Introduction
Chronic hepatitis B (CHB) is an infectious disease
that severely harms individuals worldwide. Although new cases of
hepatitis B virus (HBV) infection are greatly reduced by the
application of a hepatitis B vaccine, >350 million individuals
are infected with HBV worldwide. Persistent HBV infection may lead
to cirrhosis or hepatocellular carcinoma, which threaten the lives
of patients (1). Virus-host
interactions, particularly the virus-specific T-cell response, are
the key factors accounting for the pathogenesis of HBV infection.
In contrast to the strong and multispecific T-cell responses
observed during acute self-limited HBV infection, patients with CHB
tend to have weak and narrowly focused immune responses (2).
CD4+ T cells play a vital role in
adaptive immune responses. They help B cells produce antibodies and
undergo class-switching, as well as affinity maturation. They
recruit and activate CD8+ T cells, macrophages and other
effector cells. T helper cells, differentiated from naive
CD4+ T cells, are classified into four major lineages
based on their function, pattern of cytokine secretion and
expression of specific transcription factors. The lineages are Th1,
Th2, Th17 and T regulatory cells (3,4). The
assistance of antibody production by T cells is a fundamental
aspect of immune responses. An improved understanding of the
cellular and molecular mechanisms of T cell actions has only
recently emerged. A subset of T cells named T follicular helper
cells (TFH cells) aid B cells and represents one of the largest and
most important subsets of effector T cells in lymphoid tissues
(5,6). The features of TFH cells include CXC
chemokine receptor 5 (CXCR5) expression, inducible co-stimulator
(ICOS), location/migration (B cell follicles) and function (B cell
help). TFH cells produce a ‘helper’ cytokine, interleukin (IL)-21,
which stimulates B cells to differentiate into antibody-forming
cells via the IL-21 receptor. The dysregulation of TFH cell
function likely contributes to the pathogenesis of immune-related
diseases (7).
Humoural immune responses following HBV infection
are significant in the pathogenesis of HBV infection. Hepatitis B
surface antigen (HBsAg)-specific antibodies neutralise and mediate
protective immunity. HBV-specific antibodies are indicators of
specific stages of the disease. Hepatitis B core antigen
(HBcAg)-specific immunoglobulin G (IgG) and HBsAg-specific
antibodies persist for life following clinical recovery (8). TFH cells are a special subset of T
helper cells that regulate humoural immune responses. However, the
role of TFH cells in the pathogenesis of HBV infection is unclear.
Therefore, in the present study, the levels of TFH cells and
related molecules were detected in various types of chronic HBV
infection by flow cytometry and enzyme-linked immunosorbent assay
(ELISA). The purpose was to investigate the role of TFH cells and
related molecules in the pathogenesis of CHB.
Materials and methods
Subjects
Blood samples were obtained with informed consent
from 85 patients infected with HBV and 44 healthy controls at the
Taizhou People’s Hospital from June to December 2011. There were 42
patients with CHB (male to female ratio, 29:13; average age,
40.7±11.2 years) and 43 HBV carriers (male to female ratio, 28:15;
average age, 41.3±11.6 years). Of the 42 patients with CHB, 18
hepatitis B extracellular antigen (HBeAg)+ patients and
24 HBeAg− patients were included. Of the 43 HBV
carriers, 21 chronic HBV carriers and 22 inactive HBsAg carriers
were included. The protocol was approved by the ethics committee of
the hospital. The diagnostic criteria were based on the 2010
Chronic Hepatitis B Prevention Guide of China (9). All patients tested negative for
antibodies against hepatitis A, C, D and E viruses, as well as
human immunodeficiency virus. Patients with a history and clinical
features of drug-induced liver injury, alcoholic hepatitis and
steatohepatitis were also excluded. Any patients who had been
treated with nucleoside/nucleotide analog antiviral or
immunomodulatory drugs in the previous six months were excluded.
There were 22 cases that were hepatitis B surface antibody
(HBsAb)+ following inoculation with a hepatitis B
vaccine (male to female ratio, 13:9; average age, 38.7±10.3 years)
and 22 HBsAb− cases who had not been inoculated with a
hepatitis B vaccine (male to female ratio, 10:12; average age,
40.5±10.6 years) included as healthy controls. Subjects who were
HBeAb− and/or HBcAb+ were excluded.
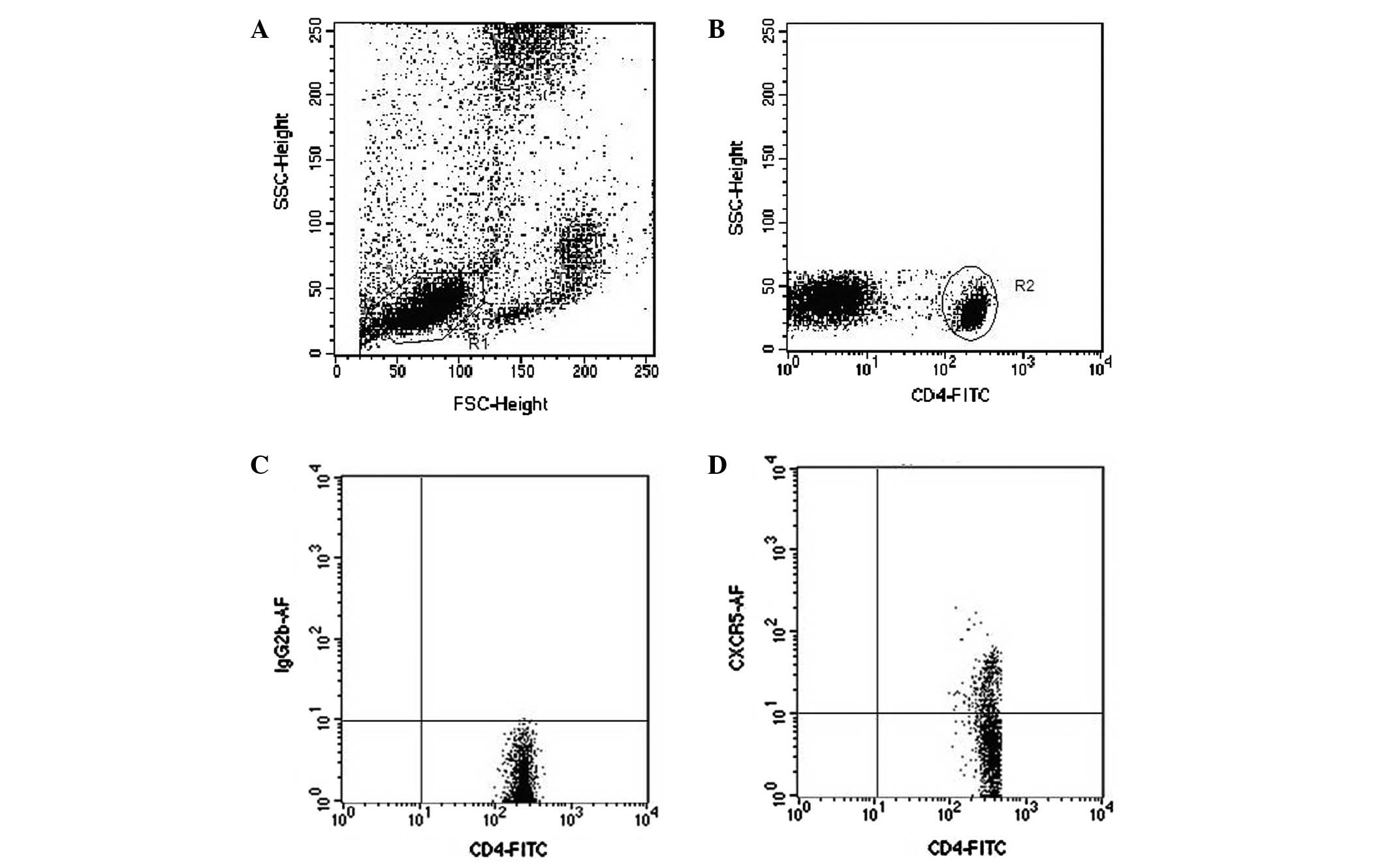
Flow cytometry analysis
Sodium citrate-treated whole blood (100 μl)
was added to 10 μl Alexa Fluor 647-conjugated anti-CXCR5 (BD
Company, San Jose, CA, USA) and 10 μl fluorescein
isothiocyanate (FITC)-conjugated anti-CD4 (eBioscience, San Diego,
CA, USA), then mixed and incubated for 30 min at room temperature.
Erythrocytes were lysed by adding 2 ml fluorescence-activated cell
sorting (FACS) lysing solution. The samples were analyzed on a FACS
cytometer using CellQuest™ software (Fig. 1). CD40L-PE/CD40-PE and CD19-FITC
were purchased from eBioscience. The expression of CD40L on the
surface of TFH cells and CD40 on the surface of CD19+ B
cells were detected as described above.
Cytokine detection
The level of IL-21 in stored peripheral plasma was
evaluated by ELISA. The kits were purchased from eBioscience and
used according to the manufacturer’s instructions. The detection
range for IL-21 in this kit was 16-2000 ng/l.
Detection of HBV DNA and serum
markers
The levels of HBV DNA were detected by fluorescence
quantitative polymerase chain reaction (PCR; lower detection limit,
103 copies/ml; Applied Biosystems, Foster City, CA,
USA). HBV PCR fluorescence quantitative detection kits were
purchased from Biological Engineering Co., Ltd. (Shanghai, China).
The serum markers of HBV, anti-HAV, anti-HCV, anti-HDV and
anti-HEV, were detected by ELISA. The kits were purchased from
Beijing Yuanpinghao Biotechnology Co., Ltd. (Beijing, China).
Statistical analysis
All values are expressed as the median and quartile
interval. Data analysis was conducted using SPSS 17.0 (SPSS Inc.,
Chicago, IL, USA). Nonparametric tests (Kruskal-Wallis H test) were
used for multiple group comparison. The Mann-Whitney U test was
used for two independent data. The Spearman correlation was used
between variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Proportion of TFH cells and levels of
CD40L expression on the surface of TFH cells
The proportion of TFH cells gated with
CD4+ T cells and CD40L expression level were detected by
flow cytometry in 42 patients with CHB, 43 HBV carriers and 44
healthy controls. Compared with the HBsAb− and
HBsAb+ healthy controls, patients with chronic HBV
infection had significantly increased percentages of TFH cells
(P<0.01). The percentage of TFH cells was higher in the patients
with CHB than in the chronic HBV carriers (P<0.01). The
percentage of CD40L in chronic HBV infected individuals was
significantly higher than in the HBsAb− and
HBsAb+ healthy controls (P<0.01). The pattern of
coexpression of CXCR5 and CD40L in the CD4+ T cells in
the different groups was similar to that of the TFH cells. No
significant difference was observed in the percentage of TFH cells
or CD40L between the HBsAb− and HBsAb+
healthy controls (Fig. 2).
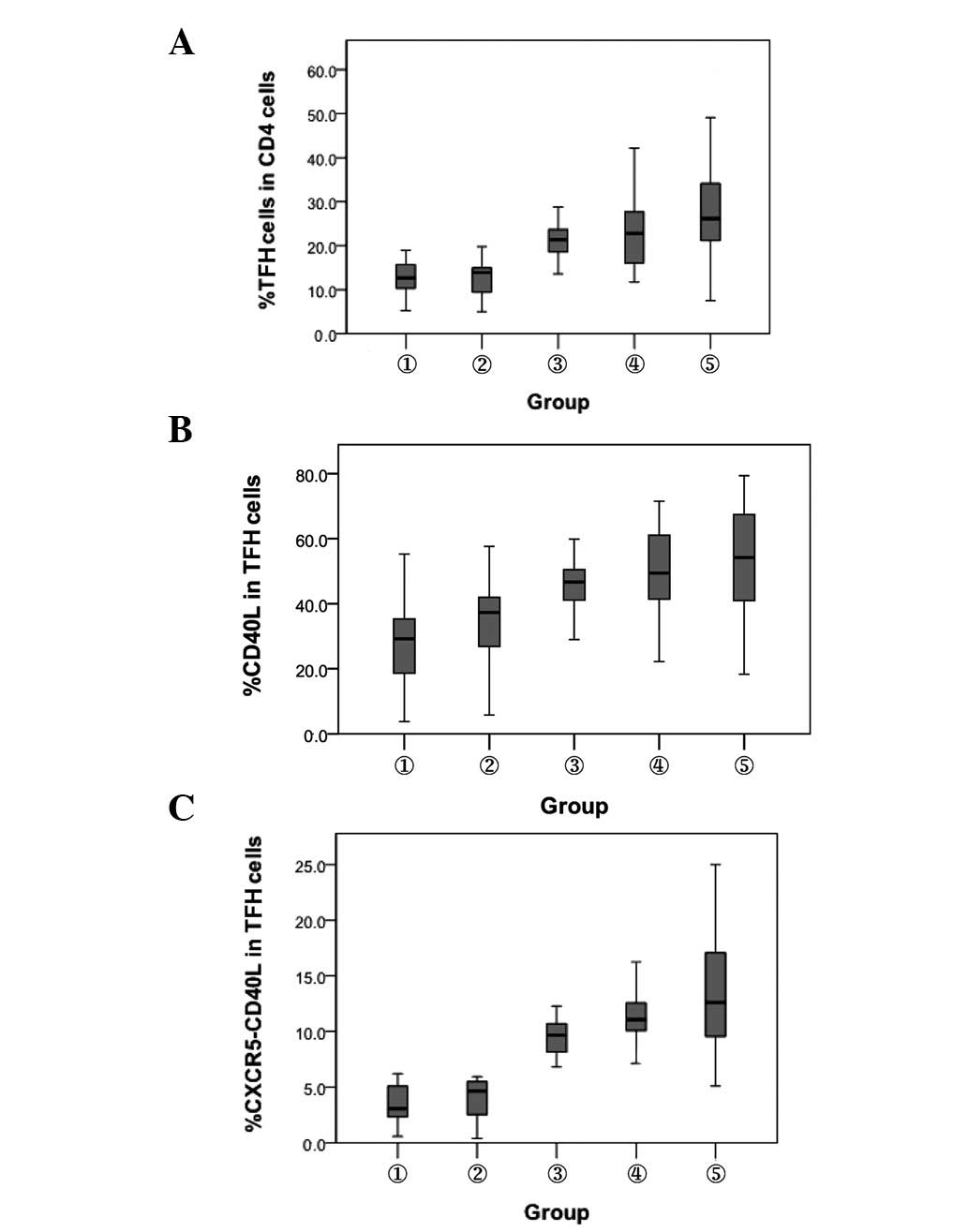 | Figure 2.Comparison of the expression of TFH
cells and their surface CD40L molecule. 1, HBsAb−
healthy controls; 2, HBsAb+ healthy controls; 3, chronic
HBV carriers; 4, inactive HBsAg carriers and 5, chronic hepatitis
B. (A) Percentage of TFH cells. P<0.01, between groups 1, 2 and
3, 4, 5. (B) Percentage of CD40L. P<0.01, between groups 1,2 and
3, 4, 5. (C) Coexpression of CXCR5+ CD40L+
cells. P<0.01, between groups 1, 2 and 3, 4, 5. TFH, T
follicular helper; HBsAb, hepatitis B surface antibody; HBV,
hepatitis B virus; HBsAg, hepatitis B surface antigen; CXCR5, CXC
chemokine receptor 5. |
Detection of the expression of
CD19+ B cells and their surface CD40 molecules in
different subjects
The percentage of CD19+ B cells and their
surface CD40 molecule expression were detected by flow cytometry in
different subjects. Compared with the HBsAb− and
HBsAb+ healthy controls, the percentage of
CD19+ B cells in patients with CHB increased
significantly (P<0.05). No significant difference was observed
between chronic HBV carriers and inactive HBsAg carriers
(P>0.05). The percentage of CD40 molecules on the surface of
CD19+ B cells in the CHB patients was lower than that in
the HBsAb− healthy controls (P<0.01). No significant
difference was observed among the remaining groups (P>0.05;
Fig. 3).
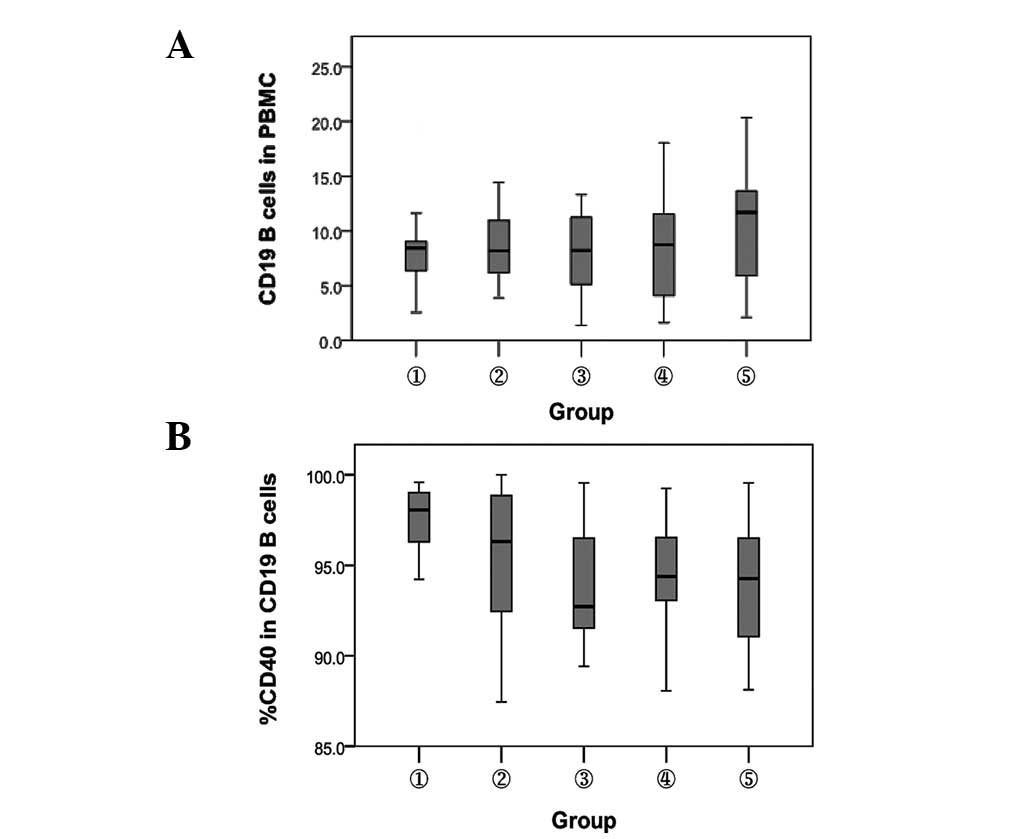 | Figure 3.Comparison of the expression of
CD19+ B cells and their surface CD40 molecule. 1,
HBsAb− healthy controls; 2, HBsAb+ healthy
controls; 3, chronic HBV carriers; 4, inactive HBsAg carriers and
5, chronic hepatitis B. (A) Percentage of CD19+ B cells.
P<0.05, between groups 3, 4 and 5. (B) Percentage of CD40.
P<0.01, between groups 1 and 3, 4 and 5. HBsAb, hepatitis B
surface antibody; HBV, heptitis B virus; HBsAg, hepatitis B surface
antigen; PBMC, peripheral blood mononuclear cells. |
Detection of plasma IL-21 expression in
different subjects
The plasma IL-21 expression level in the different
subjects was detected by ELISA. Compared with the HBsAb−
and HBsAb+ healthy controls (332.7±202.5 and 295.3±108.6
ng/l), plasma IL-21 expression was markedly decreased in the HBV
carriers and inactive HBsAg carriers (239.6±195.9 and 215.5±132.0
ng/l, respectively; P<0.05). However, plasma IL-21 expression in
the CHB patients (375.6±192.3 ng/l) was significantly higher than
that in the HBsAb+ healthy controls (P<0.05) and HBV
carriers or inactive HBsAg carriers (P<0.01). No significant
difference was identified among the other groups (P>0.05;
Fig. 4).
 | Figure 4.Comparison of IL-21 expression levels.
1, HBsAb− healthy controls; 2, HBsAb+ healthy
controls; 3, chronic HBV carriers; 4, inactive HBsAg carriers and
5, chronic hepatitis B. P<0.05, between the levels of IL-21 in
groups 1 and 3; 2 and 4, 5; P<0.01, between the levels of IL-21
in groups 1 and 4; 3, 4 and 5. IL, interleukin; HBsAb, hepatitis B
surface antibody; HBV, hepatitis B virus; HBsAg, hepatitis B
surface antigen. |
Correlation of TFH cells,
CD19+ B cells and IL-21 level with the clinical
indicators of CHB patients
The levels of HBV viral load, alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) of the
CHB patients were 5.7±2.8 (log10 copies/ml), 102±227.1 U/l and
88±152.2 U/l, respectively. No significant correlation was
identifed among the percentage of TFH cells, CD19+ B
cells, IL-21 level, HBV viral load level, ALT and AST (P>0.05).
There were 24 HBeAg− and 18 HBeAg+ cases
among 42 patients with CHB. No significant differences were
identified in the percentage of TFH cells and the expression level
of CD40L molecules between the HBeAg− and
HBeAg+ groups (P>0.05). The IL-21 expression level
was 402.2±156.7 ng/l in the HBeAg− group and 344.5±261.2
ng/l in the HBeAg+ group. No significant difference was
identified between the two groups (P>0.05; Table I).
 | Table I.Comparison of the levels of TFH and
IL-21 between the HBeAg− and HBeAg+
groups. |
Table I.
Comparison of the levels of TFH and
IL-21 between the HBeAg− and HBeAg+
groups.
| Group | n | TFH cell (%) | CD40L (%) | IL-21 (ng/l) |
|---|
|
HBeAg− | 24 | 25.2±15.2 | 51.4±18.0 | 402.2±156.7 |
|
HBeAg+ | 18 | 28.0±12.0a | 54.8±31.5a | 344.5±261.2a |
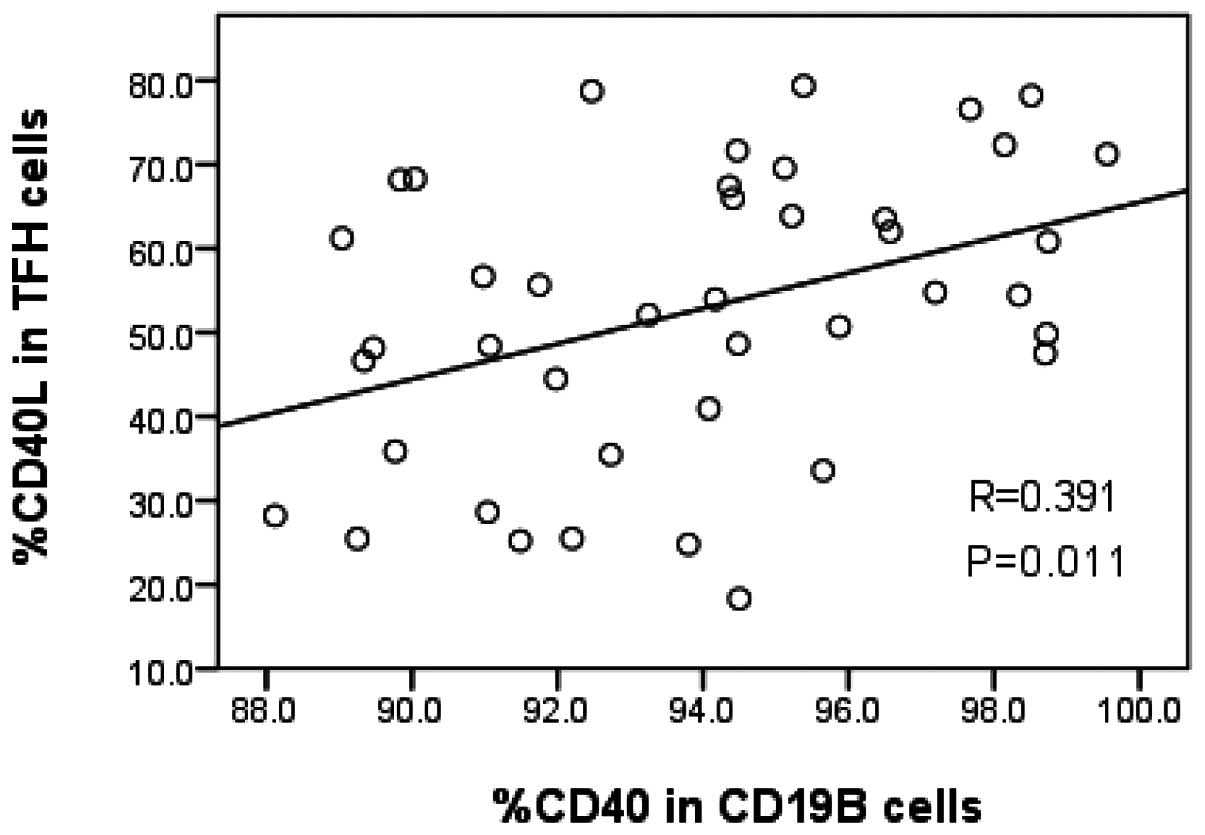
Correlation between the TFH cells and B
cells in patients with CHB
The percentage of TFH cells in CD4+ T
cells was 26.1±13.3% in the 42 patients with CHB. The level of
CD40L expression in the TFH cells was 54.2±27.9%. The percentage of
CD19+ B cells in peripheral blood mononuclear cells was
11.7±7.8%. The level of CD40 molecular expression in
CD19+ B cells was 94.3±5.4%. A positive correlation was
observed between CD40L expression in TFH cells and CD40 expression
in CD19+ B cells (r=0.391, P=0.011; Fig. 5). No correlation was observed
between the percentage of TFH cells and CD19+ B cells
(r=0.172, P=0.276).
Discussion
T helper cells are required for B cell-mediated
humoural immune responses. Previous studies have shown that Th2
cells play a key role in aiding B cell responses; however, TFH
cells have been recently recognised as the major subset that aids B
cell responses. A number of studies have reported on the role of
TFH cells in immune-related disease; however, few studies have
described their role in chronic HBV infection (7,10).
Feng et al(11) identified
that TFH cells are involved in the immune response in HBV infection
and their increase in number reflects the activation of the immune
response. The results of the present study revealed that the
percentage of TFH cells increased in patients with chronic HBV
infection compared with healthy subjects. The percentage of TFH
cells in the CHB patients was higher than that in the chronic HBV
carriers and inactive HBsAg carriers. These results suggest that
the elevation of TFH cells in CHB patients is associated with the
activation of anti-HBV immune responses and are consistent with the
study by Feng et al. Some studies showed that the expression
of CXCR5 in the activation of T cells is transient and rare, and is
only persistently expressed in TFH cells (6,12).
Therefore, the effect of CD4+ T cell activation on the
changes of TFH during HBV infection may be excluded.
The high expression level of CD40L in TFH cells
binding to CD40 in B cells plays an important role in stimulating B
cell proliferation, differentiation and immunoglobulin class
switching (13). Wu and Wen
demonstrated that the percentage of CD19+ B cells in CHB
atients was significantly higher than in healthy controls (14). In the present study, compared with
HBsAb− healthy controls, the percentage of
CD19+ B cells was elevated and the percentage of CD40
molecules on the surface of CD19+ B cells decreased in
patients with CHB. The percentage of CD40L molecules on the surface
of TFH cells in CHB patients was significantly elevated. There was
a positive correlation between the level of CD40L expression in TFH
cells and CD40 expression in CD19+ B cells. These
results suggest that the activation of B lymphocytes in patients
with CHB may be involved in the dysfunction of the humoural immune
response of CHB.
TFH cells produce numerous cytokines, including
IL-4, -10, -17 and -21, among which the most important is IL-21.
IL-21 is the major cytokine of TFH cells and also a key factor
affecting the formation of germinal centres. IL-21 is also known as
TFH cell helper factor (15). Hu
et al(16) reported that
IL-21 promotes B-cell proliferation and HBeAg− IgG
secretion in CHB patients and may play a role in the serological
conversion of HBeAg to HBeAb. The results of the current study
revealed that the IL-21 level decreased in the plasma of HBV and
inactive HBsAg carriers; however, it increased in CHB patients.
These results suggest that IL-21 expression may be correlated with
the immune response against HBV infection, similar to the
alteration of TFH cells. There was no clear difference between the
IL-21 levels of the HBeAg+ and HBeAg−
patients. No significant correlation was identified between IL-21
expression and the levels of HBV DNA, ALT and AST, which differs
from the results of the study by Feng et al(11). The cause of these discrepancies may
be related to patient selection and the research methods used.
In addition to promoting the differentiation of TFH
cells and stimulating B cell proliferation, IL-21 also promotes the
generation of interferon (IFN)-γ and counteracts regulatory T
cell-mediated immune suppression. Additionally, it enhances
CD8+ T cell and natural killer (NK) cell cytotoxicity
(17). A previous study
demonstrated that IL-21 participates in the immune response of
viral infection clearance in acute HBV infection. However, this
phenomenon is not observed in CHB patients (18). Decreased IL-21 production may block
the key function of CD8+ T cells and B cell response,
influencing the immune response against HBV. The results of the
current study revealed that the IL-21 level decreased in chronic
HBV carriers and inactive HBsAg+ carriers and the
percentage of TFH cells was significantly elevated. These results
suggest that the activity of TFH cells may decrease in chronic HBV
and inactive HBsAg+ carriers. The level of IL-21 and TFH
cells synchronously increased in the CHB patients. Yi et
al(19) reported that IL-21
and IL-21-producing cells (TFH cells) are important in generating
and maintaining multi-functional CD8+ T cells to clear
the viral infection. The TFH cell number and/or abnormal function,
as well as IL-21 expression deficiency, may be closely associated
with the chronicity of hepatitis B virus infection. However,
several studies have demonstrated that Th17 cells, CD8+
T cells and NK T cells also produce amounts of IL-21, in addition
to TFH cells (20). The effect of
these cells on the expression level of IL-21 requires further
research.
In conclusion, the results of the present study
suggest that the abnormal expression of TFH cells and IL-21 is
related to the dysfunction of the immune response during chronic
HBV infection. The interaction of CD19+ B cells with TFH
cells via their CD40 and CD40L molecules may be significant in this
process.
References
|
1.
|
Hong Y, Peng Y, Mi M, Xiao H, et al:
Lentivector expressing HBsAg and immunoglobulin Fc fusion antigen
induces potent immune responses and results in seroconversion in
HBsAg transgenic mice. Vaccine. 29:3909–3916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chisari FV: Rous-Whipple Award Lecture.
Viruses, immunity and cancer: lessons from hepatitis. B Am J
Pathol. 156:1117–1132. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhu J and Paul WE: Heterogeneity and
plasticity of T helper cells. Cell Res. 20:4–12. 2010. View Article : Google Scholar
|
|
4.
|
Durrant DM and Metzger DW: Emerging roles
of T helper subsets in the pathogenesis of asthma. Immunol Invest.
39:526–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Breitfeld D, Ohl L, Kremmer E, Ellwart J,
et al: Follicular B helper T cells express CXC chemokine receptor
5, localize to B cell follicles and support immunoglobulin
production. J Exp Med. 192:1545–1552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Schaerli P, Willimann K, Lang AB, Lipp M,
et al: CXC chemokine receptor 5 expression defines follicular
homing T cells with B cell helper function. J Exp Med.
192:1553–1562. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Crotty S: Follicular helper CD4 T cells
(TFH). Annu Rev Immunol. 29:621–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rehermann B and Nascimbeni M: Immunology
of hepatitis B virus and hepatitis C virus infection. Nat Rev
Immunol. 5:215–229. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chinese Society of Hepatology and Chinese
Society of Infectious Diseases, Chinese Medical Association: The
guideline of prevention and treatment of chronic hepatitis B (2010
version). Zhonghua Gan Zang Bing Za Zhi. 19:13–24. 2011.(In
Chinese).
|
|
10.
|
Fazilleau N, Mark L, McHeyzer-Wilimas LJ
and McHeyzer-Williams MG: Follicular helper cells: lineage and
locations. Immunity. 30:324–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Feng J, Lu L, Hua C, Qin L, et al: High
frequency of CD4+ CXCR5+ TFH cells in
patients with immune-active chronic hepatitis B. PLoS One.
6:e216982011.
|
|
12.
|
Haynes NM, Allen CD, Lesley R, Ansel KM,
et al: Role of CXCR5 and CCR7 in follicular Th cell positioning and
appearance of a programmed cell death gene-1 high germinal
center-associated subpopulation. J Immunol. 179:5099–5108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Vinuesa CG, Tangye SG, Moser B and Mackay
CR: Follicular B helper T cells in antibody responses and auto
immunity. Nature Rev Immunol. 5:853–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wu Y and Wen J: Role of B lymphocytes in
patients with chronic hepatitis B. Clinical Focus. 21:31–33.
2006.(In Chinese).
|
|
15.
|
Spolski R and Leonard WJ: IL-21 and T
follicular helper cells. Int Immunol. 22:7–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hu C, Chen C, Tan X and Shi T: Effects of
IL-21 on B lymphocytes proliferation and HBeAb production in
chronic hepatitis B patients. J Immunol. 27:126–129. 2011.(In
Chinese).
|
|
17.
|
Yi JS, Cox MA and Zajac AJ:
Interleukin-21: a multifunctional regulator of immunity to
infections. Microbes Infect. 12:1111–1119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Publicover J, Goodsell A, Nishimura S,
Vilarinho S, et al: IL-21 is pivotal in determining age-dependent
effectiveness of immune responses in a mouse model of human
hepatitis B. J Clin Invest. 121:1154–1162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yi JS, Du M and Zajac AJ: A vital role for
interleukin-21 in the control of a chronic viral infection.
Science. 324:1572–1576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Coquet JM, Kyparissoudis K, Pellicci DG,
Besra G, et al: IL-21 is produced by NKT cells and modulates NKT
cell activation and cytokine production. J Immunol. 178:2827–2834.
2007. View Article : Google Scholar : PubMed/NCBI
|