Introduction
Type 2 diabetes mellitus (T2DM) is a major risk
factor for cardiovascular disease (CVD). The rising incidence of
T2DM has resulted in CVD becoming the leading cause of morbidity
and mortality worldwide (1). This
increased risk of CVD is due to a complex cluster of risk factors
associated with T2DM including insulin resistance, hyperglycemia,
diabetic dyslipidemia, hypertension, hyperinsulinemia, systemic
inflammation and adipose tissue-derived factors (2). Changes in the mass and metabolism of
adipose tissue may be related to insulin resistance and visceral
obesity commonly associated with T2DM (3).
Adipose tissue is no longer considered to be an
inactive organ, which only stores lipids and serves as an energy
reservoir. Numerous studies have shown that it is an active
endocrine organ and secretes many substances that are involved in
the regulation of several metabolic and physiologic processes.
These chemical messengers, known as ‘adipocytokines’ or
‘adipokines’, include tumor necrosis factor α (TNF-α), adiponectin,
leptin, resistin and visfatin (4).
Adiponectin, also known as adipocyte
complement-related protein (Acrp 30), gelatin-binding protein 28
(Gbp 28), adipose most abundant gene transcript (apM1) or adipo Q
(5), is considered to be a
protective protein with antidiabetic, anti-inflammatory and
anti-atherogenic effects (4).
Reduced plasma adiponectin levels have been reported in obese
individuals, particularly in those with visceral obesity, and have
been negatively correlated with insulin resistance. Furthermore,
decreased adiponectin levels were found to be associated with a
higher incidence of T2DM (5).
Leptin is the first identified endocrine product of adipose tissue
and was found to regulate vascular function through local and
central mechanisms (2). The
primary role of leptin is to provide a signal to the central
nervous system concerning the balance of body energy, which helps
to control appetite and food intake, and to maintain stable body
weight. However, leptin receptors are located throughout the body,
suggesting that leptin is also involved in the regul ation of
various processes (4). There is
also some evidence supporting the effects of leptin on the
cardiovascular system. Leptin was shown to promote the development
of atherosclerosis by inducing oxidative stress in endothelial
cells, increasing platelet aggregation, and hypertrophy and
proliferation of vascular smooth muscle cells (4). Additionally, it was shown that a high
leptin level predicts subsequent development of T2DM (6). Resistin belongs to a family of
cystein-rich secretory proteins called resistin-like molecules or
FIZZ (found in inflammatory zones) proteins. The term ‘resistin’
comes from the development of insulin resistance in mice following
injection of resistin. Resistin has been suggested to form a
biochemical relationship between obesity and T2DM (7). Visfatin, which was originally termed
pre-β cell colony-enhancing factor (PBEF), enhances the maturation
of β cell precursors and displays nicotinamide
phosphoribosyltransferase (Nampt) activity, and was found to be
substantially correlated with the amount of visceral fat in humans
(7). Although several clinical
studies have analyzed the relationship of visfatin with insulin
resistance, diabetes, and obesity, its role in predicting diabetes
remains unclear (8).
Adipocytokines, as pro-inflammatory mediators, and several other
inflammatory markers have been found to be elevated in obese
subjects. Pro-inflammatory adipocytokines including adiponectin,
leptin, resistin and visfatin seem to contribute to the ‘low-grade
inflammatory state’ of obese individuals and to several metabolic
disorders including cardiovascular complications (9). To our knowledge, there are few study
concerning the relationship between adipocytokines and
non-traditional cardiovascular risk factors such as homocysteine
(Hcy) and asymmetric dimethyarginine (ADMA) levels.
Thus, the aim of this study was to explore the
relationship of serum profiles of adipocytokines (adiponectin,
leptin, resistin and visfatin) with traditional and non-traditional
risk factors and with anthropometric variables in patients with
T2DM.
Materials and methods
Study subjects
This study was performed at Eskişehir Osmangazi
University, Faculty of Medicine in Turkey. Between January 2007 and
March 2008, 85 subjects with T2DM were enrolled in the study. T2DM
was diagnosed according to the American Diabetes Association
Criteria. Of these patients, 45 had established cardiovascular
disease and 40 had no evidence of CVD. Clinical evidence of CVD
included myocardial infarction or coronary artery by-pass surgery
(n=17), stroke (n=8), and peripheral arterial disease (n=20).
Patients in the group without vascular disease were T2DM patients
who had no history of vascular disease, and those with normal ECG
findings at exercise and normal peripheral artery Doppler
ultrasonography findings. The clinical features of the patients are
listed in Table I. All patients
were receiving antidiabetic and antihypertensive therapies and some
were receiving antilipidemic drugs and/or aspirin for at least the
previous 6 months (Table I).
Exclusion criteria were the presence of sustained type 1 DM, acute
and chronic infections, malignancy, hepatic or renal disease,
diabetic retinopathy and nephropathy, and other endocrine
dysfunctions.
 | Table I.Clinical characteristics of the type
2 diabetic patients and controls. |
Table I.
Clinical characteristics of the type
2 diabetic patients and controls.
| Controls | T2DM | P-value |
|---|
| Total no. | 30 | 85 | |
| Gender
(male/female) | 14/16 | 40/45 | |
| Mean age
(years) | 58.00±12.31 | 61.76±11.67 | NS |
| Duration of DM
(years) | - | 9.00
(4.00–15.00) | - |
| BMI
(kg/m2) | 27.37±4.21 | 28.59±4.48 | NS |
| Glucose
(mg/dl) | 81.50
(76.00–86.25) | 140.00
(116.50–187.50) | <0.001 |
| HbA1c (%) | 4.15
(3.8–5.20) | 7.76
(6.69–6.79) | <0.001 |
| Insulin
(μU/ml) | 6.90
(4.43–9.15) | 8.80
(5.70–12.55) | <0.001 |
| HOMA-IR | 1.37
(0.90–1.97) | 3.05
(1.90–5.06) | <0.001 |
| SBP (mmHg) | 122.50
(120.00–130.00) | 130.00
(120.00–147.50) | <0.001 |
| DBP (mmHg) | 80.00
(73.50–81.25) | 80.00
(72.50–90.00) | NS |
| Medications | | | |
|
INS1/INS2/INS3 | - | 2/6/8 | |
|
SU1/SU2/SU3/SU4/SU5 | - | 5/12/8/11/5 | |
| Metformin
alone | - | 7 | |
| Metformin and
α-GI | - | 11 | |
| Diet alone | - | 10 | |
|
A/B/C/D/E/F/G/H/I | - |
6/5/4/8/6/12/7/5/14 | |
|
Statins/Fiber | - | 25/14 | |
| ASA | - | 42 | |
The control group consisted of 30 healthy control
subjects with no history of T2DM, other endocrine dysfunctions,
hyperlipidemia, hypertension, or coronary heart diseases. None of
the subjects had received any medication (hormone replacement
therapy, corticosteroids, vitamin supplements, antioxidant
formulations and thiazolidinediones) which may have affected
insulin resistance and/or endothelial function and none of the
subjects were current smokers and consumers of alcohol. Blood
pressure of all subjects was measured twice with a random zero
mercury sphygmomanometer after 10 min of rest.
All subjects were informed in regards to the aim of
the study. The study was approved by the Ethics Committee of
Eskişehir Osmangazi University Medical Faculty (2006/638).
Blood sample collection
Overnight fasting blood samples were drawn from the
patients and controls between 7.00 and 9.00 a.m. via the
venipuncture of an antecubital vein. Blood samples were collected
in vacutainer tubes with a gel separator and in heparinized tubes
for HbA1C measurements and were centrifugated at 2000 rpm for 15
min at 4°C after an incubation period of 30 min. All biochemical
variables were measured on the same day of the blood collection.
Remaining serum specimens were stored at −20°C until analysis of
adiponectin, leptin, resistin, visfatin and ADMA levels.
Biochemical analyses
Serum glucose, total cholesterol (TC),
HDL-cholesterol (HDL-C), triglyceride (TG) were measured by an
enzymatic colorimetric method and Apo-A1, Apo-B, lipo-protein (a)
and hs-CRP levels using an immunoassay method on a Hitachi Modular
analyzer using Roche Diagnostic kits. Insulin levels were measured
by chemiluminescence immunoassay method using DPC Immulite-I
analyzer (Diagnostic Products Corp., Los Angeles, CA, USA) kits.
HbA1C levels were measured by a turbidimetry immunoassay method
using Hitachi Modular analyzer with Roche Diagnostic kits. Serum
homocystein levels were measured using DPC kits on an Immulite-2000
autoanalyzer.
Serum adiponectin levels were measured by enzyme
linked immunosorbent assay (ELISA) using AviBion Human Adiponectin
(Orgenium Laboratories, Helsinki), with a sensitivity of 3 ng/ml.
Serum leptin levels were measured by an active human leptin ELISA
(DSL, Diagnostic System Laboratories, USA) with a sensitivity of
0.05 ng/ml. Serum resistin levels were measured by ELISA (Linco
Research, USA) with a sensitivity of 0.16 ng/ml. Serum visfatin
levels were measured by ELISA using the Biosource Immunoassay test
(Biosource International, Inc., USA) with a sensitivity of 602
pg/ml. Asymmetric dimethylarginine (ADMA) was measured using an
ELISA kit (Immundiagnostik AG, Bensheim) with a sensitivity 0.05
μg/ml.
Calculations
Low-density lipoprotein-cholesterol (LDL-C) was
calculated using the Friedewald formula. Standing height and body
weight were measured with the subjects dressed in light indoor
clothing without shoes. Body mass index (BMI) was calculated as
weight divided by the square of the height (kg/m2). In
order to determine insulin resistance, HOMA-IR index (Homeostasis
model assessment) was calculated as: HOMA = [(fasting serum glucose
(mmol/l) x fasting insulin (μU/ml)/22.5]. Another criteria for
insulin resistance, the ratio of leptin/adiponectin (L/A), was also
calculated.
Statistical analysis
Statistical analysis was performed using SPSS for
Windows (Statistical Package for the Social Sciences, version 20.0;
SSPS Inc. Chicago, IL, USA). The normal distribution of the
variables was evaluated using the Shapiro-Wilk test. The
Mann-Whitney U test was used for the comparison of variables which
were not normally distributed, and the independent Student’s t-test
for the comparison of variables which were normally distributed.
The Pearson and Spearman tests were used for the evaluation of
correlations among the variables according to the distribution of
variables. P<0.05 was accepted as indicative of statistical
significance. The results of variables with a normal distribution
were expressed as mean ± SD and those with a non-Gaussian
distribution were expressed as median (25th–75th percentile).
Results
Subject characteristics and anthropometric variables
are summarized in Table I. There
were no significant differences in age, BMI and DBP levels between
the T2DM patients and controls (P>0.05). In the T2DM patients,
glucose, insulin, HbA1C, HOMA-IR and SBP levels were significantly
higher than the values in the controls (P<0.001, for all).
Table II summarizes blood lipid
parameters and non-traditional CVD markers. T2DM patients had
significantly higher TC, LDL-C, TG, Lp (a), Apo-A1, Apo-B, Hcy and
ADMA levels, and L/A ratios than the controls (P<0.001 for each
comparison; for ADMA P<0.05).
 | Table II.Traditional and non-traditional
cardiovascular risk markers of the study groups. |
Table II.
Traditional and non-traditional
cardiovascular risk markers of the study groups.
| Controls | T2DM | P-value |
|---|
| TC (mg/dl) | 171.50
(162.00–185.25) | 192.00
(168.00–231.50) | <0.001 |
| TG (mg/dl) | 119.00
(98.50–144.25) | 200.00
(138.50–268.50) | <0.001 |
| HDL-C (mg/dl) | 56.43±9.15 | 41.45±9.64 | <0.001 |
| LDL-C (mg/dl) | 92.00
(80.45–105.70) | 112.80
(85.00–145.80) | <0.001 |
| Apo-A1(mg/dl) | 106.75
(146.10–172.45) | 131.20
(118.10–156.50) | <0.001 |
| Apo-B (mg/dl) | 95.45
(78.45–103.03) | 109.80
(91.40–122.90) | <0.001 |
| Lp (a) (mg/dl) | 21.25±5.14 | 45.07±16.37 | <0.001 |
| hs-CRP (mg/dl) | 0.30
(0.18–0.40) | 1.45
(0.87–2.85) | <0.001 |
| Hcy (mg/dl) | 7.67±1.86 | 10.92±3.39 | <0.001 |
| ADMA (μg/ml) | 0.56
(0.54–0.70) | 0.76
(0.59–0.99) | <0.05 |
| L/A ratio | 0.58
(0.47–0.86) | 2.01
(1.03–3.82) | <0.001 |
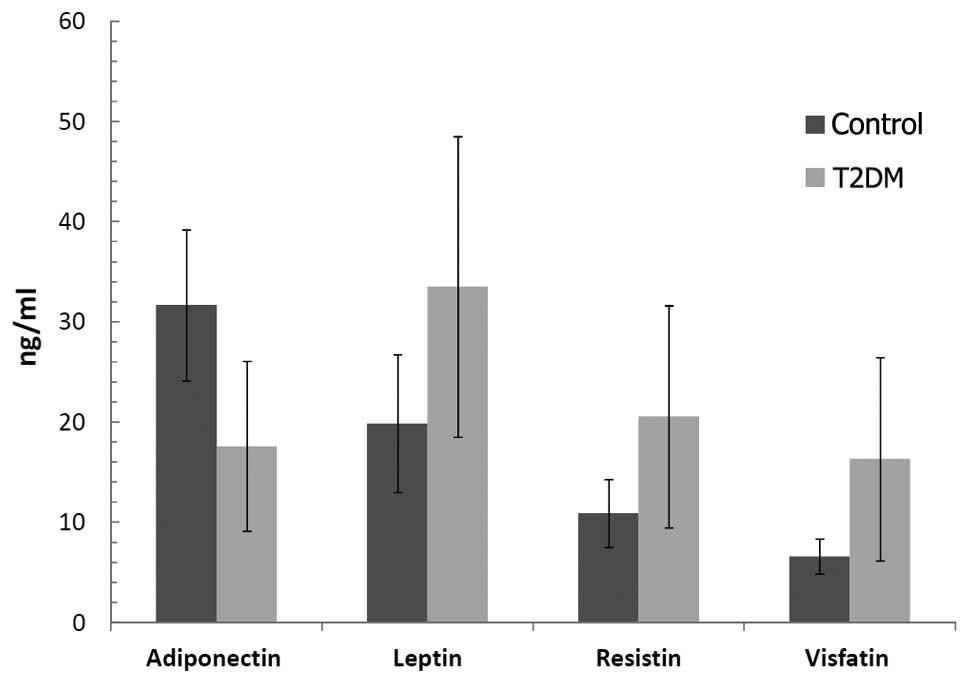
Fig. 1 illustrates
the comparison of adiponectin, leptin, resistin and visfatin levels
in the T2DM patients and control subjects. Serum adiponectin levels
were decreased significantly (P<0.001), while leptin, resistin
and visfatin levels were increased in the T2DM patients compared to
these levels in the controls (P<0.001, for each comparison).
Table III
represents the relationship between serum adiponectin, leptin,
resistin and visfatin levels and anthropometric variables.
Adiponectin was significantly and negatively correlated with HbA1C
levels, BMI, HOMA-IR, SBP and DBP. Leptin was positively correlated
with age, BMI, glucose and insulin levels, HOMA-IR, SBP and DBP.
Resistin was positively correlated with DM duration, BMI, HOMA-IR,
SBP and DBP. Visfatin was positively correlated with BMI, insulin,
HbA1C, HOMA-IR, SBP and DBP levels.
 | Table III.Relationship between adiponectin,
leptin, resistin and visfatin and anthropometric variablesa. |
Table III.
Relationship between adiponectin,
leptin, resistin and visfatin and anthropometric variablesa.
| Age (years) | DM duration
(years) | BMI
(kg/m2) | HOMA-IR | Glucose
(mg/dl) | Insulin (μU/l) | HbA1C (%) | SBP (mmHg) | DBP (mmHg) |
|---|
| A, (ng/ml) | −0.18 | −0.13 | −0.32c | −0.26b | −0.13 | −0.20 | −0.22b | −0.27b | −0.26b |
| L, (ng/ml) | 0.30b | 0.21 | 0.37c | 0.37c | 0.24b | 0.34c | 0.24b | 0.22b | 0.24b |
| R, (ng/ml) | −0.21 | −0.12 | −0.30c | −0.25b | −0.14 | −0.20 | −0.14 | −0.31c | −0.26b |
| V, (ng/ml) | −0.19 | −0.14 | −0.23b | −0.26b | −0.17 | −0.26b | −0.27b | −0.21b | −0.26b |
Table IV represents
the relationship between serum levels of adiponectin, leptin,
resistin and visfatin and traditional and non-traditional CVD risk
markers in T2DM patients. Adiponectin was positively correlated
with TG, LDL-C, Apo-A1, Hcy, ADMA and TG, hs-CRP, DBP and L/A ratio
and negatively with HDL-C. Leptin was positively correlated with
TG, Lp (a), Apo-A1, Apo-B, hs-CRP, Hcy, ADMA and L/A ratio and
negatively correlated with HDL-C. Resistin was positively with TC,
TG, Apo-A1, Apo-B, hs-CRP, Hcy and L/A ratio and negatively
correlated with HDL-C. Visfatin was correlated positively with TC,
TG, LDL-C, hs-CRP, Hcy and L/A ratio.
 | Table IV.Relationship between adiponectin,
leptin, resistin and visfatin with traditional and non-traditional
cardiovascular risk markersa. |
Table IV.
Relationship between adiponectin,
leptin, resistin and visfatin with traditional and non-traditional
cardiovascular risk markersa.
| Adiponectin
(ng/ml) | Leptin (ng/ml) | Resistin
(ng/ml) | Visfatin
(ng/ml) |
|---|
| TC (mg/dl) | −0.17 | 0.13 | 0.24b | 0.37c |
| TG (mg/dl) | −0.35c | 0.33c | 0.25b | 0.36c |
| HDL-C (mg/dl) | 0.36c | −0.25b | −0.28b | −0.16 |
| LDL-C (mg/dl) | −0.10c | 0.14 | 0.13 | 0.30c |
| Lp (a) (mg/dl) | −0.17 | 0.23b | 0.11 | 0.19 |
| Apo-A1 (mg/dl) | −0.30c | 0.24b | 0.21b | 0.20 |
| Apo-B (mg/dl) | −0.12 | 0.16 | 0.24b | 0.11 |
| hs-CRP (mg/dl) | −0.30c | 0.27b | 0.26b | 0.31c |
| Hcy (mg/dl) | −0.26b | 0.30c | 0.23b | 0.25b |
| ADMA (μg/ml) | 0.25b | 0.24b | 0.10 | 0.16 |
| L/A ratio | −0.77c | 0.63c | 0.45c | 0.49c |
| Visfatin
(ng/ml) | −0.55c | 0.52c | 0.39c | - |
| Resistin
(ng/ml) | −0.57c | 0.43c | - | |
| Leptin (ng/ml) | −0.55c | - | | |
Discussion
During the past few years, much attention has been
focused on the potential role of adipose tissue in the development
of vascular complications of diabetes. This study was designed to
explore the relationship between adipocytokines and traditional and
non-traditional risk markers and with anthropometric measurements
in T2DM. Adiponectin, an adipocytokine secreted by fat cells, has
regulatory functions on energy metabolism; its low levels are
predictive of future development of T2DM. Moreover, it is likely to
have a central role in the pathogenesis of T2DM (10).
In a recent meta-analysis, Li et al observed
a significant inverse relationship between plasma adiponectin
levels and the incidence of T2DM. Risk of T2DM appeared to decrease
with increasing adiponectin levels. Currently, adiponectin is one
of the strongest biochemical predictors of T2DM (11). In this study, diabetic patients
showed significantly decreased serum adiponectin levels compared to
the controls. Also, serum levels of lipids and lipoproteins (TG,
LDL-C, Apo-A1 and Apo-B) were significantly increased but HDL-C was
decreased with decreasing adiponectin levels, as reported in
previous studies (12–14). Several studies have also reported a
positive correlation between plasma adiponectin and HDL-C levels
(15). These data strongly suggest
a possible direct link between adiponectin and HDL-C. Moreover,
Apo-A1 and HDL-C levels were found to be inversely correlated with
the incidence of metabolic syndrome, which is associated with an
excess risk of T2DM and cardiovascular disease (16). Low adiponectin levels and increased
HDL core TG content resulting from increased neutral lipid exchange
between triglyceride-rich lipoproteins and HDL in
hypertriglyceridemic, insulin-resistant states may decrease HDL
particle numbers and cholesterol content, potentially by enhancing
catabolism of HDL particles (15,17).
Furthermore, we found a correlation between serum levels of
adiponectin and increased LDL-C levels in T2DM patients. Lautamäki
et al suggest that antioxidant effects of adiponectin on LDL
particles in the arterial wall may result in decreased production
of oxidized LDL lipoprotein (OxLDL), which may be beneficial in
patients with coronary artery disease by promoting plaque stability
(12).
Serum adiponectin levels were found to be negatively
correlated with BMI and HbA1C levels and no significant correlation
was found between serum adiponectin levels and age, DM duration,
glucose and insulin levels, as reported in previous studies
(12). In the present study, we
also examined the association between adipocytokines and hs-CRP, an
inflammatory marker. Chronic inflammation is likely to play a role
in the pathogenesis of T2DM. We found that adiponectin was
negatively correlated with hs-CRP levels. Krakoff et al have
revealed that adiponectin may be an important link between
adiposity and inflammation and T2DM (18). Elevated serum hs-CRP may play a
role in the development of insulin resistance syndrome and T2DM.
However, this elevation is accompanied by decreased levels of
adiponectin, suggesting an anti-inflammatory effect of adiponectin.
Since adiponectin could counteract the pro-inflammatory effects of
TNF-α in vascular cell components and adipose tissue, it may also
influence IL-6 and CRP production through the modulation of TNF-α
action. Therefore, adiponectin may directly or indirectly affect
CRP levels in plasma and adipose tissue through modulating
inflammatory cascades (19).
There is a growing body of evidence that leptin is
an independent risk factor for CVD and is likely to be an important
link in the development of cardiovascular risk and obesity
(20). Leptin plays an important
role in the long-term regulation of body weight. Paradoxically,
markedly increased plasma leptin levels were found in obese
individuals, suggesting a resistance to its effects on target
organs when produced excessively (21). We found that increased leptin
levels were correlated with BMI and insulin resistance (HOMA-IR) in
T2DM patients, as reported in previous studies (20,21).
Insulin resistance was reported to contribute to hyperleptinemia
indirectly (22). Abdella et
al reported that the hyperinsulinemia that frequently
accompanies obesity is likely to result in increased ob gene
expression and higher plasma leptin levels. Therefore, the
association found between leptin and insulin may simply reflect the
size of adipose tissue stores (23). High leptin levels generally
associated with high insulin levels could be partially explained by
resistance to leptin such that chronically elevated leptin levels
in obesity may result in decreased responsiveness of the receptor
system in pancreatic β cells, leading to increased insulin
secretion. The resulting hyperinsulinemia in turn could exacerbate
obesity and further increase leptin levels, resulting in a positive
feedback loop that could promote the development of diabetes
(24,25). Accordingly, we found a close
relationship between insulin and leptin levels in T2DM patients.
Additionally, leptin levels positively correlated with TG, Lp (a),
Apo-A1, glucose, SBP and DBP levels and negatively with HDL-C
levels in T2DM patients. Notably, hyperleptinemia in obesity is
suggested to dysregulate blood pressure that results in
hypertension, suggesting that leptin may be a potential predictor
of hypertension (26).
Another potential physiological role for leptin is
that the leptin-mediated T-cell immune response stimulates
proliferation of T-helper cells and increases production of
pro-inflammatory cytokines through regulation of immune cells
(20). We found an association
between serum leptin levels and hs-CRP, an inflammatory marker that
is related to both metabolic syndrome and cardiovascular events.
Adipose tissue is the source of circulating leptin and also
synthesizes IL-6, which induces CRP synthesis in the liver. Leptin
may also directly induce production of IL-6, resulting in further
upregulation of hepatic CRP production (20). Thus, serum leptin levels may be
used as an integrated marker of adiposity, insulin resistance and
vascular dysfunction that will be useful in clinical practice for
cardiovascular risk stratification. In addition, leptin and
adiponectin have opposing effects on subclinical inflammation.
Leptin upregulates cytokines such as tumor necrosis factor-α
(TNF-α) and IL-6, that are associated with insulin resistance in
T2DM and it can be considered as a pro-inflammatory cytokine. In
contrast, adiponectin downregulates the expression and release of
many pro-inflammatory immune mediators and exerts anti-inflammatory
properties (21). Thorand et
al indicated that the two adipokines, leptin and adiponectin,
interact with each other in the modulation of T2DM risk, but
adiponectin is likely to have a stronger association with T2DM risk
compared to leptin (25). We also
found an inverse relationship between leptin and adiponectin in
T2DM patients. Although leptin or adiponectin was separately
associated with the risk of T2DM, the association of T2DM risk with
leptin/adiponectin ratio (L/A) was stronger than that with leptin
alone or with adiponectin alone (27). Accordingly, Oda et al
suggested that the L/A ratio may be a useful index for insulin
resistance in clinical practice. In addition, it may also be a good
indicator for assessing the effectiveness of antidiabetic therapy.
We, therefore, calculated the HOMA-IR index and L/A ratio to reveal
insulin resistance. As expected, adiponectin and leptin were
correlated with L/A ratio negatively and positively,
respectively.
Resistin is another protein secreted by adipocytes
and leads to insulin resistance in vitro and in vivo,
constituting a possible link between obesity and diabetes (28). In the present study, we found
markedly higher serum resistin levels in T2DM patients compared to
controls. Mean serum resistin levels were correlated positively
with BMI and insulin resistance (as assessed by HOMA-IR and the L/A
ratio). The initial concept of resistin being the link between
obesity, insulin resistance and diabetes is currently debated
(29). Mojiminiyi et al
found that resistin is positively associated with markers of
obesity, inflammation and insulin resistance but negatively
correlated with markers of insulin sensitivity. Furthermore, these
associations were likely to be dependent on BMI, suggesting a
possible link between resistin levels and the insulin resistance
and low-grade inflammation accompanying obesity (30). As resistin may have a role in the
development of insulin resistance, serum resistin levels could be a
surrogate marker for the determination of insulin resistance
(31).
In the present study, serum resistin levels were
positively correlated with BMI, HOMA-IR, SBP, DBP, TC, TG, Lp (a),
Apo-A1, leptin and negatively correlated with HDL-C and adiponectin
levels in patients with T2DM. There were no correlations with age,
DM duration, glucose, insulin and HbA1c levels (28,29).
Hyperresistinemia would contribute to the pathogenesis of
hypertension in patients with T2DM (31). On the other hand, resistin levels
were related with hs-CRP, similar to the results of Mojiminiyi
et al suggesting a possible link between resistin and
obesity and insulin resistance via pro-inflammatory pathways
(29). Collectively, these data
suggest that resistin is related to the cardiovascular inflammatory
state in humans. Accordingly, CRP production induced by resistin
may explain the correlation between obesity (BMI) and resistin
levels (33). Several studies have
shown that pro-inflammatory cytokines such as IL-6, TNF-α, leptin
and possibly resistin induce CRP production (34). The positive correlation found
between leptin and resistin in our study may also be explained by
the same mechanism. We also found a negative correlation between
serum adiponectin and resistin levels. Lau et al reported
similar results and also suggested that the novel
adiponectin-resistin (AR) and insulin resistance (IRAR) indices are
cost-effective, precise, reproducible and reliable measures of
insulin sensitivity (35). In
addition, resistin has a pro-inflammatory role, which was shown to
stimulate several factors, such as ET-1 (endothelin-1), VCAM-1
(vascular cell adhesion molecule-1),and MCP-1 (monocyte
chemoattractant protein-1). Several studies have suggested resistin
as a cardiovascular risk factor and examined its role in
endothelial dysregulation and atherosclerotic lesion formation
(9).
Visfatin is a newly discovered adipocytokine
secreted by intra-abdominal adipose tissue. Several clinical
studies have analyzed the relationship between visfatin and insulin
resistance, diabetes and obesity. However, these studies have
provided disparate results (9,36,37).
In a meta-analysis, Chang et al suggested that the use of
visfatin may predict obesity, diabetes status, insulin resistance,
metabolic syndrome and cardiovascular disease (38). In this study, we found increased
visfatin levels in T2DM patients, as reported in previous studies
(39,40). In addition, visfatin levels were
positively correlated with HOMA-IR, L/A ratio and insulin levels in
T2DM patients (41). El-Masallamy
et al provided several explanations for the increased
visfatin levels noted in patients with T2DM. Firstly, increased
visfatin levels in diabetic patients may be due to impaired
visfatin signaling in target tissues. Secondly, due to the insulin
mimetic effects, increased plasma visfatin levels could be a
compensatory mechanism in response to hyperglycemia that
ameliorates the functional consequences of insulin deficiency or
resistance. Thirdly, visfatin-mediated NAD biosynthesis that
regulates glucose-stimulated insulin secretion may explain
increased levels of visfatin in T2DM patients as a compensatory
mechanism for β-cell functioning. Finally, because of the
pro-inflammatory properties, these elevated levels could be
attributed to the chronic low-grade inflammation present in T2DM
(39).
On the other hand, a direct or indirect relationship
may exist between visfatin and lipid metabolism. The relationship
of visfatin with lipid profile might be a compensatory mechanism
for diabetic dyslipidemia, since visfatin upregulates peroxi-some
proliferator-activated receptor γ activity (39). We found that visfatin levels were
positively correlated with markers of lipid metabolism such as TC,
TG and LDL-C, as reported in previous studies (42). Also, serum glucose was an
independent predictor of serum visfatin levels. Consistent with our
findings, Sandeep et al showed that the visfatin secretion
from adipocytes depends on the duration and extent of hyperglycemia
(40). Accordingly, Shaker et
al reported that high visfatin levels were positively related
to glycemic control (41).
Therefore, higher glucose and HbA1C levels may contribute to, at
least partly, the increased visfatin levels found in T2DM
patients.
Several studies have demonstrated that peripheral
insulin resistance plays an important role in the development of
endothelial dysfunction and cardiovascular disease. In addition,
impaired nitric oxide (NO) bioavailability may also be responsible
for the vascular complications noted in T2DM (43). At this point, asymmetric
dimethylarginine (ADMA), the major endogenous inhibitor of NO
synthase (NOS), may be suggested as a causative factor for
endothelial dysfunction. Increased plasma levels of ADMA are
associated with several clinical conditions involving endothelial
dysfunction, including hypertension, hypercholesterolemia, diabetes
mellitus and cardiovascular disease (44,45).
Hyperglycemia and poor glycemic control may be the underlying
mechanism of endothelial dysfunction in T2DM (46). Hyperglycemia impairs
dimethylarginine dimethylaminohydrolase (DDAH) and causes the
accumulation of ADMA, resulting in disturbances in NO pathways in
vessels (46). We found increased
ADMA levels in T2DM patients and the correlation of glucose,
insulin and HbA1C levels with ADMA levels (data not shown).
Notably, ADMA levels were correlated with hypoadiponectinemia and
hyperleptinemia but not with hyperresistinemia and
hypervisfatinemia. In fact, adiponectin and leptin increases NO
production in vascular endothelium and inhibits endothelial cell
activation, and both effects would be impaired due to decreased
adiponectin levels, leptin resistance and/or increased ADMA levels.
Because of the correlation found between all adipocytokines and
systolic and diastolic blood pressures, we also expected to find a
correlation between ADMA levels and visfatin and resistin, but
there was no such correlation.
Another factor that can affect endothelial function
is hyperhomocysteinemia (HHcy) (43). High Hcy levels have been associated
with an increased risk of stroke and other CV events (47). In the present study, T2DM patients
had higher Hcy levels than the controls, similar to the results
reported by Akalin et al (48). Emerging evidence suggests that
hyperhomocysteinemia is associated with adipose tissue dysfunction.
Wang et al showed that Hcy inhibits lipolysis in adipocytes
through AMPK activation and also reported that exogenous Hcy in the
diet for 2 weeks lowered circulating glycerol and FFA levels.
Moreover, Hcy supplementation was associated with increased leptin
levels and decreased adiponectin levels in plasma (49). We found that Hcy levels were
correlated positively with leptin, resistin and visfatin levels and
negatively correlated with adiponectin level. HHcy may promote
insulin resistance through increased resistin secretion from
adipocytes via the activation of reactive oxygen species
(ROS)-protein kinase C (PKC)-nuclear factor (NF)-κβ pathway
(50).
The results of the present study demonstrated that
secretion of adiponectin leptin, resistin and visfatin is altered
in subjects with T2DM clearly suggesting that they may be related
to obesity, hypertension and cardiovascular disease. These
adipocytokines may be an important link between increased fat mass,
insulin resistance, disorders of lipid and glucose metabolism and
endothelial dysfunction in diabetic patients. Future studies on
these adipocytokines may shed new light on the prevention and
treatment of T2DM, and offer a new field for the development of
novel drugs with which to alleviate insulin resistance and obesity.
In summary, these results suggest that decreased serum adiponectin
and increased leptin, resistin and visfatin levels in T2DM may be
novel biochemical risk factors for cardiovascular
complications.
Acknowledgements
This study was supported by grants
from the Scientific Research Projects Commission of Eskişehir
Osmangazi University (2006-11031).
References
|
1
|
Bartels DW, Davidson MH and Gong WC: Type
2 diabetes and cardiovascular disease: reducing the risk. J Manag
Care Pharm. 13:S2–S15. 2007.PubMed/NCBI
|
|
2
|
Bakker W, Eringa EC, Sipkema P and van
Hinsbergh VW: Endothelial dysfunction and diabetes: roles of
hyperglycemia, impaired insulin signaling and obesity. Cell Tissue
Res. 335:165–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lebovitz HE: Insulin resistance – a common
link between type 2 diabetes and cardiovascular disease. Diabetes
Obes Metab. 8:237–249. 2006.
|
|
4
|
Kowalska I: Role of adipose tissue in the
development of vascular complications in type 2 diabetes mellitus.
Diabetes Res Clin Pract. 78:14–22. 2007. View Article : Google Scholar
|
|
5
|
Kadowaki T, Yamauchi T, Kubota N, Hara K,
Ueki K and Tobe K: Adiponectin and adiponectin receptors in insulin
resistance, diabetes, and the metabolic syndrome. J Clin Invest.
116:1784–1792. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagawa T, Taniguchi A, Fukushima M,
Nakai Y, Nagasaka S, Ohgushi M, Matsumoto K, Kuroe A, Ohya M and
Seino Y: Leptin, triglycerides, and interleukin 6 are independently
associated with C-reactive protein in Japanese type 2 diabetic
patients. Diabetes Res Clin Pract. 75:2–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rabe K, Lehrke M, Parhofer KG and Broedl
UC: Adipokines and insulin resistance. Mol Med. 14:741–751. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gulcelik NE, Usman A and Gürlek A: Role of
adipocytokines in predicting the development of diabetes and its
late complications. Endocrine. 36:397–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gualillo O, González-Juanatey JR and Lago
F: The emerging role of adipokines as mediators of cardiovascular
function: physiologic and clinical perspectives. Trends Cardiovasc
Med. 17:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Snehalatha C, Mukesh B, Simon M,
Viswanathan V, Haffner SM and Ramachandran A: Plasma adiponectin is
an independent predictor of type 2 diabetes in Asian indians.
Diabetes Care. 26:3226–3229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li S, Shin HJ, Ding EL and van Dam RM:
Adiponectin levels and risk of type 2 diabetes: a systematic review
and meta-analysis. JAMA. 302:179–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lautamäki R, Rönnemaa T, Huupponen R,
Lehtimäki T, Iozzo P, Airaksinen KE, Knuuti J and Nuutila P: Low
serum adiponectin is associated with high circulating oxidized
low-density lipo-protein in patients with type 2 diabetes mellitus
and coronary artery disease. Metabolism. 56:881–886.
2007.PubMed/NCBI
|
|
13
|
Jaleel F, Jaleel A, Aftab J and Rahman MA:
Relationship between adiponectin, glycemic control and blood lipids
in diabetic type 2 postmenopausal women with and without
complication of ischemic heart disease. Clin Chim Acta. 370:76–81.
2006. View Article : Google Scholar
|
|
14
|
Hotta K, Funahashi T, Arita Y, Takahashi
M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K,
et al: Plasma concentrations of a novel, adipose-specific protein,
adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc
Biol. 20:1595–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vergès B, Petit JM, Duvillard L, Dautin G,
Florentin E, Galland F and Gambert P: Adiponectin is an important
determinant of apoA-I catabolism. Arterioscler Thromb Vasc Biol.
26:1364–1369. 2006.
|
|
16
|
Han R, Lai R, Ding Q, Wang Z, Luo X, Zhang
Y, Cui G, He J, Liu W and Chen Y: Apolipoprotein A-I stimulates
AMP-activated protein kinase and improves glucose metabolism.
Diabetologia. 50:1960–1968. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schulze MB, Shai I, Rimm EB, Li T, Rifai N
and Hu FB: Adiponectin and future coronary heart disease events
among men with type 2 diabetes. Diabetes. 54:534–539. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krakoff J, Funahashi T, Stehouwer CD,
Schalkwijk CG, Tanaka S, Matsuzawa Y, Kobes S, Tataranni PA, Hanson
RL, Knowler WC and Lindsay RS: Inflammatory markers, adiponectin,
and risk of type 2 diabetes in the Pima Indian. Diabetes Care.
26:1745–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan G, Zhou L, Tang J, Yang Y, Gu W, Li
F, Hong J, Gu Y, Li X, Ning G and Chen M: Serum CRP levels are
equally elevated in newly diagnosed type 2 diabetes and impaired
glucose tolerance and related to adiponectin levels and insulin
sensitivity. Diabetes Res Clin Pract. 72:244–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wannamethee SG, Tchernova J, Whincup P,
Lowe GD, Kelly A, Rumley A, Wallace AM and Sattar N: Plasma leptin:
associations with metabolic, inflammatory and haemostatic risk
factors for cardiovascular disease. Atherosclerosis. 191:418–426.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reilly MP, Iqbal N, Schutta M, Wolfe ML,
Scally M, Localio AR, Rader DJ and Kimmel SE: Plasma leptin levels
are associated with coronary atherosclerosis in type 2 diabetes. J
Clin Endocrinol Metab. 89:3872–3878. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stefanović A, Kotur-Stevuljević J, Spasić
S, Bogavac-Stanojević N and Bujisić N: The influence of obesity on
the oxidative stress status and the concentration of leptin in type
2 diabetes mellitus patients. Diabetes Res Clin Pract. 79:156–163.
2008.PubMed/NCBI
|
|
23
|
Abdella NA, Mojiminiyi OA, Moussa MA, Zaki
M, Al Mohammedi H, Al Ozairi ES and Al Jebely S: Plasma leptin
concentration in patients with type 2 diabetes: relationship to
cardiovascular disease risk factors and insulin resistance. Diabet
Med. 22:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asakawa H, Tokunaga K and Kawakami F:
Relationship of leptin level with metabolic disorders and
hypertension in Japanese type 2 diabetes mellitus patients. J
Diabetes Complications. 15:57–62. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thorand B, Zierer A, Baumert J, Meisinger
C, Herder C and Koenig W: Associations between leptin and the
leptin/adiponectin ratio and incident type 2 diabetes in
middle-aged men and women: results from the MONICA/KORA Augsburg
study 1984–2002. Diabet Med. 27:1004–1011. 2010.PubMed/NCBI
|
|
26
|
Maenhaut N and van de Voorde J: Regulation
of vascular tone by adipocytes. BMC Med. 16:9–25. 2011.
|
|
27
|
Oda N, Imamura S, Fujita T, Uchida Y,
Inagaki K, Kakizawa H, Hayakawa N, Suzuki A, Takeda J, Horikawa Y
and Itoh M: The ratio of leptin to adiponectin can be used as an
index of insulin resistance. Metabolism. 57:268–273. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu HL, Wang HW, Wen Y, Zhang MX and Lin
HH: Roles of adipocyte derived hormone adiponectin and resistin in
insulin resistance of type 2 diabetes. World J Gastroenterol.
12:1747–1751. 2006.PubMed/NCBI
|
|
29
|
Mojiminiyi OA and Abdella NA: Associations
of resistin with inflammation and insulin resistance in patients
with type 2 diabetes mellitus. Scand J Clin Lab Invest. 67:215–225.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kusminski CM, McTernan PG and Kumar S:
Role of resistin in obesity, insulin resistance and Type II
diabetes. Clin Sci (Lond). 109:243–256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tokuyama Y, Osawa H, Ishizuka T, Onuma H,
Matsui K, Egashira T, Makino H and Kanatsuka A: Serum resistin
level is associated with insulin sensitivity in Japanese patients
with type 2 diabetes mellitus. Metabolism. 56:693–698. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takata Y, Osawa H, Kurata M, Kurokawa M,
Yamauchi J, Ochi M, Nishida W, Okura T, Higaki J and Makino H:
Hyperresistinemia is associated with coexistence of hypertension
and type 2 diabetes. Hypertension. 5:534–539. 2008. View Article : Google Scholar
|
|
33
|
de Luis DA, Sagrado MG, Conde R, Aller R,
Izaola O, de la Fuente B, Castrillón JL and Romero E: Relation of
resistin levels with cardiovascular risk factors, insulin
resistance and inflammation in naïve diabetes obese patients.
Diabetes Res Clin Pract. 89:110–114. 2010.PubMed/NCBI
|
|
34
|
Al-Daghri N, Chetty R, McTernan PG,
Al-Rubean K, Al-Attas O, Jones AF and Kumar S: Serum resistin is
associated with C-reactive protein and LDL cholesterol in type 2
diabetes and coronary artery disease in a Saudi population.
Cardiovasc Diabetol. 4:1–6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lau CH and Muniandy S: Novel
adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are
useful integrated diagnostic biomarkers for insulin resistance,
type 2 diabetes and metabolic syndrome: a case control study.
Cardiovasc Diabetol. 10:82011. View Article : Google Scholar
|
|
36
|
Esteghamati A, Alamdari A, Zandieh A,
Elahi S, Khalilzadeh O, Nakhjavani M and Meysamie A: Serum visfatin
is associated with type 2 diabetes mellitus independent of insulin
resistance and obesity. Diabetes Res Clin Pract. 91:154–158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alghasham AA and Barakat YA: Serum
visfatin and its relation to insulin resistance and inflammation in
type 2 diabetic patients with and without macroangiopathy. Saudi
Med J. 29:185–192. 2008.PubMed/NCBI
|
|
38
|
Chang YH, Chang DM, Lin KC, Shin SJ and
Lee YJ: Visfatin in overweight/obesity, type 2 diabetes mellitus,
insulin resistance, metabolic syndrome and cardiovascular diseases:
a meta-analysis and systemic review. Diabetes Metab Res Rev.
27:515–527. 2011. View Article : Google Scholar
|
|
39
|
El-Mesallamy HO, Kassem DH, El-Demerdash E
and Amin AI: Vaspin and visfatin/Nampt are interesting interrelated
adipokines playing a role in the pathogenesis of type 2 diabetes
mellitus. Metabolism. 60:63–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sandeep S, Velmurugan K, Deepa R and Mohan
V: Serum visfatin in relation to visceral fat, obesity, and type 2
diabetes mellitus in Asian Indians. Metabolism. 56:565–570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shaker O, El-Shehaby A, Zakaria A, Mostafa
N, Talaat S, Katsiki N and Mikhailidis DP: Plasma visfatin and
retinol binding protein-4 levels in patients with type 2 diabetes
mellitus and their relationship to adiposity and fatty liver. Clin
Biochem. 44:1457–1463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen MP, Chung FM, Chang DM, Tsai JC,
Huang HF, Shin SJ and Lee YJ: Elevated plasma level of
visfatin/pre-B cell colony-enhancing factor in patients with type 2
diabetes mellitus. J Clin Endocrinol Metab. 91:295–299. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pereira EC, Ferderbar S, Bertolami MC,
Faludi AA, Monte O, Xavier HT, Pereira TV and Abdalla DS:
Biomarkers of oxidative stress and endothelial dysfunction in
glucose intolerance and diabetes mellitus. Clin Biochem.
41:1454–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Krzyzanowska K, Mittermayer F, Wolzt M and
Schernthaner G: ADMA, cardiovascular disease and diabetes. Diabetes
Res Clin Pract. 15:122–126. 2008. View Article : Google Scholar
|
|
45
|
Kanazawa I, Yano S, Notsu Y, Yamaguchi T,
Nabika T and Sugimoto T: Asymmetric dimethylarginine as a risk
factor for cardiovascular disease in Japanese patients with type 2
diabetes mellitus. Clin Endocrinol (Oxf). 74:467–472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yasuda S, Miyazaki S, Kanda M, Goto Y,
Suzuki M, Harano Y and Nonogi H: Intensive treatment of risk
factors in patients with type-2 diabetes mellitus is associated
with improvement of endothelial function coupled with a reduction
in the levels of plasma asymmetric dimethylarginine and endogenous
inhibitor of nitric oxide synthase. Eur Heart J. 27:1159–1165.
2006.
|
|
47
|
de Luis DA, Fernandez N, Arranz ML, Aller
R, Izaola O and Romero E: Total homocysteine levels relation with
chronic complications of diabetes, body composition, and other
cardiovascular risk factors in a population of patients with
diabetes mellitus type 2. J Diabetes Complications. 19:42–46.
2005.PubMed/NCBI
|
|
48
|
Akalin A, Alatas O and Colak O: Relation
of plasma homocysteine levels to atherosclerotic vascular disease
and inflammation markers in type 2 diabetic patients. Eur J
Endocrinol. 158:47–52. 2008. View Article : Google Scholar
|
|
49
|
Wang Z, Pini M, Yao T, Zhou Z, Sun C,
Fantuzzi G and Song Z: Homocysteine suppresses lipolysis in
adipocytes by activating the AMPK pathway. Am J Physiol Endocrinol
Metab. 301:703–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Jiang C, Xu G, Wang N, Zhu Y, Tang C
and Wang X: Homocysteine upregulates resistin production from
adipocytes in vivo and in vitro. Diabetes. 57:817–827. 2008.
View Article : Google Scholar : PubMed/NCBI
|