Introduction
Cervical cancer is the fourth most common cancer in
women worldwide, and most cases occur in low- and middle-income
countries (1). Prophylactic
vaccination significantly reduces the risk of cervical cancer
development, and regular screening through Pap test and human
papillomavirus detection enable early diagnosis by detecting
premalignant lesions or early stages of cervical cancer (2). In México, cervical cancer is the
second leading cause of cancer mortality in women, which suggests
that, despite the available screening methods, cervical cancer
remains a public health problem (3). There are three known histological
types of cervical cancer: Squamous cell carcinoma (SCC),
adenocarcinoma (AC) and adenosquamous carcinoma (ASC), with SCC and
AC being the most prevalent (4). To
determine the prognostic outcome and treatment schemes, oncologists
consider the clinical stage of cervical cancer, which is determined
according to the criteria of the International Federation of
Gynaecology and Obstetrics (FIGO) (5). For patients with early stages of
cervical cancer, the treatment is surgery, while for patients with
locally advanced cervical cancer, the treatment is concomitant
chemoradiotherapy (2). For patients
with recurrent cervical cancer, different therapeutic strategies
have been developed, including immune checkpoint inhibitors and DNA
damage repair inhibitors (6). The
anti-programmed death 1 (PD-1) monoclonal antibody pembrolizumab
was the first immunotherapy strategy approved by the US Food and
Drug Administration for recurrent or metastatic cervical cancer
(6).
The immune system plays crucial roles in both cancer
control and development. It has been demonstrated that immune
checkpoint proteins that are involved in the negative regulation of
the immune response are linked to evasion mechanisms in cancer
(7). Exhausted T cells that are
unable to eliminate cancer cells overexpress certain immune
checkpoints, such as PD-1, cytotoxic T-lymphocyte-associated
antigen-4 (CTLA-4), lymphocyte activation gene-3 (LAG-3) and T-cell
immunoglobulin and mucin domain-containing protein-3 (Tim-3)
(7).
Tim-3 is normally expressed on the membrane of
several immune cells, including T cells. The binding of Tim-3 to
its ligand, galectin-9, results in the suppression of T-cell
responses and may lead to CD8+ T-cell exhaustion, which
impairs the cytotoxic activity responsible for cancer cell
eradication (8,9). Previous studies have shown that
membrane Tim-3 expression is significantly greater in the
peripheral CD4+ and CD8+ T cells of patients
with cervical cancer compared with the controls, with the highest
Tim-3 expression found in patients with stage III-IV cervical
cancer than in those with stage I-II (10). Compared with premalignant lesions
and cervicitis, membrane Tim-3 is also overexpressed in cervical
cancer. In addition, the higher expression of membrane Tim-3 was
associated with a shorter overall survival in patients with
cervical cancer (11). Therefore,
the immune checkpoint Tim-3/galectin-9 has been proposed by certain
authors as a potential immunotherapy target for patients with
cervical cancer (10-12).
Soluble immune checkpoints (sICs) have been
identified in the serum, and these soluble forms of some membrane
proteins can be produced by alternative splicing, cleavage with
proteases or secretion by shedding the cell membrane. Increased
levels of sICs have been reported in different cancer types
(13). In most cases, the increased
expression of sICs has been found to be associated with disease
severity and poor overall survival (14).
Therefore, sICs could be potential biomarkers
associated with prognosis and treatment response (13). The soluble form of Tim-3 (sTim-3)
may be produced in different ways, such as alternative splicing,
cleavage from the cell surface by diverse enzymes, including
disintegrins and matrix metalloproteases, and passive release from
apoptotic cells (15-17).
Increased levels of sTim-3 have been reported in different cancer
types, such as thyroid carcinoma (7), hepatocarcinoma (18), oral SCC (19) and non-small cell lung cancer (NSCLC)
(20). Higher levels of sTim-3 were
detected in patients with thyroid carcinoma with lymph node
metastasis (7). In contrast to most
types of cancer, patients with breast cancer presented lower levels
of sTim-3 compared with healthy individuals (21). This indicated that sTim-3 levels are
regulated in a tissue-dependent manner.
To the best of our knowledge, the role of sTim-3 in
cervical cancer has not been assessed; the only information
reported for sTim-3 and cervical cancer corresponds to a study
conducted in patients with locally advanced cervical cancer. In
this study, the effect of concurrent chemoradiotherapy in the
levels of different sICs including sTim-3 were evaluated, and an
increase in sTim-3 levels was reported following treatment,
emphasizing the importance of designing improved treatment
strategies when considering their immunomodulatory effects.
Nevertheless, the clinical implications of this increase were not
analysed (22).
In a previous study, the presence of galectin-9 was
evaluated in the serum of patients with cervical cancer, and it was
reported that the serum galectin-9 level was higher in patients
with cervical cancer compared with the control group, which
increased with advanced clinical stages (23).
The serum levels of Tim-3 need to be explored in
patients with cervical cancer to assess whether they are associated
with clinicopathological characteristics or clinical outcomes and
to evaluate its potential as a diagnostic or prognostic biomarker.
Furthermore, the association between the serum levels of sTim-3 and
galectin-9 could provide information about their possible role in
immune regulation and treatment response.
Materials and methods
Patients and study protocol
A total of 108 patients with cervical cancer and 40
women with a normal cytology report were included in the present
study.
Inclusion and exclusion criteria,
diagnostic criteria, and description of control subjects
The patients with cervical cancer included in the
study were from the High Specialty Medical Unit, Mexican Institute
of Social Security (IMSS), in Puebla City, Mexico, and the study
was carried out between November 14, 2017 and October 18, 2023,
(except for the period from November 14, 2018 to December 17, 2018,
in which the project did not have the authorized extension for
follow up) in accordance with the Declaration of Helsinki. The
ethical regulations were approved by the National Commission for
Scientific Research, under the registration number R-2017-785-119.
The results presented in the present study correspond to the
protocol approved by the Local Committee of Health Research 2106 of
the IMSS, under the registration number R-2023-2106-004, in which
the use of data and serum samples previously collected, was
authorized.
The cervical cancer group included women first
diagnosed according to the Bethesda System (24), and the diagnosis was confirmed
through the analysis of a biopsy by a pathologist. Women who were
previously diagnosed with another cancer type, had acute
infections, were pregnant or had autoimmune diseases were excluded
from the study. Patients with cervical cancer were staged according
to the FIGO criteria (5). The serum
samples from patients with cervical cancer were obtained at the
beginning of the intervention prior to the commencement of any
treatment.
Clinicopathological information was obtained from
the clinical records of the patients. The clinical outcomes
considered in the present study were complete remission or death
during the follow-up period following medical intervention.
Women included in the control group were negative
for premalignant lesions or cervical cancer according to their
cytology report. Women who were pregnant, had acute infections or
had autoimmune diseases were excluded. All the participants were
informed about the study and signed informed consent.
Serum sTim-3 concentration
Venous blood (5 ml) was collected from each
participant and centrifuged at 2,000 x g for 10 min. The serum was
stored at -20˚C until use.
The serum concentration of sTim-3 was determined
using a Quantikine Human Tim-3 enzyme-linked immunosorbent assay
(ELISA) kit (cat. no. DTIM30; R&D Systems Inc.), according to
the manufacturer's instructions. Serum samples were diluted at a
1:5 ratio, and each dilution was performed in duplicate.
The serum galectin-9 concentration was determined
using a Quantikine Human Gal-9 ELISA kit (cat. no. DGAL90; R&D
Systems, Inc.), according to the manufacturer's instructions. Serum
samples were diluted at a 1:2 ratio, and each dilution was
performed in duplicate (23).
The microplates were read at absorbances of 450 and
540 nm using a Synergy 4 microplate reader (BioTek Instruments,
Inc.). Wavelength corrections were performed by subtracting the
reading at 540 nm from the reading at 450 nm. For the analysis, an
average of the duplicates of each sample was obtained, and the
serum sTim-3 and galectin-9 concentrations were determined by
comparison against their respective standard curves, and the values
were multiplied by the dilution factor.
Statistical analysis
The Mann-Whitney test was performed to compare the
serum sTim-3 concentration between the control and cervical cancer
groups, between the control and cervical cancer groups by the age
range and between clinical outcomes. The Kruskal-Wallis test
followed by Dunn's test was performed to compare sTim-3
concentration by age ranges in cervical cancer groups and in the
different clinicopathological characteristics. P≤0.05 was
considered to indicate a statistically significant difference. In
addition, correlation analysis was performed for sTim-3 and
galectin-9 concentrations using Spearman's rank correlation
coefficient. A normality test was performed on the raw data to
evaluate its distribution, and the corresponding test was applied.
Statistical analysis was performed using Prism software version 10
(GraphPad software, Inc.). Receiver operating characteristic (ROC)
curve analysis was performed following the method by DeLong et
al (25) using MedCalc software
version 22.030 (MedCalc Software bvba).
Results
Characteristics of the study
subjects
Serum samples from 108 patients with a diagnosis of
cervical cancer and 40 women with a normal cytology report were
included in the present study. The patients with cervical cancer
had a mean age of 52.3±13.4 (26-87) years, and the control group
had a mean age of 31.7±10.2 (21-60) years (Mann-Whitney,
P<0.0001). The clinicopathological characteristics of the
patients with cervical cancer are described in Table I.
 | Table ISerum levels of sTim-3 and
clinicopathological characteristics of patients with cervical
cancer. |
Table I
Serum levels of sTim-3 and
clinicopathological characteristics of patients with cervical
cancer.
| A,
Clinicopathological characteristics analysed by Kruskal Wallis and
Dunn't tests |
|---|
| Clinicopathological
characteristics | n | Median
concentration (range) | P-value
(Kruskal-Wallis test) | P-value (Dunn's
test) |
|---|
| Histological
type | | | | |
|
SCC | 83 | 3.04
(0.84-12.70) | 0.6179 | SCC vs. AC
>0.9999 |
|
AC | 11 | 3.48
(1.32-10.70) | | SCC vs. AS
>0.9999 |
|
ASC | 6 | 2.71
(0.96-5.82) | | AS vs. AC
>0.9999 |
| Differentiation
grade | | | | |
|
G1 | 4 | 2.56
(1.35-4.14) | 0.6445 | G1 vs. G2
>0.9999 |
|
G2 | 56 | 3.10
(0.94-12.55) | | G1 vs. G3
>0.9999 |
|
G3 | 25 | 3.27
(.84-12.24) | | G2 vs. G3
>0.9999 |
| FIGO stage | | | | |
|
I | 8 | 2.16
(0.94-6.93) | 0.0371a | I vs. II
>0.9999 |
|
II | 27 | 2.96
(0.84-12.14) | | I vs. III
0.7395 |
|
III | 40 | 3.29
(1.17-10.26) | | I vs. IV
0.0338a |
|
IV | 13 | 5.07
(1.23-12.70) | | II vs. III
>0.9999 |
| | | | | II vs. IV
0.1784 |
| | | | | III vs. IV
0.2562 |
| B,
Clinicopathological characteristics assessed by Mann-Whitney U
test |
| Clinicopathological
characteristics | n | Median
concentration (range) | P-value
(Mann-Whitney U test) | N/A |
| Keratinization | | | | |
|
NKSCC | 46 | 3.01
(0.94-12.14) | 0.9495 | N/A |
|
KSCC | 21 | 3.26
(0.84-12.70) | | |
| Clinical
outcome | | | | |
|
Total
remission of disease | 15 | 2.8
(0.98-4.72) | 0.1583 | N/A |
|
Deceased | 14 | 3.7
(1.17-10.70) | | |
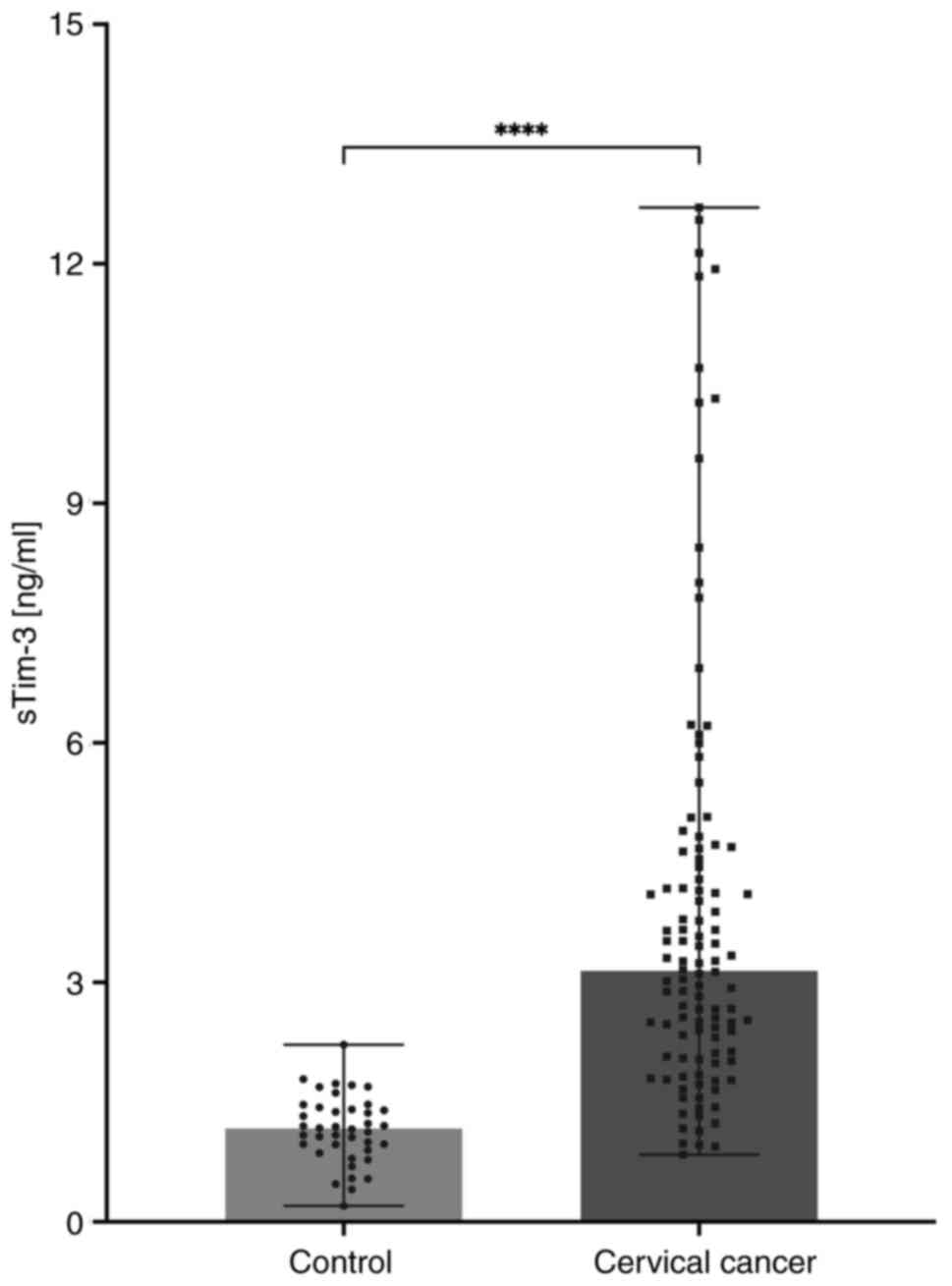
Serum sTim-3 concentration is
increased in patients with cervical cancer
The serum levels of sTim-3 were significantly higher
in the patients with cervical cancer compared with the controls, as
shown in Fig. 1. The median
concentration in the control group was 1.169 ng/ml compared with
3.146 ng/ml in the cervical cancer group.
As significant differences in age between control
vs. cervical cancer samples were obtained, it was determined if
there were differences in the concentration of sTim-3 with respect
to age. For this, age ranges in the control and in the cervical
cancer groups were established and it was determined if there were
significant differences between the age groups. The mean
concentrations of sTim-3 in control and the median concentrations
in cervical cancer groups by age ranges are shown in Table II. No statistical differences were
obtained between age groups in the control and the cervical cancer
groups (Table II). The
concentrations of sTim did not change with respect to age neither
in the control group nor in the cervical cancer group.
 | Table IISerum concentration of sTim-3 with
respect to the age range. |
Table II
Serum concentration of sTim-3 with
respect to the age range.
| A, Concentration of
sTim-3 with respect to age range in control and cervical cancer
groups |
|---|
| Group | Age range | Median and range
concentration of sTim3 | P-value
(Kruskall-Wallis test) |
|---|
| Control | 21-30 years | 0.99 ng/ml
(0.41-2.21) | a0.525 |
| | 31-40 years | 1.14 ng/ml
(0.20-1.73) | |
| | 41-60 years | 1.54 ng/ml
(0.47-1.69) | |
| Cervical
cancer | 21-30 years | 4.69 ng/ml
(2.88-6.10) | a0.6786 |
| | 31-40 years | 3.26 ng/ml
(1.17-10.31) | |
| | 41-60 years | 3.10 ng/ml
(0.94-12.14) | |
| | >60 years | 3.04 ng/ml
(0.84-12.70) | |
| B, Concentration of
sTim-3 in control vs cervical cancer groups by age range |
| Group | Age range | Mann-Whitney U test
P- value | |
| Control vs.
Cervical cancer | 21-30 years | 0.0008 | |
| | 31-40 years | 0.0001 | |
| | 41-60 years | 0.0016 | |
The levels of sTim-3 by the age range 21-30 years,
31-40 years and 41-60 years between the control and cervical cancer
groups were also compared and significant differences were obtained
(Table II). The previous analysis
supports that the sTim-3 concentration is not modified by age and
the concentration changes appeared to be associated with the
disease.
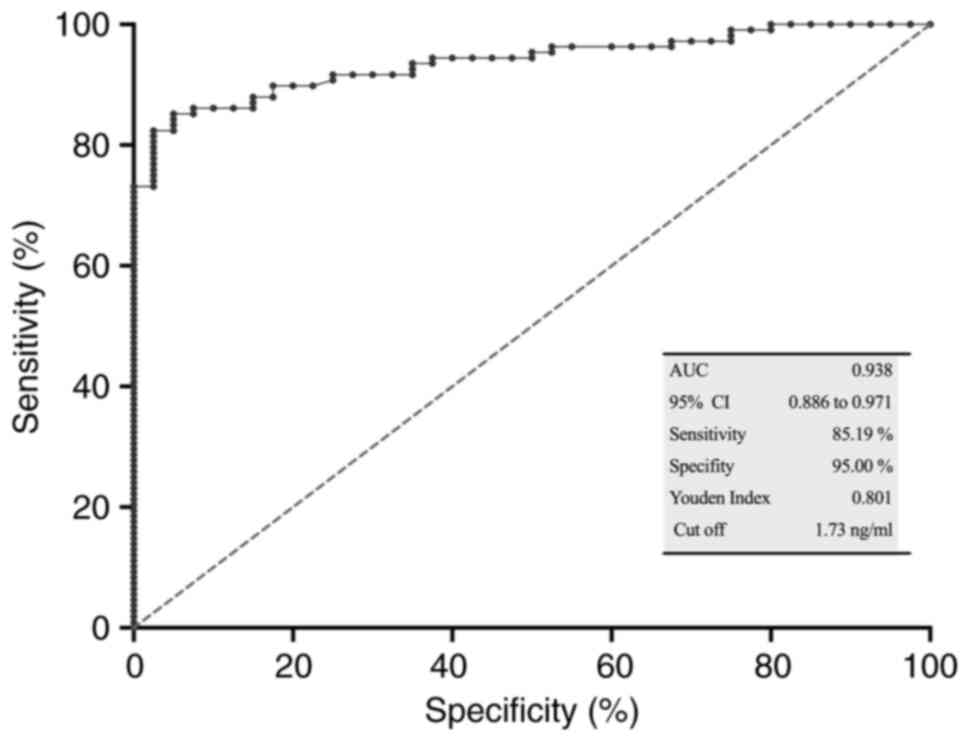
ROC analysis and diagnostic efficacy
of sTim-3
To determine the diagnostic potential of sTim-3 as a
biomarker, ROC analysis was performed for serum sTim-3 in patients
with cervical cancer (n=108) vs. the control group (n=40). The area
under the curve (AUC) was 0.938 (P<0.0001), the Youden index was
0.8019, the optimal cut-off value was >1.7313 ng/ml, specificity
was 95.00% and sensitivity was 85.19% (Fig. 2).
Association between serum sTim-3
levels, and clinicopathological characteristics and clinical
stage
The serum sTim-3 concentration was compared between
groups with different histological types of cervical cancer (SCC,
ACs and ASCs), differentiation grades, and (for the SCC group)
between the keratinization and non-keratinization status. The
median concentration of sTim-3 for each group is shown in Table I. Statistical analysis did not
reveal differences in these characteristics.
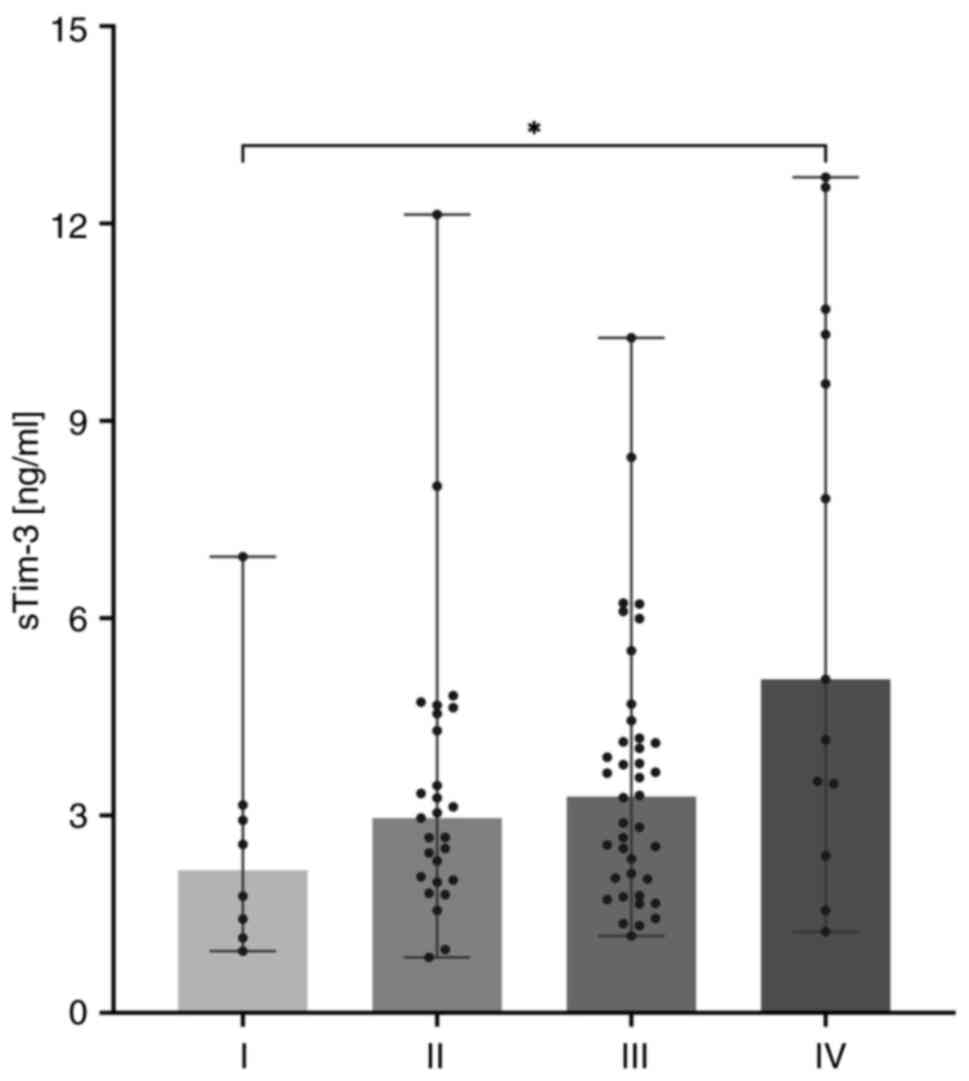
The serum sTim-3 concentration increased
progressively with increasing clinical stages; the median
concentration and the range of sTim-3 for each group are shown in
Table I. The Kruskal-Wallis test
results revealed a significant difference among the groups
(P=0.0371). Post-hoc Dunn analysis showed that sTim-3 concentration
in stage IV was significantly higher compared with that in stage I
(P=0.0338; Fig. 3).
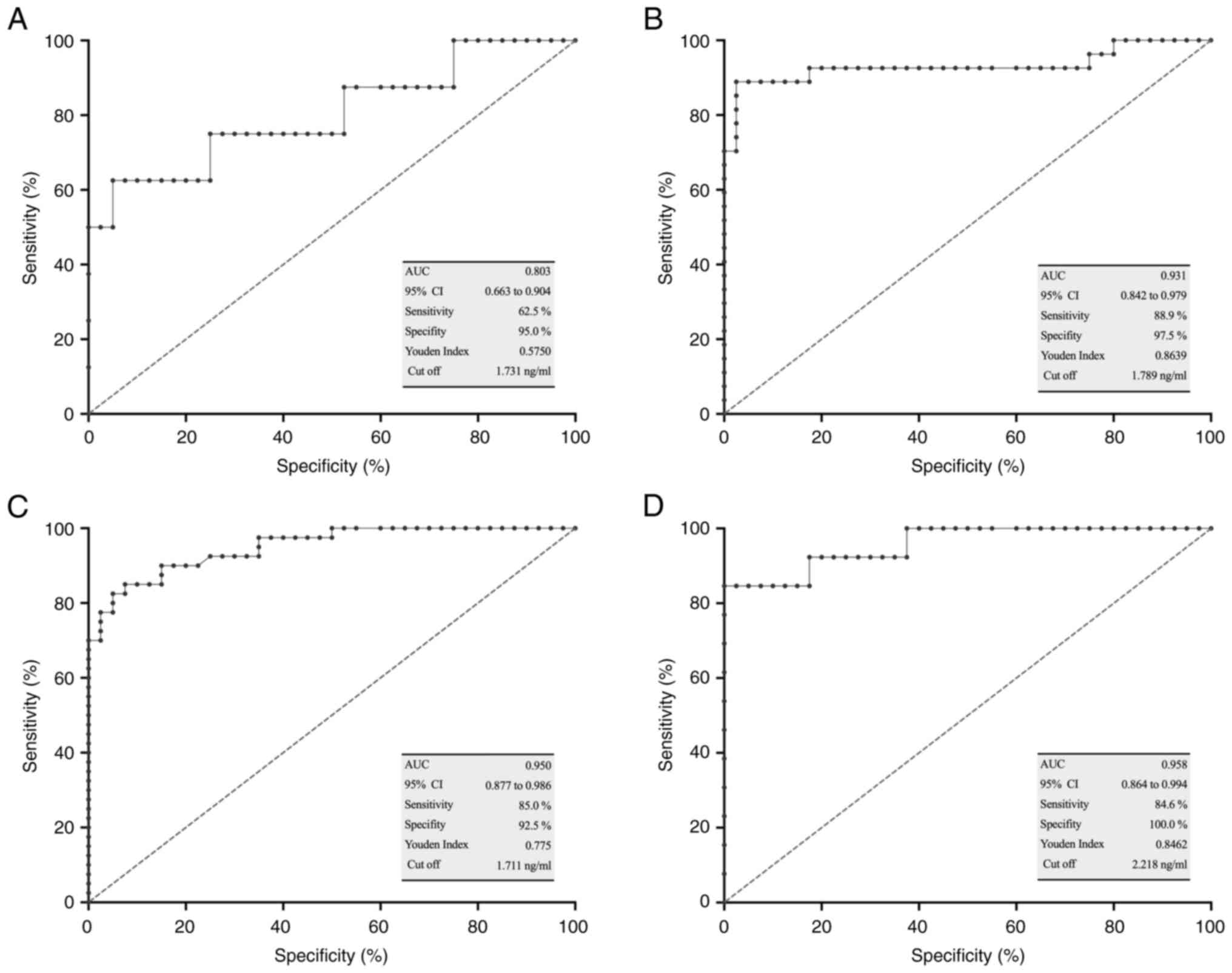
ROC curve analysis and diagnostic
efficacy of sTim-3 in different clinical stage groups
ROC curve analysis was performed for each clinical
stage group vs. the control group. The AUC, the sensitivity and
specificity are shown in Fig. 4.
The optimal sensitivity values were obtained for clinical stages
II, III and IV (Fig. 4B-D), while
the specificity values were exceptional for all stages, and notably
100% for stage IV (Fig. 4A-D).
sTim-3 and clinical outcomes
Since serum sTim-3 concentrations were evaluated
before any treatment in patients with cervical cancer, the outcome
was defined as total remission of disease or death during the
follow-up period. No statistical association was observed between
sTim-3 levels and patient outcomes; however, a trend towards
increasing sTim-3 concentration was observed in the group of
deceased patients, as shown in Table
I.
Serum galectin-9 concentration in
patients with cervical cancer
The serum concentrations of galectin-9 were
previously reported in this cohort of patients with cervical cancer
(23); however, as new patients
were subsequently included, the serum concentrations of galectin-9
were also determined in this study to perform a correlation
analysis of the serum levels of galectin-9 and sTim-3. The median
concentration of galectin-9 in patients with cervical cancer was
9.64±3.18-34.97 ng/ml. A notably increased concentration of
galectin-9 was detected in patients with FIGO stage IV (13.85
ng/ml) compared with stage I (7.73 ng/ml), stage II (8.13 ng/ml)
and stage III (8.58 ng/ml) cervical cancer (Table III).
 | Table IIIConcentration of serum galectin-9
with respect to the FIGO stage. |
Table III
Concentration of serum galectin-9
with respect to the FIGO stage.
| FIGO | Median
concentration of serum galectin-9 | Range
concentration |
|---|
| Stage I | 7.73 ng/ml | 3.18-17.23
ng/ml |
| Stage II | 8.13 ng/ml | 3.25-23.45
ng/ml |
| Stage III | 8.58 ng/ml | 3.48-23.85
ng/ml |
| Stage IV | 13.85 ng/ml | 3.30-34.97
ng/ml |
Correlation of the serum levels of
sTim-3 and galectin-9
To determine whether there was a correlation between
the serum levels of sTim-3 and galectin-9, Spearman's rank
correlation coefficient was performed using the data of 102
patients' sera. A moderate positive correlation was identified
between sTim-3 and galectin-9 concentrations, with a correlation
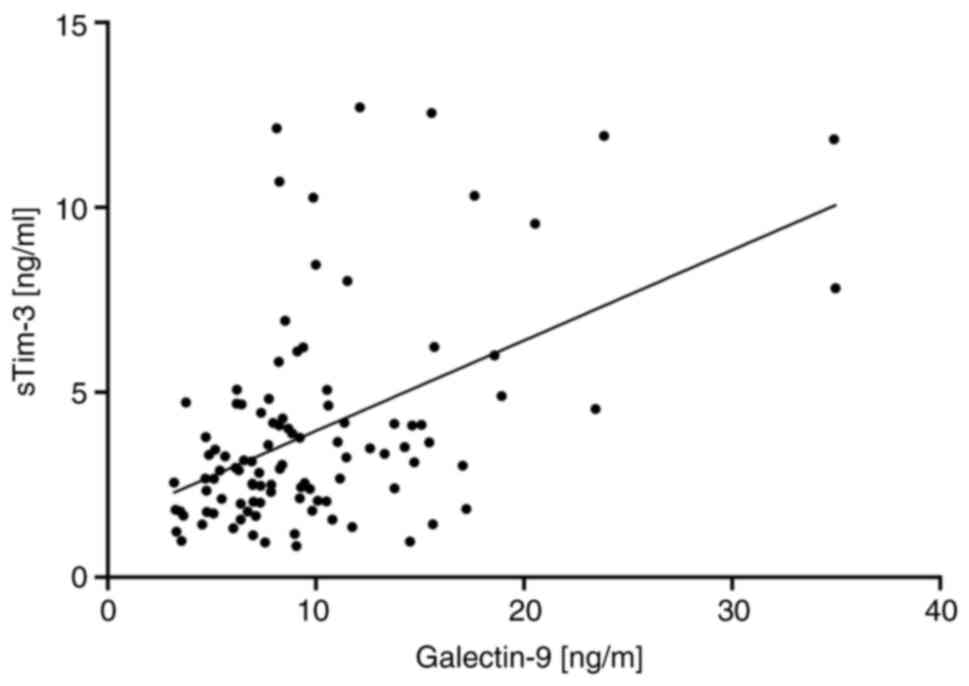
coefficient of ρ=0.41 and high significance (P<0.0001; Fig. 5).
Discussion
To the best of our knowledge, this was the first
time that the serum levels of sTim-3 were compared between patients
with cervical cancer and healthy women. The results revealed
significantly increased concentrations of sTim-3 in patients with
cervical cancer. ROC curve analysis revealed that sTim-3
effectively differentiates patients with cervical cancer from
healthy women. These results highlighted its potential use for the
diagnosis of cervical cancer, possibly as a complementary cervical
cytology test to improve sensitivity.
These results resembled those of other studies that
analysed sTim-3 in several types of cancer, including oral SCC,
skin basal cell carcinoma, colorectal cancer, gastric cancer, and
osteosarcoma, and those studies reported higher levels of sTim-3 in
patients with cancer compared with healthy individuals (26-30).
By contrast, patients with breast cancer presented lower levels of
sTim-3 compared with healthy individuals (21). This indicated that sTim-3 levels
were regulated in a tissue-dependent manner.
However, an analysis of sTim-3 levels among several
clinical groups revealed that this protein was not associated with
the histological type, differentiation grade or keratinizing
status; thus, sTim-3 did not appear to be associated with tumour
histological characteristics, at least in cervical cancer.
Similarly, in a previous study, no association was found between
sTim-3 and the histological subtype of the tumour in patients with
differentiated thyroid carcinoma (7).
Nonetheless, in the present study, increased levels
of sTim-3 were detected in women with advanced clinical stages of
cervical cancer. Indeed, significant differences in sTim-3 were
identified between patients with FIGO clinical stage IV disease and
those with stage I disease. Of note, this increase could be linked
to an immune dysregulation and a blocking immune response that is
more noticeable as the disease progresses. However, there were no
statistical differences between clinical stages I, II and III;
including a larger number of patients per group could make the
differences observed statistically significant, but this should be
explored in future studies. The ROC curve analysis performed for
each clinical stage versus the control group revealed that the
concentration of sTim-3 was an effective discriminator for clinical
stages II, III and IV, supporting that at the early stage of
disease the dysregulation of the immune response is less. In a
previous study with patients with cervical cancer, Tim-3 expression
was evaluated in peripheral T cells and was significantly increased
compared with the control group. The expression of Tim-3 on
peripheral CD4+ and CD8+ T cells was
significantly greater in patients with stages III-IV disease than
in those with stages I-II disease (12).
The association of sTim-3 with cancer progression
has also been observed in gastric carcinoma, where the highest
levels of sTim-3 were found in patients with carcinoma, followed by
patients with benign gastric disease, and the lowest levels were
found in the healthy control group (29). Of note, the serum levels of sTim-3
have not been evaluated in patients with premalignant lesions of
the cervix; this is to be the focus of our future studies.
In patients with osteosarcoma, sTim-3 was also found
to be associated with the risk of disease progression (30). In patients with colorectal cancer,
the levels of sTim-3 are greater in those with postoperative
recurrence (28). In clear cell
renal cell carcinoma, high levels of sTim-3 were found to be
associated with decreased survival (31).
In combination, in the present study, the serum
levels of sTim-3 were analysed with respect to the clinical
outcome, and higher concentrations were observed in patients who
succumbed to disease than in patients with total remission. Given
the limited number of samples in the present study, it is important
to plan further studies that include a higher number of patients to
effectively determine the association of sTim-3 with clinical
outcome.
Although the present study did not include patients
treated with immunotherapy, it is important to mention that in a
study performed on patients with NSCLC and anti-PD-1 immunotherapy,
high expression levels of sTim-3 were reported in patients that did
not respond to treatment, and an increase in serum levels was
linked to tumour progression (32).
Considering the anti-PD-1 immunotherapy for patients with
metastatic cervical cancer, the study of sTim-3 as a negative
factor affecting treatment response highlights the importance of
studying this soluble protein in patients with cervical cancer. In
addition, it has been proposed to combine anti-Tim-3 therapy with
anti-PD-1 therapy, to increase treatment response; therefore,
further studying the role of sTim-3 with cervical cancer may
provide valuable insights.
The results of the present study indicated that
sTim-3 is associated with cancer progression, but the mechanism
remains to be elucidated. Tim-3 is a negative regulator of the
anti-tumour immune response, and its overexpression is part of a
series of biochemical mechanisms that are activated, favouring
cancer development. It was hypothesized that sTim-3 is a soluble
signal that suppresses anti-tumour immune response. The soluble
form of Tim-3 may be produced in different ways, such as
alternative splicing, passive release from apoptotic cells, and
proteolytic cleavage by two A disintegrin and metalloprotease
metalloproteases (15-17).
However, sTim-3 can also be secreted by T cells, as has been
reported in differentiated thyroid carcinoma, which highlights that
CD3+ and CD8+ T-cell subsets isolated from
metastatic lymph nodes exhibit increased secretion of sTim-3
compared with those isolated from normal lymph nodes (7).
The major ligand of Tim-3 is galectin-9, which
participates in multiple cellular processes through interaction
with carbohydrates in glycoproteins (33,34).
It has been reported that the binding of galectin-9 to membrane
Tim-3 induces T-cell apoptosis (35,36).
In our previous study, serum galectin-9 concentrations were
evaluated, and higher levels were reported in patients with
cervical cancer compared with those in healthy women. The highest
serum galectin-9 levels were reported in patients with advanced
clinical stage disease (23).
The previous analysis of serum galectin-9 compared
with the results obtained for sTim-3 suggested that sTim-3 is a
better diagnostic biomarker, as the sensitivity to discriminate
between control and patients with cervical cancer was 85.19% for
sTim-3 compared with 68.2% for galectin-9(21). With respect to disease progression,
both proteins exhibited a similar behavior, with disease
progression being linked to an increase in concentration; sTim-3
showed significant differences between clinical stage I vs.
clinical stage IV, and serum galectin-9 showed significant
differences between clinical stage I vs. IV, and clinical stage II
vs. VI (23). The difference in
sensitivity of these soluble proteins could be the result of their
biological roles; Tim-3 is a receptor that has different ligands
(galectin-9, phospathidylserine, carcinoembryonic antigen-related
cell adhesion molecule 1, and high mobility group protein B1),
these ligands have been studied in cancer and the results have
shown that they are related with disease progression. Thus, in
addition to galectin-9, other ligands could be participating in
cervical cancer progression through their interaction with sTim-3,
which could explain the difference in sensitivity observed between
sTim-3 and serum galectin-9 (37-39).
Given that new patients were subsequently included
in this cohort and considering the relevant interactions of
galectin-9 with Tim-3, the serum concentrations of galectin-9 were
also determined, and a correlation analysis of the serum levels of
both proteins was performed. These results revealed a positive
moderate correlation, suggesting a possible role of this immune
checkpoint complex in the progression of the disease.
Tim-3/galectin-9 signalling was involved in immune regulation, and
changes in serum levels could affect the development, prognosis and
response to cancer, which highlighted the importance of
Tim-3/galectin-9 as potential biomarkers and therapeutic targets
(13).
sICs play important roles in immune modulation and
are involved in the development and prognosis of cancer; thus, they
are considered potential biomarkers for improving cancer diagnosis,
as well as therapeutic targets (13). To date there is no biomarker that is
routinely used for the diagnosis and follow-up of patients with
cancer, but different serum biomarkers have been proposed for
prognosis and follow up of patients with cervical cancer. One of
the most studied is squamous cell carcinoma antigen (SCC); the
serum levels of this antigen have been reported to be increased in
patients with cervical cancer, with higher levels in advanced
clinical stages. This antigen has been proposed for monitoring the
response to treatment (40,41). One of the limitations of the present
study is the lack of evaluation of other serum biomarkers studied
previously (42), which would have
been useful to compare the results obtained for sTim-3. This would
have enriched the insights of the present findings.
In conclusion, sTim-3 may be an effective
discriminator of patients with cervical cancer and a potential
diagnostic biomarker. In addition, the increased expression of
sTim-3 in patients with advanced clinical stage highlights its
potential as a biomarker of progression. Serum immune checkpoints
must be studied to elucidate their functions in cancer progression,
treatment response and survival.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from the
Mexican Institute of Social Security (grant no. R-2023-2106-004).
The funder had no role in the design, data collection, data
analysis, or reporting of this study.
Availability of data and materials
All data generated or analysed in this study are
included in this published article.
Authors' contributions
VVR and JRL designed the study. VJVZ, ICR and SPP
performed the investigation and data collection. VVR and JRL
drafted the manuscript and provided approval of the final version
of the manuscript. ICR, JRL and VVR performed the data analysis,
and created the figures and tables. ICR and SPP confirm the
authenticity of all raw data. All authors have read and agreed to
the published version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Human Ethics and Local
Committee of Health Research number 2106 from the Mexican Institute
of Social Security with the registration number R-2023-2106-004.
Written informed consent was obtained from all the participants in
this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
D'Augè TG, Di Donato V and Giannini A:
Strategic approaches in management of early-stage cervical cancer:
A comprehensive editorial. Clin Exp Obstet Gynecol. 51:235–237.
2024.
|
|
3
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Piñeros M, Znaor A, Soerjomataram I and Bray F: Global cancer
observatory: Cancer today. Available from: https://gco.iarc.fr/today (open in a new window).
Accessed January 2, 2020.
|
|
4
|
Meng Y, Chu T, Lin S and Wu P, Zhi W, Peng
T, Ding W, Luo D and Wu P: Clinicopathological characteristics and
prognosis of cervical cancer with different histological types: A
population-based cohort study. Gynecol Oncol. 163:545–551.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mohamud A, Høgdall C and Schnack T:
Prognostic value of the 2018 FIGO staging system for cervical
cancer. Gynecol Oncol. 165:506–513. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
D'Oria O, Bogani G, Cuccu I, D'Auge TG, Di
Donato V, Caserta D and Giannini A: Pharmacotherapy for the
treatment of recurrent cervical cancer: An update of the
literature. Expert Opin Pharmacother. 25:55–65. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shao Y, Gui X, Wang Y, Sheng L, Sun D,
Zeng Q and Wang H: Serum soluble immune checkpoint levels predict
cervical lymph node metastasis of differentiated thyroid carcinoma
patients. Cancer Med. 12:17648–17659. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cedeno-Laurent F and Dimitroff CJ:
Galectins and their ligands: Negative regulators of antitumor
immunity. Glycoconj J. 29:619–625. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ohue Y, Kurose K, Nozawa R, Isobe M,
Nishio Y, Tanaka T, Doki Y, Hori T, Fukuoka J, Oka M and Nakayama
E: Survival of lung adenocarcinoma patients predicted from
expression of PD-L1, galectin-9, and XAGE1 (GAGED2a) on tumor cells
and tumor-infiltrating T cells. Cancer Immunol Res. 4:1049–1060.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li J, Sun XF, Shen Y, Yang Q and Dai SY:
Elevated expression of T-cell immunoglobulin and mucin domain 3 on
T cells from peripheral blood in patients with cervical carcinoma.
Gynecol Obstet Invest. 86:63–70. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang
L, Huang M and Zhou J: Tim-3 expression in cervical cancer promotes
tumor metastasis. PLoS One. 8(e53834)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen Z, Dong D, Zhu Y, Pang N and Ding J:
The role of Tim-3/Galectin-9 pathway in T-cell function and
prognosis of patients with human papilloma virus-associated
cervical carcinoma. FASEB J. 35(e21401)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gu D, Ao X, Yang Y, Chen Z and Xu X:
Soluble immune checkpoints in cancer: Production, function and
biological significance. J Immunother Cancer. 6(132)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khan M, Arooj S and Wang H: Soluble
B7-CD28 family inhibitory immune checkpoint proteins and
anti-cancer immunotherapy. Front Immunol. 12(651634)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Geng H, Zhang GM, Li D, Zhang H, Yuan Y,
Zhu HG, Xiao H, Han LF and Feng ZH: Soluble form of T cell Ig mucin
3 is an inhibitory molecule in T cell-mediated immune response. J
Immunol. 176:1411–1420. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hansen JA, Hanash SM, Tabellini L, Baik C,
Lawler RL, Grogan BM, Storer B, Chin A, Johnson M, Wong CH, et al:
A novel soluble form of Tim-3 associated with severe
graft-versus-host disease. Biol Blood Marrow Transplant.
19:1323–1330. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Möller-Hackbarth K, Dewitz C, Schweigert
O, Trad A, Garbers C, Rose-John S and Scheller J: A disintegrin and
metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell
immunoglobulin and mucin domain 3 (Tim-3). J Biol Chem.
288:34529–34544. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li F, Li N, Sang J, Fan X, Deng H, Zhang
X, Han Q, Lv Y and Liu Z: Highly elevated soluble Tim-3 levels
correlate with increased hepatocellular carcinoma risk and poor
survival of hepatocellular carcinoma patients in chronic hepatitis
B virus infection. Cancer Manag Res. 10:941–951. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lu K, Ma H, Wang T, Huang Y and Ru M: The
diagnostic value of soluble TIM-3 in oral squamous cell carcinoma.
Future Oncol. 18:1381–1390. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Peng Y, Zhang C, Rui Z, Tang W, Xu Y, Tao
X, Zhao Q and Tong X: A comprehensive profiling of soluble immune
checkpoints from the sera of patients with non-small cell lung
cancer. J Clin Lab Anal. 36(e24224)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rapoport BL, Steel HC, Benn CA, Nayler S,
Smit T, Heyman L, Theron AJ, Hlatshwayo N, Kwofie LLI, Meyer PWA
and Anderson R: Dysregulation of systemic soluble immune
checkpoints in early breast cancer is attenuated following
administration of neoadjuvant chemotherapy and is associated with
recovery of CD27, CD28, CD40, CD80, ICOS and GITR and substantially
increased levels of PD-L1, LAG-3 and TIM-3. Front Oncol.
13(1097309)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu C, Li X, Li A, Zou W, Huang R, Hu X,
Yu J, Zhang X and Yue J: Concurrent chemoradiotherapy Increases the
levels of soluble immune checkpoint proteins in patients with
locally advanced cervical cancer. J Immunol Res.
2022(9621466)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reyes-Vallejo T, Conde-Rodríguez I,
Serna-Villalobos J, Ramírez-Díaz I, Pérez-Villalobos G,
Delgado-López G, Vazquez-Zamora VJ, Gutiérrez-Quiroz CT,
Ávila-Jiménez L, García-Carrancá A, et al: Serum levels of
galectin-9 are increased in cervical cancer patients and are higher
in advanced clinical stages. Onco Targets Ther. 15:1211–1220.
2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nayar R and Wilbur DC: The bethesda system
for reporting cervical cytology: A historical perspective. Acta
Cytol. 61:359–372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988.PubMed/NCBI
|
|
26
|
Bailly C, Thuru X, Goossens L and Goossens
JF: Soluble TIM-3 as a biomarker of progression and therapeutic
response in cancers and other of human diseases. Biochem Pharmacol.
209(115445)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Malinga NZ, Siwele SC, Steel HC, Kwofie
LLI, Meyer PWA, Smit T, Anderson R, Rapoport BL and Kgokolo MCM:
Systemic levels of the soluble co-inhibitory immune checkpoints,
CTLA-4, LAG-3, PD-1/PD-L1 and TIM-3 are markedly increased in basal
cell carcinoma. Transl Oncol. 19(101384)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hong J, Chen X, Chen L, Wang Y, Huang B
and Fang H: Clinical value of combined detection of serum sTim-3
and CEA or CA19-9 for postoperative recurrence of colorectal cancer
diagnosis. Cancer Manag Res. 15:563–572. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen L, Hong J, Hu R, Yu X, Chen X, Zheng
S, Qin Y, Zhou X, Wang Y, Zheng L, et al: Clinical value of
combined detection of serum sTim-3 and pepsinogen for gastric
cancer diagnosis. Cancer Manag Res. 13:7759–7769. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li B, Wang Q, Luo Y, Wang S, Pan S, Zhao
W, Ye Z and Wu X: Peripheral soluble immune checkpoint-related
proteins were associated with survival and treatment efficacy of
osteosarcoma patients, a cohort study. Cancers (Basel).
16(1628)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Q, Zhang J, Tu H, Liang D, Chang DW,
Ye Y and Wu X: Soluble immune checkpoint-related proteins as
predictors of tumor recurrence, survival, and T cell phenotypes in
clear cell renal cell carcinoma patients. J Immunother Cancer.
7(334)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen C, Zhao F, Peng J, Zhao D, Xu L, Li
H, Ma S, Peng X, Sheng X, Sun Y, et al: Soluble Tim-3 serves as a
tumor prognostic marker and therapeutic target for CD8+ T cell
exhaustion and anti-PD-1 resistance. Cell Rep Med.
5(101686)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yasinska IM, Sakhnevych SS, Pavlova L,
Selnø AT, Abeleira AM, Benlaouer O, Silva IG, Mosimann M, Varani L,
Bardelli M, et al: The Tim-3-Galectin-9 pathway and its regulatory
mechanisms in human breast cancer. Front Immunol.
10(1594)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Müllerová M, Hovorková M, Závodná T, Št
Astná LC, Krupková A, Hamala V, Nováková K, Topinka J, Bojarová P
and Strašák T: Lactose-functionalized carbosilane glycodendrimers
are highly potent multivalent ligands for Galectin-9 binding:
Increased glycan affinity to galectins correlates with aggregation
behavior. Biomacromolecules. 24:4705–4717. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Yang R, Sun L, Li CF, Wang YH, Yao J, Li
H, Yan M, Chang WC, Hsu JM, Cha JH, et al: Galectin-9 interacts
with PD-1 and TIM-3 to regulate T cell death and is a target for
cancer immunotherapy. Nat Commun. 12(832)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lake CM, Voss K, Bauman BM, Pohida K,
Jiang T, Dveksler G and Snow AL: TIM-3 drives temporal differences
in restimulation-induced cell death sensitivity in effector CD8+ T
cells in conjunction with CEACAM1. Cell Death Dis.
12(400)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Smith CM, Li A, Krishnamurthy N and Lemmon
MA: Phosphatidylserine binding directly regulates TIM-3 function.
Biochem J. 478:3331–3349. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang J, Li H, Kulkarni A, Anderson JL,
Upadhyay P, Onyekachi OV, Arantes LMRB, Banerjee H, Kane LP, Zhang
X, et al: Differential impact of TIM-3 ligands on NK cell function.
J Immunother Cancer. 13(e010618)2025.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yim EK and Park JS: Biomarkers in cervical
cancer. Biomark Insights. 1:215–225. 2007.PubMed/NCBI
|
|
41
|
Chen W, Xiu S, Xie X, Guo H, Xu Y, Bai P
and Xia X: Prognostic value of tumor measurement parameters and
SCC-Ag changes in patients with locally-advanced cervical cancer.
Radiat Oncol. 17(6)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fu J, Wang W, Wang Y, Liu C and Wang P:
The role of squamous cell carcinoma antigen (SCC Ag) in outcome
prediction after concurrent chemoradiotherapy and treatment
decisions for patients with cervical cancer. Radiat Oncol.
14(146)2019.PubMed/NCBI View Article : Google Scholar
|