Introduction
Cardiac disease is a leading cause of connective
tissue disease (CTD) mortality and has attracted considerable
attention (1). Most patients with
CTD present with nonspecific cardiac symptoms, normal
electrocardiogram (ECG) findings and a preserved left ventricle
(LV) ejection fraction (2,3). Therefore, they do not receive an early
cardiac diagnosis. Pulmonary arterial hypertension (PAH), right
ventricle (RV) dilatation and hypertrophy are common complications
of CTD with critical consequences (4). The prevalence of CTD-associated PAH
based on right heart catheterization (RHC) is estimated to be as
high as 13% (5). The incidence of
systemic lupus erythematosus (SLE)-associated PAH ranges between
0.5 and 43%, and this leads to compromised RV functions (6). The prevalence of PAH in patients with
scleroderma is between 5 and 12% (7). Among patients with mixed CTD, 20-30%
have cardiac manifestations (8).
The most severe cardiopulmonary complication of mixed CTD is PAH,
which results in increased RV pressure and has a fatal outcome in
approximately half of patients (9).
These late-stage phenomena, including endocarditis, atherosclerosis
and pericarditis, may eventually lead to death or right heart
failure in patients with CTD (10).
Therefore, the detection of early-stage markers rather than
late-stage markers in CTD development is a critical issue. RV
abnormalities are associated with a risk of heart failure and
cardiovascular death (11). RV
dilatation and RV hypertrophy (RVH) are frequently observed in
patients with CTD (12,13). Clinical evidence has shown that the
RV structure can deteriorate despite a reduction in pulmonary
vascular resistance after PAH-targeted therapies (14). RVH progression persists even when
CTD-associated PAH is alleviated (15). This finding suggests that PAH may
not be the sole indicator of RVH.
Cardiovascular magnetic resonance (CMR) can depict
myocardial structure and characteristics using cine and late
gadolinium enhancement (LGE) sequences, T1 mapping, and T1-derived
ventricular extracellular volume (ECV) estimation (16). T1-mapping can be used to
homogenously detect diffuse cardiac impairment (17) and predict the prognostic
significance of CTD (18). Tissue
tracking, a post processing method for strain analysis based on
cine images from CMR, has been employed to assess the nature and
function of myocardial tissue deformation (19).
Few studies have focused on cardiac involvement in
patients with CTD (20) and fewer
have focused on early detection of cardiac impairment (21). The present study explored factors
that may predict the presence of RVH to reduce major adverse
cardiovascular events in patients with CTD. Using clinical
assessments and multi-imaging tests, the present study aimed to
identify markers for the early detection of cardiac involvement
preceding RVH.
Materials and methods
Study participants
All participants provided written informed consent
and the study protocol was approved by the Institutional Review
Boards of the Renji Hospital [approval no. (2017)083; Shanghai,
China]. Consecutive participants, including patients with CTD
without RVH, patients with CTD with RVH and control subjects, were
prospectively enrolled in the three cohorts at Renji Hospital
(Shanghai, China) between September 2017 and July 2018. The age
range of patients in the control, non-RVH and RVH groups was 24-54,
23-57 and 30-54 years, respectively. The diagnosis of CTD was based
on the clinical classification criteria, laboratory findings and
imaging data (22). The inclusion
criteria for patients with non-right ventricular hypertrophy
(non-RVH) were as follows: i) Consecutive patients who presented to
the outpatient clinic with SLE, polymyositis, systemic scleroderma,
Sjögren's syndrome or mixed CTD diagnosis; ii) participants with
CTD whose RV wall thickness was ≤4 mm were assigned to the non-RVH
group according to echocardiography; iii) SLE duration was >6
months irrespective of cardiac symptoms. SLE activity and disease
severity did not affect enrolment in the study; and iv)
participants with RVH with an RV wall thickness >4 mm diagnosed
using echocardiography (23).
Age-matched candidates served as healthy controls and were used to
establish the baseline myocardial T1 and strain values. Healthy
volunteers with normal echocardiographic results and CMR findings
were used as controls. The exclusion criteria were as follows: i)
Age <18 or >80 years; ii) documented coronary artery disease
and prior angiography for coronary artery disease (>50%
stenosis); iii) patients with known congenital heart disease or
other systemic diseases-induced RVH, including coronary artery
disease, chronic obstructive pulmonary disease, primary pulmonary
hypertension and valve disease; and iv) patients with standard
metallic contraindications to CMR or an estimated glomerular
filtration rate of <30 ml/min/1.73 m2 and severe
infection were excluded due to the consideration of CMR safety.
Clinical symptom assessment of
patients with CTD
Clinical cardiac involvement of patients with CTD
included clinical symptoms such as chest tightness, pectoralgia,
heart palpitations, orthopnea, shortness of breath and edema. No
specific myocardial enzymes were measured, and patients with CTD
that may coexist with coronary artery disease, myocarditis,
pericarditis, valvular heart disease, heart failure and PAH were
not included in the present cohort study. The etiology of CTD and
drug use were recorded.
ECG and RHC assessment
CTD-induced PAH was defined as an increase in mean
pulmonary arterial pressure (PAP) of ≥20 mmHg at rest, as assessed
by RHC (24). Within 48 h,
pulmonary pressure was double-checked by echocardiography (E9; GE
Healthcare). A CMR scan was scheduled within 6 h of
echocardiography.
Follow-up
Telephone or outpatient consultations were used for
the follow-up of the patients who survived every 3 months. When a
patient died, cardiac death was identified from the death
certificate. The shortest follow-up duration was >8 months.
CMR protocol
All CMR examinations were performed using a Philips
3T Ingenia MR system (Philips Healthcare).
Biventricular function
RV and LV volumetric assessments were obtained using
cine imaging with whole-heart coverage of short-axis slices (7 mm
thick with a 3-mm gap). Additionally, three long-axis views of the
LV (4-, 2- and 3-chamber views) and two long-axis views of the RV
(2- and 3-chamber views) were acquired. Detailed sequences and
parameter settings are shown in Data
S1.
Native and post-contrast T1 mapping. Native
and post-contrast myocardial T1 mapping was used for ECV
determination. A MOLLI R5.1 sequence was used for T1 mapping, and a
‘5-3-3’ scheme (in sec) was chosen (details are presented in
Data S1). The scheme was performed
on three mid-diastolic LV short-axis slices (basal, mid-ventricle
and apex) before and 15 min after a bolus intravenous injection of
0.15 mmol/kg gadobutrol (Bayer AG). Typical acquisition parameters
are presented in Data S1.
Evaluation of LGE. LGE imaging was performed
with no slice gap and whole heart coverage of short-axis slices 10
min after gadobutrol administration. Segmented LGE images with at
least three matching slices and native T1 images were obtained. A
visual assessment of LGE positivity was performed. The acquisition
parameters are shown in Data
S1.
Cardiac image analysis: Bi-ventricular
morphology, function and myocardial deformation
LV and RV function parameters, mass, volume,
ejection fractions, and strain were assessed using Circle (cvi42
version 5.5.6.1; Circle Cardiovascular Imaging, Inc.). CMR data
analysis was performed by two observers who are experienced in CMR
and were blinded to the clinical information. For the LV and RV
volumes and masses, the endocardial borders were manually traced at
end-diastole and end-systole. The papillary muscles were included
as part of the myocardium. All volumetric indices were normalized
to body surface area (BSA). An RV/LV volume ratio >1.27 was
defined as RV enlargement (25). An
RV wall thickness >4 mm indicates RVH and RV pressure overload
in the absence of other explanatory pathologies (23).
The LV and RV global circumferential strain and
global radial strain were obtained in the short-axis view at the
apical, midventricular and basal levels, and the global
longitudinal strain was derived from the long-axis view (2-, 3- and
4-chamber images). Myocardial deformation was voxel-tracked, and
software-integrated contours automatically tracked distinctive
features within a user-defined region of interest throughout the
cardiac cycle. All contours were manually examined, adjusted when
necessary, and assessed.
Fibrosis assessment: LGE, ECV
quantification and quality assessment
LGE, T1 and ECV images were used to examine the
presence and extent of regional and diffuse fibrosis (cvi42 version
5.5.6.1). The methods for creating parametric maps and quality
assessments are provided in Data
S1. The reported T1 values were derived by an operator blinded
to the LGE images. Hematocrit was measured on the same day for all
participants. The ECV was calculated using the ECV formula:
ECV=1-hematocrit x (1/T1 myo post-1/T1 myo pre)/(1/T1 blood
post/1/T1 blood pre). T1 and ECV quantification details are
provided in Data S1.
Statistical analysis
Quantitative data are presented as the mean ± SD or
the median (interquartile range), and categorical data are
presented as numbers and percentages. Quantitative data were
checked for normality using the Kolmogorov-Smirnov test, and the
three groups were compared using one-way analysis of variance and
post hoc Bonferroni correction. The Kruskal-Wallis test and post
hoc Bonferroni test were used for among-group comparisons. For
categorical data, comparisons among the three groups were performed
using Pearson's χ2 test or Fisher's exact test.
Furthermore, a second blinded operator calculated >50% of the
randomly selected cases. The same analysis technique was used, and
the inter-observer reproducibility of volume, strain, T1 mapping
and ECV measurements was assessed using intra-class correlation
coefficient analysis. Univariate and multivariate regression
analyses of RV myocardial infarction (RVMI) outcomes were performed
to identify statistically significant determinants. All analyses
were performed using IBM SPSS Statistics software (version 17.0;
SPSS, Inc.). P<0.05 (two-tailed) was considered to indicate a
statistically significant difference.
Results
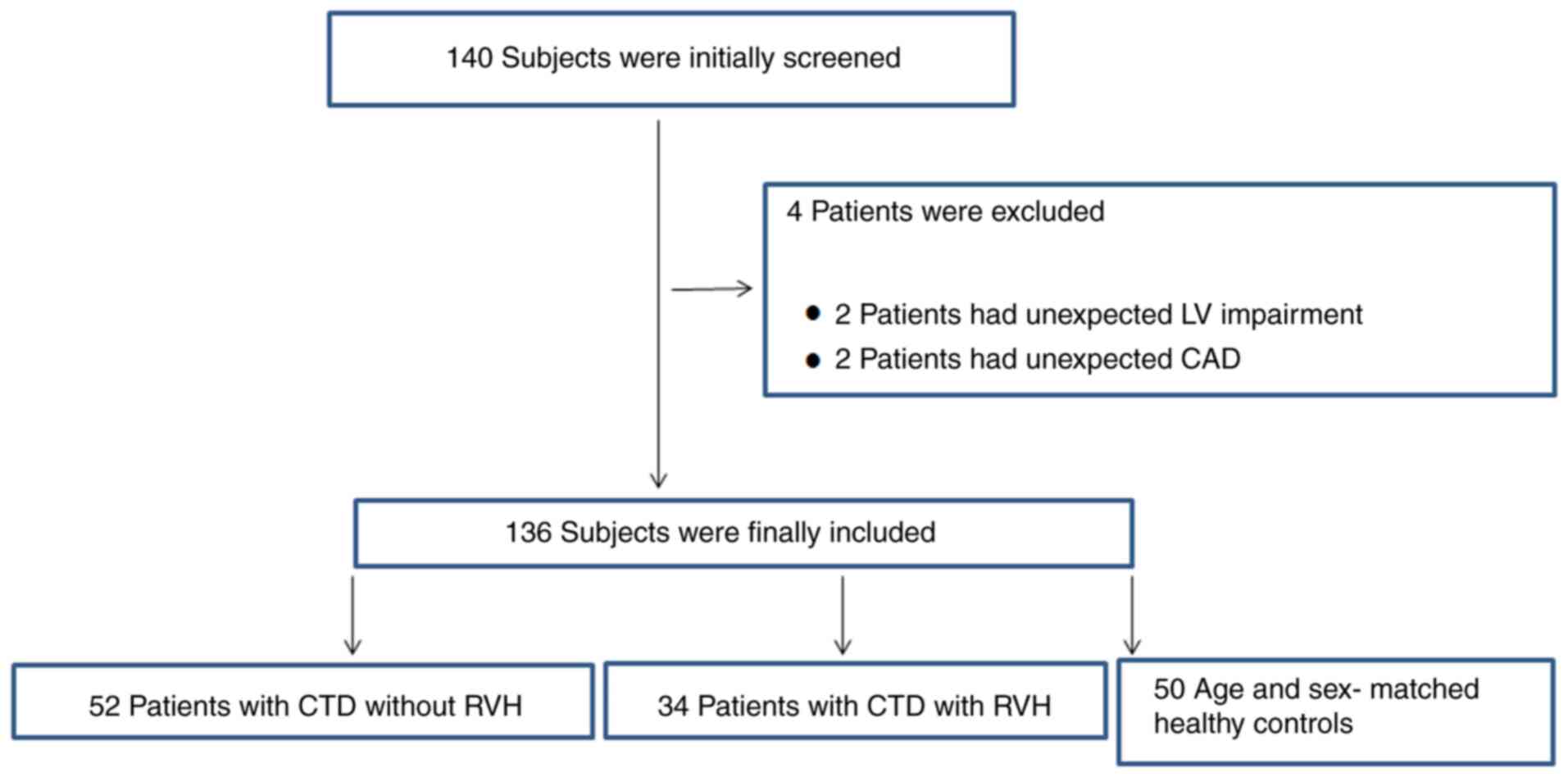
Screening of enrolled patients
A total of 140 participants were recruited based on
the inclusion and exclusion criteria. A total of 4 patients were
excluded: 2 patients had unexpected LV impairment and 2 patients
had unexpected coronary artery disease. Consequently, 136 patients
were recruited, including 52 patients without RVH, 34 patients with
RVH and 50 age-matched healthy controls. The final patient
selection flowchart is shown in Fig.
1.
Inter-observer assessment of
reproducibility
Table I summarizes
the inter-observer variability in biventricular volumes, strain and
T1 values. Intraclass correlation coefficient analysis indicated a
strong association, except for the global longitudinal strain of
the right ventricle, between the two operators.
 | Table IInter-observer agreement for strain,
volume and T1 values. |
Table I
Inter-observer agreement for strain,
volume and T1 values.
| Variables | ICC (95% CI) | F statistic | P-value |
|---|
| Strains | | | |
|
Global
radial strain (%) | | | |
|
Left
ventricle | 0.924
(0.841-0.964) | 14.207 | <0.001 |
|
Right
ventricle | 0.856
(0.697-0.931) | 0.528 | <0.001 |
|
Global
circumferential strain (%) | | | |
|
Left
ventricle | 0.955
(0.905-0.979) | 66.683 | <0.001 |
|
Right
ventricle | 0.899
(0.787-0.952) | 4.637 | <0.001 |
|
Global
longitudinal strain (%) | | | |
|
Left
ventricle | 0.895
(0.780-0.950) | 66.495 | <0.001 |
|
Right
ventricle | 0.588
(0.135-0.804) | 0.141 | 0.010 |
| Biventricular
morphology and function | | | |
|
Right
ventricle | | | |
|
RVEF | 0.984
(0.967-0.992) | 2.163 | <0.001 |
|
RVEDV/BSA | 0.997
(0.994-0.999) | 2.592 | <0.001 |
|
RVESV/BSA | 0.994
(0.987-0.997) | 0.319 | <0.001 |
|
Left
ventricle | | | |
|
LVEF | 0.987
(0.973-0.994) | 0.982 | <0.001 |
|
LVEDV/BSA | 0.972
(0.941-0.987) | 3.526 | <0.001 |
|
LVESV/BSA | 0.991
(0.981-0.996) | 0.036 | <0.001 |
|
RVEDV/LVEDV | 0.813
(0.608-0.911) | 1.207 | <0.001 |
| T1 values and
ECV | | | |
|
Left
ventricle | | | |
|
Native
T1 myo | 0.981
(0.958-0.991) | 39.083 | <0.001 |
|
T1-post
myo | 0.993
(0.986-0.997) | 9.057 | <0.001 |
|
ECV | 0.958
(0.909-0.981) | 13.837 | <0.001 |
|
Right
ventricle | | | |
|
Native
T1 myo | 0.930
(0.781-0.977) | 0.002 | <0.001 |
|
T1-post
myo | 0.919
(0.749-0.974) | 0.864 | <0.001 |
|
ECV | 0.758
(0.246-0.922) | 3.163 | 0.008 |
Characteristics of patients
The baseline characteristics of the patients are
summarized in Table II. Sex and
BSA were comparable between the two diseased groups, consisting
primarily of female patients with significantly lower BSA levels
than those of the control group (P<0.05). The etiologies were
comparable between the non-RVH and RVH groups (P>0.05). More
patients with RVH tended to receive endothelial receptor
antagonists and prostacyclin medications than those in the non-RVH
group (P<0.05). Both diseased groups had accelerated heartbeats
compared with the control group (P<0.001). Except for heart
rate, the non-RVH and RVH groups also had high brain natriuretic
peptide (P<0.001), troponin I (P<0.001) and creatine
kinase-MB (P=0.004) levels, but low creatine phosphate kinase
levels (P<0.001) compared with the control group. In addition,
according to the ECG results, in the non-RVH and RVH groups, the
incidence (30/34; 88%) and values (76±23 mmHg) of pulmonary
hypertension in the RVH group were significantly increased compared
with those in the control group (0/50; 0% incidence and 22±21 mmHg;
P<0.001) (Table II).
 | Table IIBaseline characteristics. |
Table II
Baseline characteristics.
|
Characteristics | Controls
(n=50) | Non-RVH (n=52) | RVH (n=34) |
P-valuea |
|---|
| Clinical | | | | |
|
Age,
years | 39±15 | 35±12 | 42±12b | 0.450 |
|
Female, n
(%) | 30(60) | 46(88)c | 32(94)c | <0.001 |
|
BSA,
m2 | 1.76±0.21 |
1.56±0.17c |
1.58±0.18c | <0.001 |
|
Vital
signs | | | | |
|
Heart
rate, bpm | 72±13 | 78±14c | 83±13c | 0.001 |
|
Systolic
BP, mmHg | 123±18 | 119±16 | 118±19 | 0.360 |
|
Diastolic
BP, mmHg | 78±12 | 75±12 | 78±13 | 0.554 |
|
Serum
markers of cardiac injury | | | | |
|
BNP,
pg/ml | 52 (26, 167) | 89 (39,
291)c | 358 (62,
784)c | <0.001 |
|
TNI,
ng/ml | 0.00 (0.00,
0.01) | 0.01 (0.01,
0.05)c | 0.01 (0.01,
0.03)c | <0.001 |
|
CPK,
U/l | 99 (72, 134) | 50 (27,
148)c | 50 (34,
107)c | <0.001 |
|
CK-MB,
ng/ml | 1.50 (1.20,
2.20) | 8.00 (0.70,
19.50)c | 5.25 (2.80,
11.70)c | 0.004 |
|
ACC/AHA
stages C-D, n (%) | 0 (0) | 2(4) | 5(15) | 0.160 |
|
ECG
abnormal, n (%) | 0 (0) | 26(50) | 25(74)b | 0.030 |
|
Pulmonary
hypertension, n (%) | 0 (0) | 24(46) | 30(88)b | <0.001 |
|
PAH,
mmHg | 22±21 | 31±21 | 76±23b,c | <0.001 |
| Etiology | | | | |
|
Systemic
lupus erythematosus, n (%) | 0 (0) | 40(76) | 19(56) | 0.070 |
|
Polymyositis,
n (%) | 0 (0) | 6(12) | 1(3) | 0.300 |
|
Systemic
scleroderma, n (%) | 0 (0) | 2(4) | 2(6) | 0.930 |
|
Sjogren
syndrome, n (%) | 0 (0) | 1(2) | 3(9) | 0.330 |
|
Mixed
connective tissue disease, n (%) | 0 (0) | 3(6) | 9(27) | 0.080 |
| Medical
therapy | | | | |
|
None, n
(%) | 50(100) | 41(79)c | 13(38)b,c | <0.001 |
|
Calcium
antagonists, n (%) | 0 (0) | 4(8) | 3(9) | 0.850 |
|
Endothelial
receptor antagonists, n (%) | 0 (0) | 1(2) | 13(38)b | <0.001 |
|
Phosphodiesterase
indicator, n (%) | 0 (0) | 1(2) | 5(15) | 0.060 |
|
Prostacyclin,
n (%) | 0 (0) | 7(13) | 15(44)b | 0.001 |
| CMR | | | | |
|
LV
morphology and function | | | | |
|
LVEDV,
ml | 122±34 | 118±32 | 85±34b,c | <0.001 |
|
LVEDV/BSA,
ml/m2 | 69±15 | 76±22 | 54±18b,c | <0.001 |
|
LVESV,
ml | 41±15 | 45±26 | 34±24b,c | 0.004 |
|
LVESV/BSA,
ml/m2 | 23±8 | 29±19 | 21±13b | 0.016 |
|
LV
mass, g | 111±32 | 110±30 | 113±74 | 0.050 |
|
LV
mass/BSA, g/m2 | 62±13 | 71±21 | 71±46 | <0.001 |
|
LVEF,
% | 67±7 | 63±12 | 63±12 | 0.171 |
|
RV
morphology and function | | | | |
|
RVEDV,
ml | 107±32 | 112±31 | 145±45b,c | 0.003 |
|
RVEDV/BSA,
ml/m2 | 60±14 | 73±20c | 92±26b,c | <0.001 |
|
RVESV,
ml | 41±15 | 51±19c | 89±37b,c | <0.001 |
|
RVESV/BSA,
ml/m2 | 23±8 | 33±12c | 56±22b,c | <0.001 |
|
RV
mass, g | 32±12 | 34±8 | 57±21b,c | <0.001 |
|
RV
mass/BSA, g/m2 | 18±6 | 22±6c | 36±12b,c | <0.001 |
|
RVEF,
% | 62±9 | 55±10c | 40±11b,c | <0.001 |
|
RVESV/LVESV | 0.87±0.10 |
0.98±0.26c |
2.09±1.14b,c | <0.001 |
|
Myocardial
fibrosis | | | | |
|
LGE
size, % | 0 (0) | 0 (0, 0) | 4.53 (2.32,
9.79)b | <0.001 |
|
LGE
(+), n (%) | 0 (0) | 8(15) | 28(82)b | <0.001 |
| Death | | | | |
|
All-cause
mortality, n (%) | 0 (0) | 3(6) | 2(6) | 0.200 |
|
Cardiac
death, n (%) | 0 (0) | 2(4) | 1(3) | 0.140 |
Cardiac systolic function and
dimensions
Notably, more RV parameters were affected than LV
parameters (Table II). RVH was
associated and significantly increased with RV dilatation in terms
of RV end-diastolic volume (RVEDV), RV end-systolic volume (RVESV),
RVEDV/BSA and RVESV/BSA indices (P=0.016) compared with those of
the control group. The chamber dilatation led to a reduction in RV
ejection fraction (RVEF) (40±11% in the RVH group vs. 55±10% in the
non-RVH group and 62±9% in the control group for RVEF, P<0.001;
63±12% in the RVH group vs. 63±12% in the non-RVH group and 67±7%
in the control group for the LVEF, P=0.171). In the non-RVH group,
RV dimensions were normal [RV end systolic volume (RVESV)/LV end
systolic volume, 0.98±0.26 in the non-RVH group vs. 0.87±0.1 in the
control group; P=0.5], the RVEF was within the normal range
(51-71%), and no LV morphological or functional impairment was
observed.
Prevalence and extent of LV myocardial
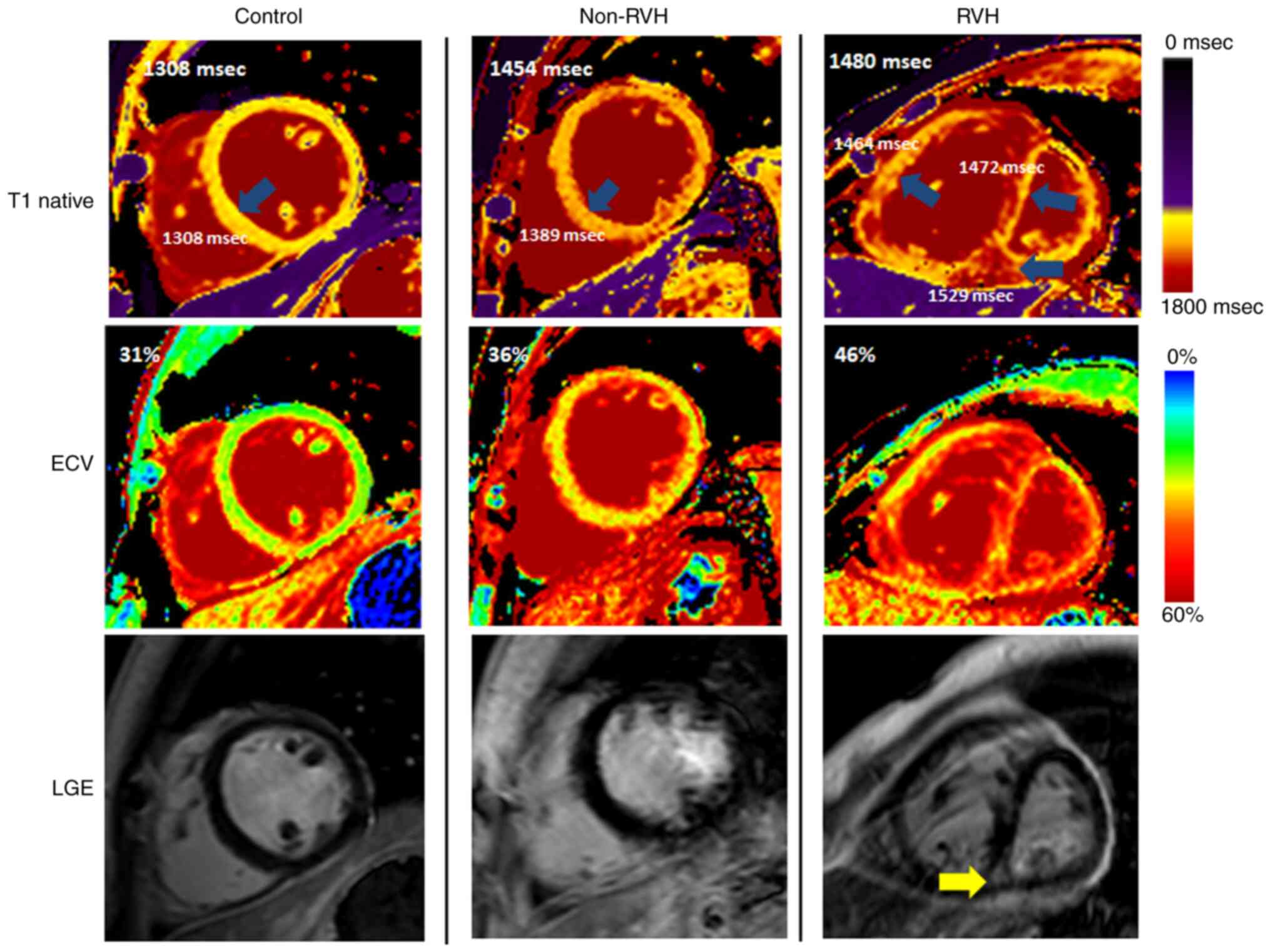
fibrosis in the non-RVH and RVH groups
LGE is a biomarker for regional myocardial fibrosis
(26). In the non-RVH group,
positive fibrosis [LGE (+)] was observed in 15.4% (8/52) of
patients and the median LV extent (LGE size) was 0.0 (0,0 for
25-75% quartile). By contrast, the LGE(+) incidence in the RVH
group was 82.4% (28/34) and the median LGE size was 4.53 (2.32-9.79
for 25-75% quartile) (Table II).
The LGE(+) incidence and LGE size in the non-RVH group were
significantly decreased compared with those in the RVH group.
Table III summarizes the T1 and
ECV values of the LV. Notably, the native LV myocardial T1 and ECV
were increased in the non-RVH group (all P<0.001; compared with
controls). The values were not significantly different between the
non-RVH and RVH groups (native myocardial T1: 1,362±72 msec in the
non-RVH group vs. 1,372±89 msec in the RVH group; P=0.61; ECV:
31±4% in the non-RVH group vs. 32±5% in the RVH group; P=0.24).
Fig. 2 shows examples of regional
and diffuse myocardial fibrosis in patients. Native myocardial T1
and ECV detected increased fibrosis through values and color maps,
even when LGE was negative (second column; a patient with
non-RVH).
 | Table IIINative and post-contrast T1
relaxation times for the LV. |
Table III
Native and post-contrast T1
relaxation times for the LV.
| | LV | |
|---|
| Variables | Controls
(n=50) | Non-RVH(n=52) | RVH (n=34) |
P-valuea |
|---|
| Native T1
myocardium, msec | 1,268±42 |
1,362±72b |
1,372±89b | <0.0001 |
| Post (15 min) T1
myocardium, msec | 613±39 | 661±66b | 585±63c | <0.001 |
| ECV, % | 25±3 | 31±4b | 32±5b | <0.001 |
Furthermore, the T1 values of the hypertrophied RV
were investigated in the RVH group (Table IV). The hypertrophied RV free wall
exhibited significantly increased native T1 (1,463±130 msec) and
ECV (37±6%) levels compared with the septum with LGE(+) (1,392±96)
and ECV (34±5) (P<0.001 for myocardial native T1; P=0.108 for
ECV). By contrast, T1 myocardium 15 min post-contrast T1 relaxation
did not significantly differ between the hypertrophied RV free wall
(576±70) and the septum with LGE(+) (560±69) (P=0.222 for post
T1).
 | Table IVRight ventricular native and
post-contrast T1 relaxation times in the right ventricular
hypertrophy group (n=34). |
Table IV
Right ventricular native and
post-contrast T1 relaxation times in the right ventricular
hypertrophy group (n=34).
| Variables | Septum with LGE
(+) | Septum without LGE
(+) | Right ventricular
free wall |
P-valuea |
|---|
| NativeT1
myocardium, msec | 1,392±96 | 1,357±93 |
1,463±130b,c | <0.001 |
| Post (15 min) T1
myocardium, msec | 560±69 | 568±66 | 576±70 | 0.222 |
| ECV, % | 34±5 | 33±4 | 37±6 | 0.108 |
Biventricular myocardial
deformation
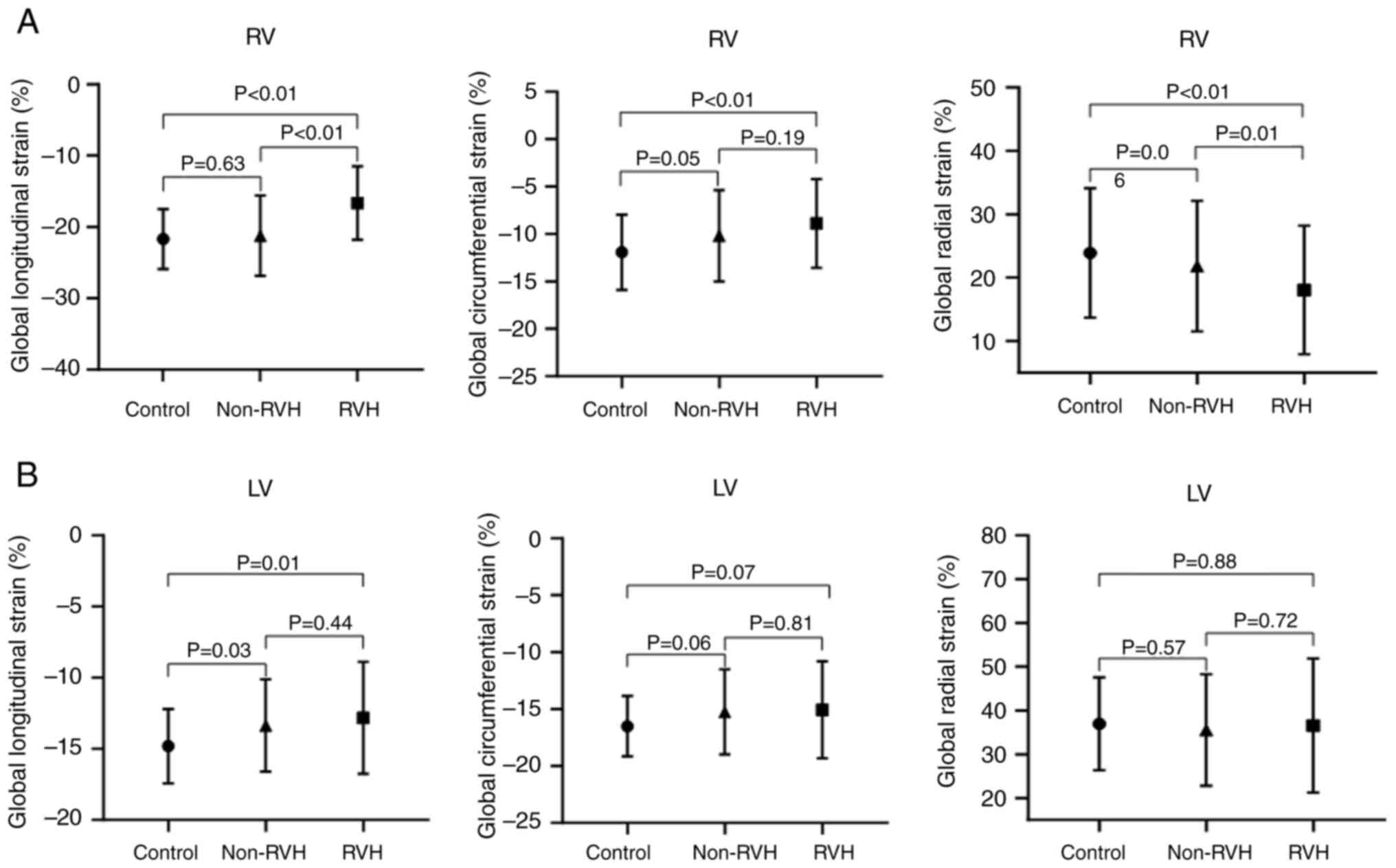
The global myocardial deformation is shown in
Fig. 3. Global longitudinal and
circumferential strains of the RVH group were significantly
increased compared with those in the control group (P<0.01).
Global circumferential strain in the RVH groups was not
significantly increased compared with that in the non-RVH group
(P=0.19). However, global radial strain in the RVH group was
markedly lower than that in the control (P<0.01) and non-RVH
groups (P=0.01) (Fig. 3A). In the
LV, LV global longitudinal strain was the only index with strain
reduction in the non-RVH and RVH groups (-14.82±2.6 msec in the
control group; -13.23±3.22 msec in the non-RVH group; -12.82±3.93
msec in the RVH group; P=0.03 for the non-RVH group and P=0.01 for
the RVH group compared with the control group). The global radial
and circumferential strains of the LV were not markedly reduced in
the non-RVH or RVH groups compared with those in the control group
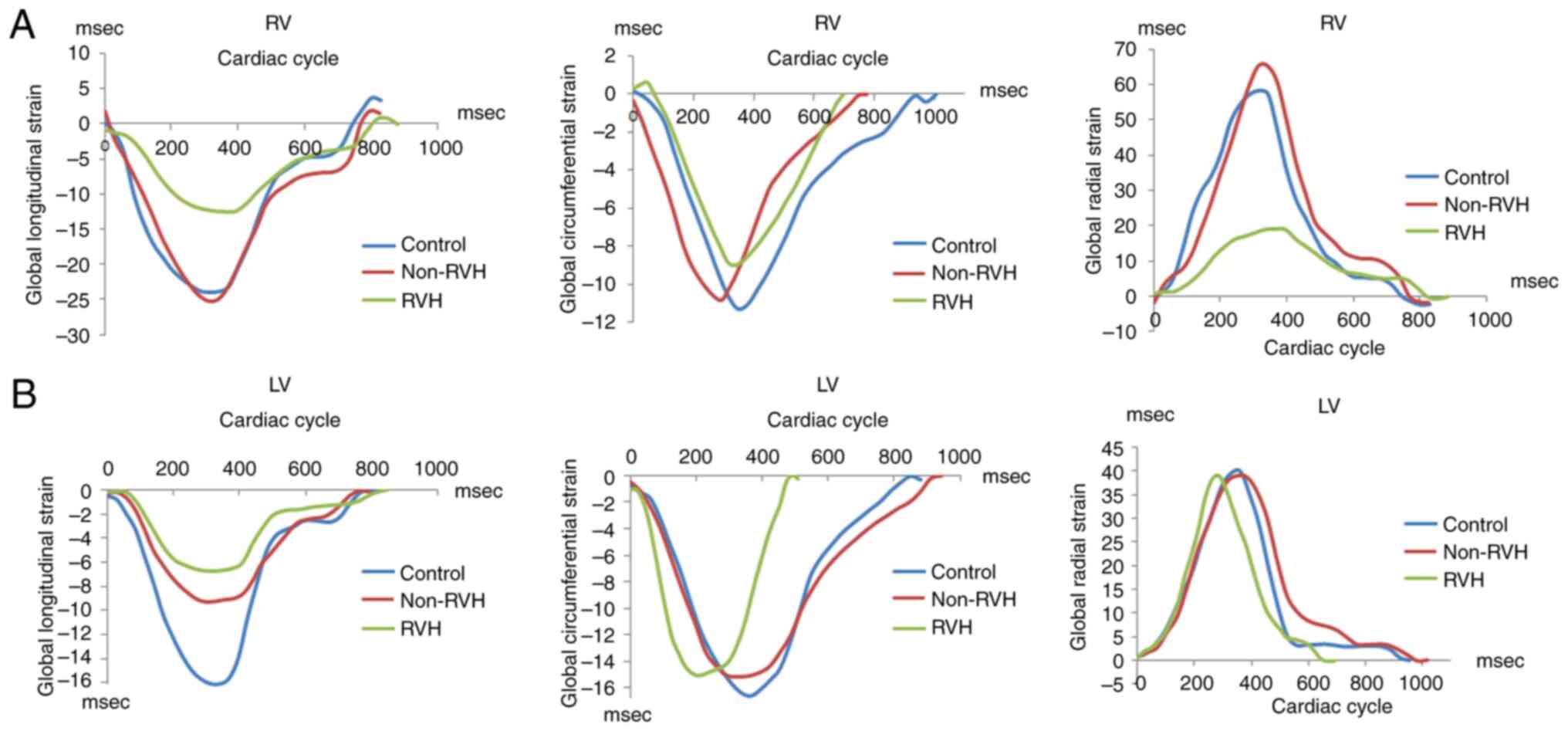
(Fig. 3B). Examples of the RV and
LV mean global longitudinal strain in the three groups of patients
are shown in Fig. 4 to demonstrate
the same results individually. The curves in Fig. 4 were representative and randomly
selected, showing data from 1 patient in each group.
Predictors of increases in the RV mass
index
Regarding the predictors of an increase in the RV
mass index, all baseline clinical and CMR parameters in Tables I and II were included in the univariate
regression analysis with RVMI outcomes (Table V). Parameters selected via the
univariate analysis (P<0.05) were included in a multivariate
regression analysis, which demonstrated that PAP predicted RVH when
the RV wall thickness was within the normal range (P=0.01). In the
group with RVH, RV end-systolic volume was a predictor of RVH
(P<0.0001). No other cardiac imaging or laboratory findings were
predictors of RVH.
 | Table VPredictors of an increase in the
right ventricular mass index. |
Table V
Predictors of an increase in the
right ventricular mass index.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Groups | Predictors | Coefficient | SE | t-statistic | P-value | Coefficient | SE | t-statistic | P-value |
|---|
| Non-RVH | RVESV | 0.12 | 0.05 | 2.20 | 0.03 | -0.21 | 0.17 | -1.25 | 0.22 |
| | RVESV/BSA | 0.21 | 0.08 | 2.82 | 0.01 | 0.04 | 0.08 | 0.44 | 0.66 |
| | RVEDV/BSA | 0.13 | 0.05 | 2.83 | 0.01 | 0.37 | 0.28 | 1.32 | 0.19 |
| | LVEDV/BSA | 11.52 | 4.12 | 2.80 | 0.01 | 0.03 | 0.05 | 1.32 | 0.19 |
| | PAP | 7.32 | 0.43 | 16.87 | 0.01 | 0.16 | 0.06 | 2.84 | 0.01 |
| RVH | RVESV | 0.23 | 0.03 | 7.98 | <0.0001 | 0.23 | 0.03 | 7.98 | <0.0001 |
| | RVESV/BSA | 0.37 | 0.05 | 7.90 | <0.0001 | -0.4 | 1.29 | -0.31 | 0.49 |
| | RVEDV | 0.15 | 0.03 | 5.92 | <0.0001 | -0.29 | 0.53 | -0.55 | 0.59 |
| | RVEDV/BSA | 0.26 | 0.04 | 5.79 | <0.0001 | 0.31 | 0.78 | 0.39 | 0.69 |
| | RVEDV/RVESV | 7.33 | 1.38 | 5.31 | <0.0001 | 0.11 | 3.09 | 0.03 | 0.97 |
| | RVEF | -0.57 | 0.15 | -3.79 | 0.01 | 0.14 | 0.36 | 0.38 | 0.71 |
| | LV cardiac
output/BSA | -6.68 | 2.73 | -2.45 | 0.02 | -1.31 | 3.09 | -0.42 | 0.67 |
| | LV stroke
volume/BSA | -0.61 | 0.21 | -2.91 | 0.01 | -0.03 | 0.33 | -0.08 | 0.93 |
Prognosis of the non-RVH and RVH
groups
The mean duration of follow-up was 12.1±4.5 and
10.8±3.5 months for the non-RVH and RVH groups, respectively.
Notably, 2 of 52 patients (4%) in the non-RVH group and 1 of 34
patients in the RVH group (3%) died due to cardiac death during
follow-up. The death rates were similar (P=0.14; Table II).
Discussion
The major findings of the present study were as
follows: i) Patients with RVH had impaired RVEF, RV dimensions and
global longitudinal strain compared with the non-RVH and control
groups; ii) fibrosis assessment by CMR revealed that native T1
values and the ECV in patients in the RVH and non-RVH groups were
significantly higher than those in the control group; and iii) PAP
in the non-RVH stage and RVESV in the RVH stage predicted RVH
progression.
A previous study has suggested that PAH may not be
the sole predictor of progressive RV failure and death in patients
with CTD (27). To further
understand other predictors of RV failure, we hypothesized that the
predictors would vary with the disease stage of the target organs.
In the non-RVH stage, RVH is primarily driven by PAH (pressure
overload), which induces an increase in the RV pressure that,
without timely intervention, may progress into compensatory RVH.
Late-stage phenomena, such as prominent RV dilatation, were not
observed in the non-RVH stage (2,28),
possibly suggesting that treatment intervention at this time might
contribute to preventing or reversing further damage to the
myocardium.
Once a patient enters the RVH stage, progressive
right-sided heart failure or sudden and unexpected death can occur,
and simple cardiac protection may not be beneficial because of the
damage to the cardiac tissue substrate and its structure and
function (29,30). Extensively increased biventricular
extracellular matrix and regional LGE accounted for the reduced RV
strain and the lack of RV contraction, which led to an increase in
RVESV. Therefore, the RVESV became a predictor of RVH
progression.
The present study primarily focused on the early
detection of myocardial damage. Pulmonary pressure measurements
from routine echocardiographic examinations are both practical and
important because routine echocardiographic examination can be used
for guidance of patient treatments. For example, once PAH was
detected, 88% of patients in the RVH group had PAH, which increased
to 76±23 (mean ± SD). In addition, the right heart structural and
functional abnormality appeared. Although PAH can guide treatment,
it is often delayed (31).
Therefore, early indicators that identify the presence of
myocardial damage, which are the focus of the present study, are
urgently needed.
Concerning all clinical and imaging parameters, only
native T1 values, T1-deduced ECV and longitudinal strain can be
used to screen for myocardial abnormalities in the non-RVH stage
(26). The first two parameters
represent the extracellular matrix, which indirectly reflects
diffuse fibrotic changes (32,33),
whereas the last parameter indicates myocardial deformation, which
may be due to LV chamber compression caused by RV dilatation
(34). Furthermore, native T1, ECV
and longitudinal strain are warning signs of heart involvement in
patients with a normal cardiac systolic ejection fraction (35). Theoretically, a histological
increase in myocardial fibrosis may occur first, followed by a
decrease in myocardial deformation; ventricular structural and
functional abnormalities may occur later (36). Notably, whether CTD treatment is
effective in reversing cardiac damage remains unclear.
Non-invasive detection of cardiac tissue
characterization may become a recommendation in the future, as it
favors the early detection of cardiac impairment. Furthermore, PAH
was not the sole predictor of progressive RV failure or death in
patients with CTD. The predictors varied according to the presence
of RVH. Finally, native myocardial T1, ECV and LV longitudinal
strains can be used as warning signs of myocardial damage in
patients with CTD.
The present study had some limitations. First, mixed
etiologies of CTD, which may complicate cardiac pathophysiological
mechanisms, were included in the present study. However, the varied
etiologies were equally distributed between the groups, which
eliminated the difference. Second, caution should be exercised
regarding mortality rates. The relatively short follow-up period
and low mortality rate make the results prone to bias. Third, the
sample size of the present study was small, which may have resulted
in sample bias. Forth, all patients were from one medical center,
which may give rise to selection bias. Finally, the present
cross-sectional study proposed markers of myocardial impairment;
however, whether they can serve as follow-up indicators and their
mechanisms require further evaluation. To overcome these
limitations, the sample size will be expanded and data from
multiple medical centers will be combined in the future. Future
studies will also focus on detecting RV contractility in PAH,
compensated heart failure and decompensated heart failure periods
of RVH. These results will reveal more detailed mechanisms of
myocardial damage in patients with CTD.
In conclusion, non-invasive examination of cardiac
tissue characterization using CMR enabled the early detection of
cardiac impairment before RVH development. Native myocardial T1,
ECV and LV longitudinal strain may serve as warning signs of
myocardial damage in patients with CTD. The predictors of RVH
varied with heart disease stages. PAP in the non-RVH stage and
RVESV in the RVH stage predicted further RVH progression.
Supplementary Material
Supplementary methods.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Science
Foundation of China (grant no. 81470391), Shanghai Municipal
Education Commission-Gaofeng Clinical Medicine Grant Support (grant
no. 20172014), and a three-year action plan to promote clinical
skills and clinical innovation in municipal hospitals (grant no.
16CR3020A).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS, MJ and SZ were responsible for the conception
and design of the study. JS, GX, SF, HY, ZZ and YX were responsible
for acquisition and analysis of the data. MJ and SZ confirmed the
authenticity of all the raw data. MJ was the main supervisor and SZ
was the co-supervisor. JS, MJ and SZ wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Institutional Review Boards of the Renji Hospital, Shanghai
Jiaotong University [approval no. (2017)083; Shanghai, China]. All
participants provided written informed consent for
participation.
Patient consent for publication
Written informed consent for publication was
obtained from all individual participants included in the
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Braun J, Krüger K, Manger B, Schneider M,
Specker C and Trappe HJ: Cardiovascular comorbidity in inflammatory
rheumatological conditions. Dtsch Arztebl Int. 114:197–203.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dawi J, Affa S, Misakyan Y, Fardeheb S,
Kades S, Kiriaki A, Mohan AS, Norris B, Yoon S and Venketaraman V:
Exploring cardiovascular implications in systemic lupus
erythematosus: A holistic analysis of complications, diagnostic
criteria, and therapeutic modalities, encompassing pharmacological
and adjuvant approaches. Biomol Concepts. 15:2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Miner JJ and Kim AH: Cardiac
manifestations of systemic lupus erythematosus. Rheum Dis Clin
North Am. 40:51–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schermuly RT, Ghofrani HA, Wilkins MR and
Grimminger F: Mechanisms of disease: Pulmonary arterial
hypertension. Nat Rev Cardiol. 8:443–455. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wigley FM, Lima JAC, Mayes M, McLain D,
Chapin JL and Ward-Able C: The prevalence of undiagnosed pulmonary
arterial hypertension in subjects with connective tissue disease at
the secondary health care level of community-based rheumatologists
(the UNCOVER study). Arthritis Rheum. 52:2125–2132. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Plazak W, Gryga K, Milewski M, Podolec M,
Kostkiewicz M, Podolec P and Musial J: Association of heart
structure and function abnormalities with laboratory findings in
patients with systemic lupus erythematosus. Lupus. 20:936–944.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hassoun PM: The right ventricle in
scleroderma (2013 grover conference series). Pulm Circ. 5:3–14.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Alpert MA, Goldberg SH, Singsen BH, Durham
JB, Sharp GC, Ahmad M, Madigan NP, Hurst DP and Sullivan WD:
Cardiovascular manifestations of mixed connective tissue disease in
adults. Circulation. 68:1182–1193. 1983.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vegh J, Hegedus I, Szegedi G, Zeher M and
Bodolay E: Diastolic function of the heart in mixed connective
tissue disease. Clin Rheumatol. 26:176–181. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leal GN, Silva KF, França CMP, Lianza AC,
Andrade JL, Campos LMA, Bonfá E and Silva CA: Subclinical right
ventricle systolic dysfunction in childhood-onset systemic lupus
erythematosus: Insights from two-dimensional speckle-tracking
echocardiography. Lupus. 24:613–620. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kawut SM, Barr RG, Lima JAC, Praestgaard
A, Johnson WC, Chahal H, Ogunyankin KO, Bristow MR, Kizer JR,
Tandri H and Bluemke DA: Right ventricular structure is associated
with the risk of heart failure and cardiovascular death: The
Multi-Ethnic Study of Atherosclerosis (MESA)-right ventricle study.
Circulation. 126:1681–1688. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tang Y, Yang Z, Wen J, Tang D, Luo Y,
Xiang C, Huang L and Xia L: Association of serum uric acid with
right cardiac chamber remodeling assessed by cardiovascular
magnetic resonance feature tracking in patients with connective
tissue disease. Front Endocrinol (Lausanne).
15(1351197)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vos JL, Butcher SC, Fortuni F, Galloo X,
Rodwell L, Vonk MC, Bax JJ, van Leuven SI, de Vries-Bouwstra JK,
Snoeren M, et al: The prognostic value of right atrial and right
ventricular functional parameters in systemic sclerosis. Front
Cardiovasc Med. 9(845359)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rich S, Pogoriler J, Husain AN, Toth PT,
Gomberg-Maitland M and Archer SL: Long-term effects of epoprostenol
on the pulmonary vasculature in idiopathic pulmonary arterial
hypertension. Chest. 138:1234–1239. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bell RD, White RJ, Garcia-Hernandez ML, Wu
E, Rahimi H, Marangoni RG, Slattery P, Duemmel S, Nuzzo M, Huertas
N, et al: Tumor necrosis factor induces obliterative pulmonary
vascular disease in a novel model of connective tissue
disease-associated pulmonary arterial hypertension. Arthritis
Rheumatol. 72:1759–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Messroghli DR, Moon JC, Ferreira VM,
Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R,
Salerno M, Schelbert EB, et al: Clinical recommendations for
cardiovascular magnetic resonance mapping of T1, T2, T2* and
extracellular volume: A consensus statement by the society for
cardiovascular magnetic resonance (SCMR) endorsed by the European
association for cardiovascular imaging (EACVI). J Cardiovasc Magn
Reson. 19(75)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
White SK, Sado DM, Flett AS and Moon JC:
Characterising the myocardial interstitial space: The clinical
relevance of non-invasive imaging. Heart. 98:773–779.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wong TC, Piehler KM, Kang IA, Kadakkal A,
Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG,
Kuller LH and Schelbert EB: Myocardial extracellular volume
fraction quantified by cardiovascular magnetic resonance is
increased in diabetes and associated with mortality and incident
heart failure admission. Eur Heart J. 35:657–664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Broberg CS, Chugh SS, Conklin C, Sahn DJ
and Jerosch-Herold M: Quantification of diffuse myocardial fibrosis
and its association with myocardial dysfunction in congenital heart
disease. Circ Cardiovasc Imaging. 3:727–734. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hachulla AL, Launay D, Gaxotte V, de
Groote P, Lamblin N, Devos P, Hatron PY, Beregi JP and Hachulla E:
Cardiac magnetic resonance imaging in systemic sclerosis: A
cross-sectional observational study of 52 patients. Ann Rheum Dis.
68:1878–1884. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mavrogeni S, Sfikakis PP, Karabela G,
Stavropoulos E, Spiliotis G, Gialafos E, Panopoulos S, Bournia V,
Manolopoulou D, Kolovou G and Kitas G: Cardiovascular magnetic
resonance imaging in asymptomatic patients with connective tissue
disease and recent onset left bundle branch block. Int J Cardiol.
171:82–87. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhernakova A, van Diemen CC and Wijmenga
C: Detecting shared pathogenesis from the shared genetics of
immune-related diseases. Nat Rev Genet. 10:43–55. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Rudski LG, Lai WW, Afilalo J, Hua L,
Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and
Schiller NB: Guidelines for the echocardiographic assessment of the
right heart in adults: A report from the American society of
echocardiography endorsed by the European association of
echocardiography, a registered branch of the European society of
cardiology, and the canadian society of echocardiography. J Am Soc
Echocardiogr. 23:685–713, 786-788. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 ESC/ERS guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Heart J.
43:3618–3731. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Altmayer SPL, Patel AR, Addetia K,
Gomberg-Maitland M, Forfia PR and Han Y: Cardiac MRI right
ventricle/left ventricle (RV/LV) volume ratio improves detection of
RV enlargement. J Magn Reson Imaging. 43:1379–1385. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Minegishi S, Kato S, Takase-Minegishi K,
Horita N, Azushima K, Wakui H, Ishigami T, Kosuge M, Kimura K and
Tamura K: Native T1 time and extracellular volume fraction in
differentiation of normal myocardium from non-ischemic dilated and
hypertrophic cardiomyopathy myocardium: A systematic review and
meta-analysis. Int J Cardiol Heart Vasc. 25(100422)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hsu S, Houston BA, Tampakakis E, Bacher
AC, Rhodes PS, Mathai SC, Damico RL, Kolb TM, Hummers LK, Shah AA,
et al: Right ventricular functional reserve in pulmonary arterial
hypertension. Circulation. 133:2413–2422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li X, Shi K, Yang ZG, Guo YK, Huang S, Xia
CC, He S, Li ZL, Li C and He Y: Assessing right ventricular
deformation in hypertrophic cardiomyopathy patients with preserved
right ventricular ejection fraction: A 3.0-T cardiovascular
magnetic resonance study. Sci Rep. 10(1967)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Konstam MA, Kiernan MS, Bernstein D,
Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani
FD, et al: Evaluation and management of right-sided heart failure:
A scientific statement from the American heart association.
Circulation. 137:e578–e622. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Monteagudo-Vela M, Tindale A,
Monguió-Santín E, Reyes-Copa G and Panoulas V: Right ventricular
failure: Current strategies and future development. Front
Cardiovasc Med. 10(998382)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ryan JJ and Archer SL: The right ventricle
in pulmonary arterial hypertension: Disorders of metabolism,
angiogenesis and adrenergic signaling in right ventricular failure.
Circ Res. 115:176–188. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mascherbauer J, Marzluf BA, Tufaro C,
Pfaffenberger S, Graf A, Wexberg P, Panzenböck A, Jakowitsch J,
Bangert C, Laimer D, et al: Cardiac magnetic resonance postcontrast
T1 time is associated with outcome in patients with heart failure
and preserved ejection fraction. Circ Cardiovasc Imaging.
6:1056–1065. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Puntmann VO, Voigt T, Chen Z, Mayr M,
Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T
and Nagel E: Native T1 mapping in differentiation of normal
myocardium from diffuse disease in hypertrophic and dilated
cardiomyopathy. JACC Cardiovasc Imaging. 6:475–484. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yeong CC, Harrop DL, Ng ACT and Wang WYS:
Global longitudinal strain manually measured from mid-myocardial
lengths is a reliable alternative to speckle tracking global
longitudinal strain. J Cardiovasc Imaging. 32(35)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Seno A, Antiochos P, Lichtenfeld H,
Rickers E, Qamar I, Ge Y, Blankstein R, Steigner M, Aghayev A,
Jerosch-Herold M and Kwong RY: Prognostic value of T1 mapping and
feature tracking by cardiac magnetic resonance in patients with
signs and symptoms suspecting heart failure and no clinical
evidence of coronary artery disease. J Am Heart Assoc.
11(e020981)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Korthals D, Chatzantonis G, Bietenbeck M,
Meier C, Stalling P and Yilmaz A: CMR-based T1-mapping offers
superior diagnostic value compared to longitudinal strain-based
assessment of relative apical sparing in cardiac amyloidosis. Sci
Rep. 11(15521)2021.PubMed/NCBI View Article : Google Scholar
|