Cerebral venous thrombosis after
lumbar puncture and intravenous high dose corticosteroids: A case
report of a childhood multiple sclerosis. Brain Dev. 35:602–605.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gazioglu S, Solmaz D and Boz C: Cerebral
venous thrombosis after high dose steroid in multiple sclerosis: A
case report. Hippokratia. 17:88–90. 2013.PubMed/NCBI
|
|
23
|
Gasparini S, Russo M, Dattola V, Ferlazzo
E and Aguglia U: Cryptogenic cerebral venous thrombosis in a
Multiple-sclerosis-patient treated with Alemtuzumab. Mult Scler
Relat Disord. 44(102246)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu M, Cui W, Huang W, Liu Z, Xu Z and
Huang H: Cerebral venous thrombosis after High-dose steroid in
patient with multiple sclerosis: A case report. Medicine
(Baltimore). 102(e34142)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Virupakshaiah A, Moseley CE, Elicegui S,
Gerwitz LM, Spencer CM, George E, Shah M, Cree BAC, Waubant E and
Zamvil SS: Life-threatening MOG Antibody-associated hemorrhagic
ADEM with elevated CSF IL-6. Neurol Neuroimmunol Neuroinflamm.
11(e200243)2024.PubMed/NCBI View Article : Google Scholar
|