Introduction
In recent years, the use of mobile phones has
increased substantially; thus, there is a requirement for the
potential effects on the central nervous system to be investigated.
Numerous studies have demonstrated that electromagnetic field (EMF)
exposure does not significantly increase the apoptosis rate in the
brain cells of rodents (1–4). However, there have also been several
studies that have revealed evidence to the contrary (5–8).
Apoptosis occurs in response to a wide range of
environmental stimuli (9,10). Caspase-3 is a key protein that is
involved in the mechanism for apoptosis in several cell types,
including neurons (11), and has
been implicated in the processes leading to neurodegeneration
(12). An imbalance of caspase-3
levels in the apoptotic pathway may promote certain human diseases.
Studies of experimental models have suggested that caspase-3 is a
reliable indicator of apoptotic rate (13–16).
However, data concerning the effects of EMF on apoptosis are
limited (1).
Several studies have investigated the acute effects
of EMF exposure during the pre- and postnatal periods (17–20).
By contrast, less is understood regarding the chronic effects of
EMF exposure. The developing human brain is now regularly exposed
to mobile telephones pre- and postnatally (17). However, a World Health Organization
(WHO) symposium (18) concluded
that, due to a paucity of relevant research, it remained unclear
whether the developing brain exhibited an increased sensitivity to
EMF exposure. A key recommendation of the symposium was to study
the impact of EMF exposure on the developing nervous system in
immature animals (19). Thus, the
aim of the present study was to determine the chronic effects of
EMF exposure on pre- and postnatal rats.
Lycopersicon esculentum (tomato) and lycopene
consumption have been demonstrated to be correlated with reduced
incidences of cancer (21–23), cardiovascular disease (24) and type 2 diabetes (25). Lycopene is a carotenoid present in
high concentrations in tomatoes (26), and has been demonstrated to provide
protection against the cellular damage caused by reactive oxygen
species (ROS) (26–28). Lycopene has a high liposolubility
and is capable of passing through the blood-brain barrier (29), suggesting that it may have
potential as a treatment for brain diseases (30). Qu et al revealed that
lycopene protected against trimethyltin-induced neurotoxicity by
inhibiting the mitochondrial apoptotic pathway. These results
indicated that tomato and lycopene may act as neuroprotective
agents, due to their anti-apoptotic, antioxidant and free radical
scavenging effects (30). In the
current study, we investigated the protective effects of
Lycopersicon esculentum extract on apoptosis and
neurodegeneration in the EMF-exposed rat cerebellum, during pre-
and postnatal development.
Materials and methods
Animals
Albino Wistar male and female rats were used for the
study. The rats were housed individually in cages, maintained under
standard conditions, and were fed with standard pelletized food and
water ad libitum. The study comprised three groups of rats,
one control and two experimental (EMF1 and EMF2) groups. In the
control group, the rats were kept in the same conditions as the
experimental groups, but without exposure to EMF. In the EMF1
group, the rats were exposed to EMF during pre- and postnatal
periods (until postnatal day 80). In the EMF2 group, the rats
received the same EMF exposure as the EMF1 group; however, they
were also provided with daily oral supplements of Lycopersicon
esculentum extract (∼2 g/kg) during the pre- and postnatal
periods. All experimental protocols received full approval from the
Animal Ethical Committee of Dumlupınar University, Turkey, No.
02.11.2009/9.
EMF exposure
A commercially available cellular telephone with
Global System for Mobile communications (GSM)-900 digital
technology was used for EMF exposure. The cell phones were placed
on the inside walls of the cages, and the rats were exposed to the
effects of the cell phones throughout the pre- and postnatal
periods, until they were 80 days old. During the study, the
exposure procedure comprised the phones being in standby mode for
the entire day, with the exception of 30 min per day when they were
in talking mode. The control group was kept in the same conditions
as the experimental groups, without exposure to GSM, in a separate
room. Therefore, the effects of the EMF on the control group were
prevented.
Immunohistochemical staining
The rats were perfused first with phosphate-buffered
saline under ethyl ether anesthesia, and then with 4% buffered
paraformaldehyde. The rat brains were dissected and postfixed in
the same fixative. Following fixation, coronal blocks of the
cerebellar cortex were embedded in paraffin and sectioned at 5
μm. The paraffin sections were studied with caspase-3
immunostaining, using the avidin-biotin peroxidase (ABC) and
horseradish peroxidase-streptavidin methods for anti-caspase-3
rabbit polyclonal antibodies (1:50 dilution; Diagnostic Biosystems,
Pleasanton, CA, USA). The paraffin-embedded tissue slices were
deparaffinized with xylene, and the endogenous peroxidase activity
was prevented by incubation in 0.3% hydrogen peroxide in methanol.
The tissue slices were hydrated with graded alcohol, treated with
10% normal serum and then incubated with the primer antibody at 4°C
overnight. They were then incubated with biotinylated anti-mouse
IgG or biotinylated anti-rabbit IgG for 30 min at room temperature.
Subsequently, the tissues were incubated with avidin-biotinylated
horseradish peroxidase or streptavidin horseradish peroxidase in
10% normal goat serum for 30 min at room temperature. Following
this, the slices were visualized using 3-amino-4-ethylcarbazole
(AEC) as a chromogen. The negative controls consisted of tissue
sections incubated in the absence of the primary antibody. The
sections were then mounted for counting.
Counting of caspase-3-labeled cells
The presence of cells undergoing apoptosis was
determined by the immunohistochemical detection of caspase-3. We
randomly selected six 200×200 μm2 fields from the
three coronal sections through the Purkinje and granule cell layers
of the cerebellum for each rat, and the caspase-3-labeled cells
were counted.
Cresyl violet staining
The paraffin sections were stained for RNA/DNA with
cresyl violet to reveal the dark neurons. The sections were stained
with 0.5% cresyl violet (Sigma, St. Louis, MO, USA), dehydrated in
graded ethanol and xylene, and then coverslipped with Permount
mounting medium (eBioscience, Inc., San Diego, CA, USA).
Statistical analysis
Statistical analysis was performed using the
computer software program SPSS for Windows (SPSS Inc., Chicago, IL,
USA). The cell count values of the groups were analyzed by analysis
of variance (ANOVA) with post hoc Tukey test calculations for
intergroup comparisons. P<0.05 was considered to indicate a
statistically significant difference. The data of this study are
presented as the mean value ± standard error of the mean (SEM).
Results
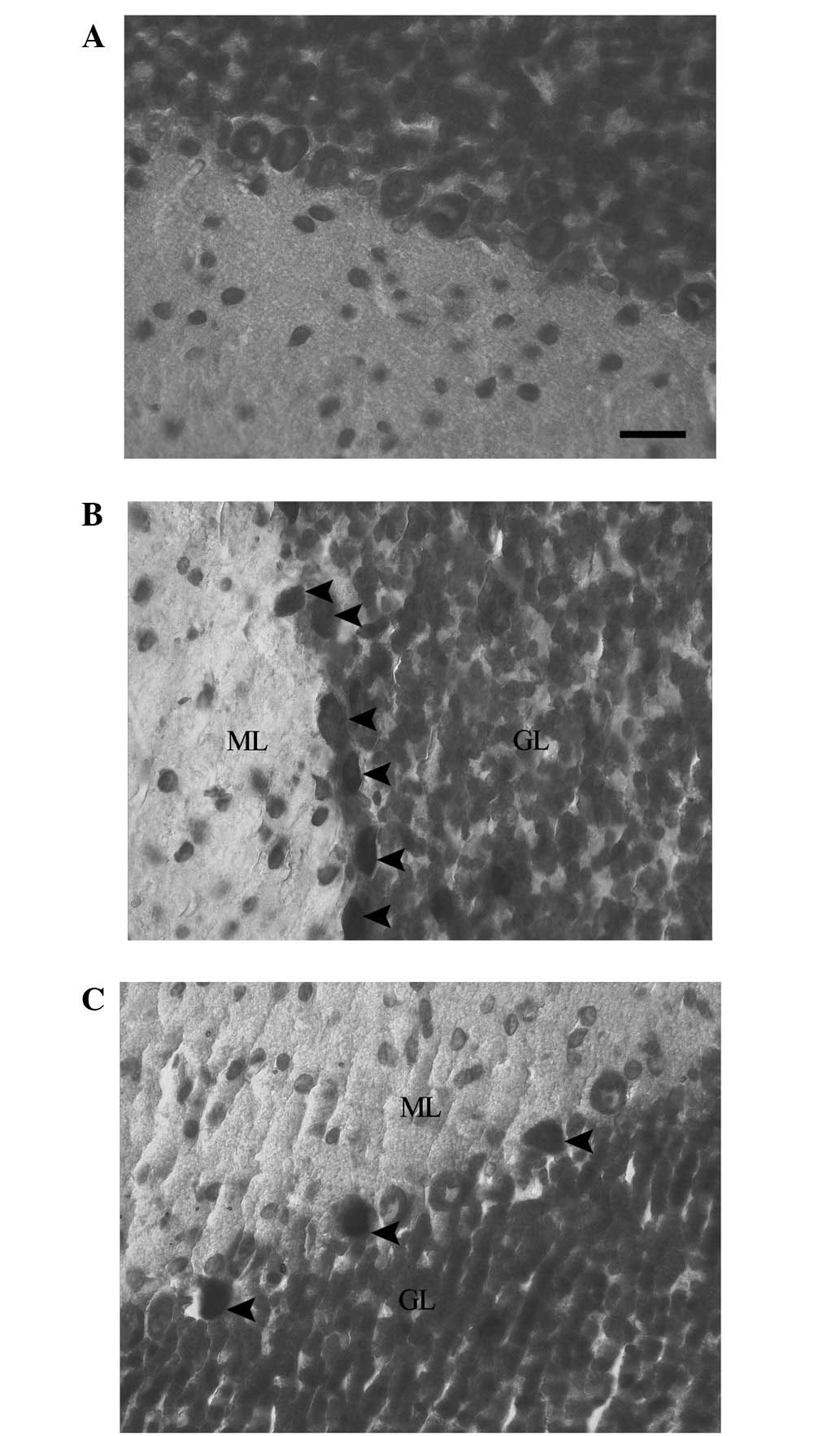
Cresyl violet staining was used for evaluation of
the presence of dark neurons. The staining of the cerebellums of
the rats in the control group revealed normal neuronal morphology
in the Purkinje neurons. This was indicated by the pale blue
appearance of the Purkinje neurons (Fig. 1A). In the EMF1 group, the Purkinje
neurons demonstrated dark neuron degenerative changes. This was
indicated by the intensively stained (dark), shrunken and irregular
neuronal cytoplasms of the grouped dark Purkinje neurons (Fig. 1B). In the EMF2 group, dark Purkinje
neurons were dispersed among intact neurons in the cerebellum.
There was a reduced number of dark Purkinje neurons in the EMF2
group, in comparison with the EMF1 group (Fig. 1C).
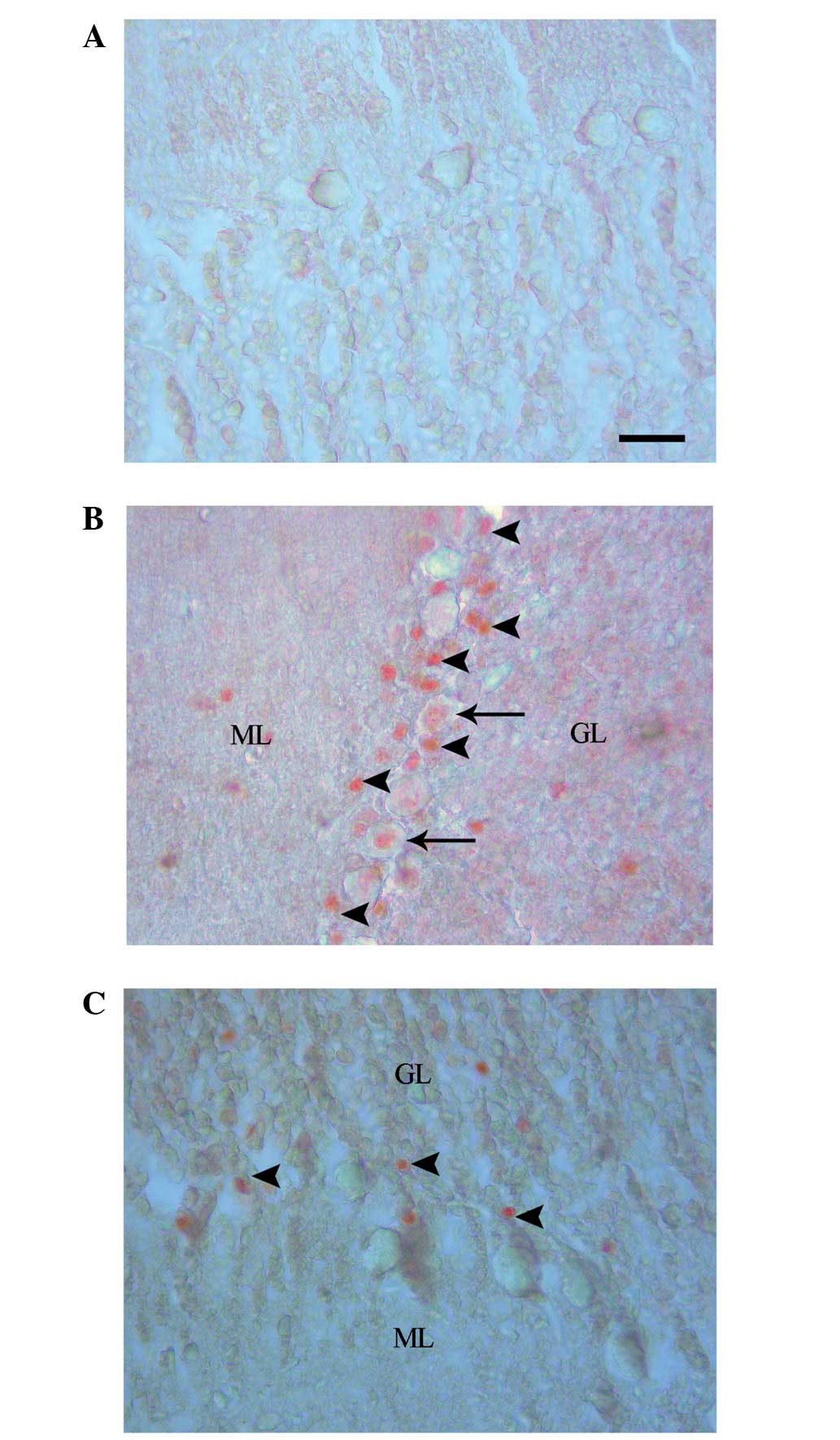
Caspase-3 labeling revealed an absence of cell
staining in the control group (Fig.
2A), and positive cell staining in the EMF1 (Fig. 2B) and EMF2 (Fig. 2C) groups. The numbers of
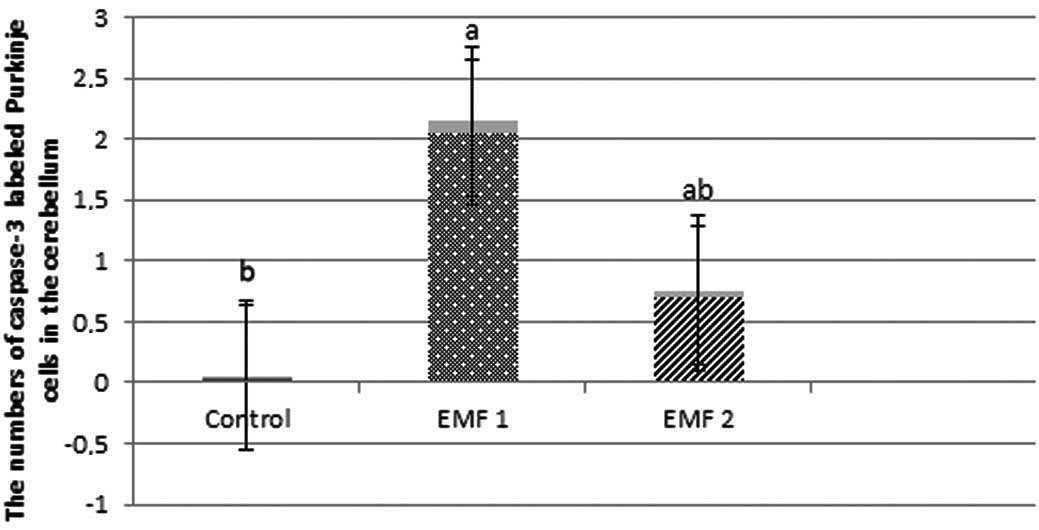
caspase-3-labeled neurons were observed to be 0.04±0.02, 2.05±0.09
and 0.69±0.06 for the control, EMF1 and EMF2 groups, respectively
(mean ± SEM; Fig. 3). In addition,
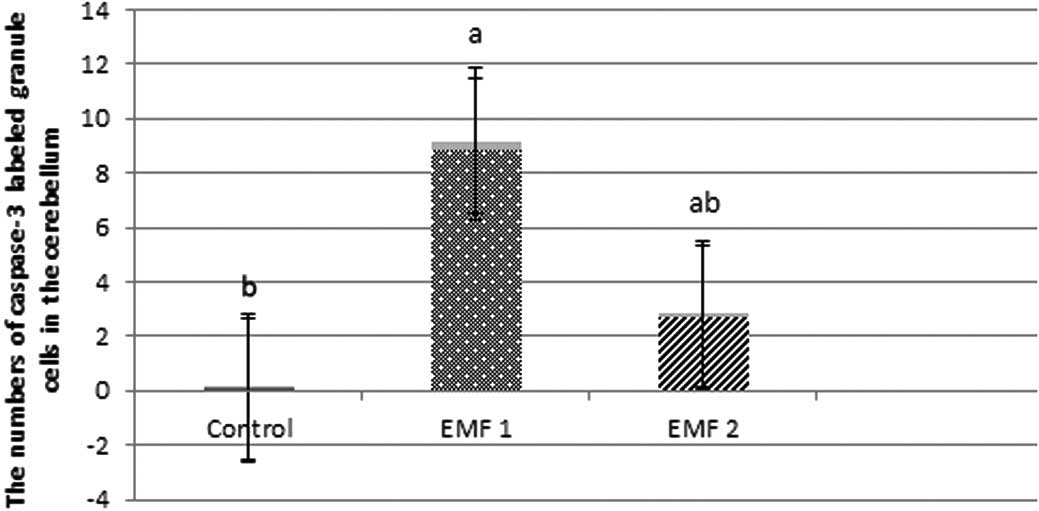
the numbers of caspase-3-labeled granule cells were observed to be
0.07±0.03, 8.86±0.32 and 2.70±0.12, for the control, EMF1 and EMF2
groups, respectively (mean ± SEM; Fig.
4).The caspase-3-labeled Purkinje neurons and granule cells
were more prevalent in the EMF1 and EMF2 groups, compared with the
control group (P<0.001; Figs. 3
and 4). There was a significant
reduction in the number of caspase-3-labeled Purkinje neurons and
granule cells in the EMF2 group, as compared with the EMF1 group
(P<0.001; Figs. 3 and 4).
Discussion
EMF exposure has been been demonstrated to induce
apoptosis in the human colon (31)
and epidermoid cancer cells (32);
however, EMF exposure was not observed to induce apoptosis in human
peripheral mononuclear blood cells (33) or lymphocytes (34). McNamee et al reported that
there was no increase in apoptosis in the cerebellums of immature
mice subjected to acute EMF exposure (4), whilst Joubert et al observed
that EMF exposure did not significantly increase the apoptosis
rates in rat primary neuronal cultures (3). However, Bas et al demonstrated
that postnatal exposure to 900-MHz EMF reduced the number of
pyramidal cells in the cornu ammonis (CA) of the female rat
hippocampus (20). In addition,
Sonmez et al determined that long-duration exposure to EMF
led to a reduction in the number of Purkinje cells in the female
rat cerebellum (8). In the present
study, it was demonstrated that there was an increase in the number
of caspase-3-labeled Purkinje neurons and granule cells in the
cerebellums of 80-day-old rats, following pre- and postnatal
exposure to 900-MHz EMF. This indicated that chronic EMF exposure
had a greater apoptotic effect on the cerebellar cells than
short-duration exposure, even at the same frequency.
Lycopersicon esculentum and lycopene
consumption are correlated with certain diseases that are
associated with oxidative stress (21–23).
Lycopene, a carotenoid predominantly present in Lycopersicon
esculentum and Lycopersicon esculentum products, has
been suggested to exhibit antioxidant activity (35). Several in vitro and in
vivo studies have demonstrated the protective potential of
lycopene against oxidative damage (36,37).
Qu et al observed that lycopene rescued hippocampal neurons
from apoptotic cell death induced by trimethyltin, a neurotoxin
that partially mimics the pathogenic mechanisms of numerous
neurodegenerative disorders (30).
Reduced terminal deoxynucleotidyl-transferase-mediated dUTP nick
end labeling (TUNEL) staining revealed that pretreatment of the
trimethyltin-treated hippocampal neurons with lycopene
significantly improved cell viability, by inhibiting neuronal
apoptosis. These results indicate that the antioxidant, lycopene,
protects against trimethyltin-induced neurotoxicity by inhibiting
the ROS-initiated mitochondrial apoptotic pathway. Caspase-3 is
considered to be a key protease responsible for a number of the
biological and morphological features of apoptosis (38). Türk et al demonstrated that
lycopene exhibited anti-peroxidative and -apoptotic effects on
cyclophosphamide-induced testicular lipid peroxidation and
apoptosis (39). The present study
revealed that Lycopersicon esculentum extract reduced
apoptosis in the Purkinje neurons and granule cells in the EMF2
group. This study also demonstrated that there were fewer dark
Purkinje neurons in the EMF2 group, in comparison with the EMF1
group. The results indicate that Lycopersicon esculentum
extract therapy may reduce apoptosis and neurodegeneration in rats
exposed to EMF pre- and postnatally.
In conclusion, this study demonstrated that
Lycopersicon esculentum exerted a protective effect against
EMF-induced apoptosis and neurodeneration in rat Purkinje neurons
and granule cells, during pre- and postnatal periods. Further
investigation is required to evaluate the neuroprotective efficacy
of Lycopersicon esculentum in vivo.
References
|
1.
|
Akdag MZ, Dasdag S, Ulukaya E, Uzunlar AK,
Kurt MA and Taşkin A: Effects of extremely low-frequency magnetic
field on caspase activities and oxidative stress values in rat
brain. Biol Trace Elem Res. 138:238–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kim TH, Huang TQ, Jang JJ, et al: Local
exposure of 849 MHz and 1763 MHz radiofrequency radiation to mouse
heads does not induce cell death or cell proliferation in brain.
Exp Mol Med. 40:294–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Joubert V, Leveque P, Cueille M,
Bourthoumieu S and Yardin C: No apoptosis is induced in rat
cortical neurons exposed to GSM phone fields. Bioelectromagnetics.
28:115–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
McNamee JP, Bellier PV, McLean JR, Marro
L, Gajda GB and Thansandote A: DNA damage and apoptosis in the
immature mouse cerebellum after acute exposure to a 1 mT, 60 Hz
magnetic field. Mutat Res. 513:121–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lai H and Singh NP: Magnetic-field-induced
DNA strand breaks in brain cells of the rat. Environ Health
Perspect. 112:687–694. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Grassi C, D’Ascenzo M, Torsello A,
Martinotti G, Wolf F, Cittadini A and Azzena GB: Effects of 50 Hz
electromagnetic fields on voltage-gated Ca2+ channels and their
role in modulation of neuroendocrine cell proliferation and death.
Cell Calcium. 35:307–315. 2004.
|
|
7.
|
Maskey D, Pradhan J, Aryal B, et al:
Chronic 835-MHz radio-frequency exposure to mice hippocampus alters
the distribution of calbindin and GFAP immunoreactivity. Brain Res.
1346:237–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sonmez OF, Odaci E, Bas O and Kaplan S:
Purkinje cell number decreases in the adult female rat cerebellum
following exposure to 900 MHz electromagnetic field. Brain Res.
1356:95–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
MacKenzie SH and Clark AC: Death by
caspase dimerization. Adv Exp Med Biol. 747:55–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wickman G, Julian L and Olson MF: How
apoptotic cells aid in the removal of their own cold dead bodies.
Cell Death Differ. 19:735–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Folch J, Alvira D, López-Querol M, et al:
Evaluation of transcriptional activity of caspase-3 gene as a
marker of acute neurotoxicity in rat cerebellar granular cells.
Toxicol In Vitro. 24:465–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Snigdha S, Smith ED, Prieto GA, et al:
Caspase-3 activation as a bifurcation point between plasticity and
cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pace V, Bellizzi D, Giordano F, et al:
Experimental testing of a mathematical model relevant to the
extrinsic pathway of apoptosis. Cell Stress Chaperones. 15:13–23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Duran-Vilaregut J, Del Valle J, Manich G,
et al: Systemic administration of 3-nitropropionic acid points out
a different role for active caspase-3 in neurons and astrocytes.
Neurochem Int. 56:443–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hwang IK, Ahn JH, Yoo DY, et al: Increased
immunoreactivities of cleaved αII-spectrin and cleaved caspase-3 in
the aged dog spinal cord. Neurochem Res. 37:480–486. 2012.
|
|
16.
|
Liu J, Lu Y and Liang J: A novel
fluorescence derivatization method combined with HPLC for
determining the activities of endogenous caspase. Analyst.
137:5097–5104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hossmann KA: Effects of electromagnetic
radiation of mobile phones on the central nervous system.
Bioelectromagnetics. 24:49–62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
The sensitivity of children to EMF
exposure. In: Proceedings of the 2004 EPRI-Cosponsored World Health
Organization Workshop; EPRI, Palo Alto, NC, USA. 1011147(3): pp.
3–13. 2004
|
|
19.
|
Rodier PM: Chronology of neuron
development: animal studies and their clinical implications. Dev
Med Child Neurol. 22:525–545. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bas O, Odaci E, Kaplan S, et al: 900 MHz
electromagnetic field exposure affects qualitative and quantitative
features of hippocampal pyramidal cells in the adult female rat.
Brain Res. 1265:178–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Giovannucci E: Tomato products, lycopene,
and prostate cancer: a review of the epidemiological literature. J
Nutr. 135:2030S–2031S. 2005.PubMed/NCBI
|
|
22.
|
Ford NA, Elsen AC, Zuniga K, Lindshield BL
and Erdman JW Jr: Lycopene and apo-12′-lycopenal reduce cell
proliferation and alter cell cycle progression in human prostate
cancer cells. Nutr Cancer. 63:256–263. 2011.PubMed/NCBI
|
|
23.
|
Teodoro AJ, Oliveira FL, Martins NB, Maia
Gde A, Martucci RB and Borojevic R: Effect of lycopene on cell
viability and cell cycle progression in human cancer cell lines.
Cancer Cell Int. 12:362012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rissanen TH, Voutilainen S, Nyyssönen K,
Salonen R, Kaplan GA and Salonen JT: Serum lycopene concentrations
and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease
Risk Factor Study. Am J Clin Nutr. 77:133–138. 2003.PubMed/NCBI
|
|
25.
|
Coyne T, Ibiebele TI, Baade PD, Dobson A,
McClintock C, Dunn S, et al: Diabetes mellitus and serum
carotenoids: findings of a population-based study in Queensland,
Australia. Am J Clin Nutr. 82:685–693. 2005.PubMed/NCBI
|
|
26.
|
Agca CA, Tuzcu M, Gencoglu H, Akdemir F,
Ali S, Sahin K and Kucuk O: Lycopene counteracts the hepatic
response to 7,12-dimethylbenz[a]anthracene by altering the
expression of Bax, Bcl-2, caspases, and oxidative stress
biomarkers. Pharm Biol. 50:1513–1518. 2012.PubMed/NCBI
|
|
27.
|
Kelkel M, Schumacher M and Dicato M:
Antioxidant and anti-proliferative properties of lycopene. Free
Radic Res. 45:925–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kong KW, Rajab NF, Prasad KN, Ismail A,
Markom M and Tan CP: Lycopene-rich fractions derived from pink
guava by-product and their potential activity towards hydrogen
peroxide-induced cellular and DNA damage. Food Chem. 123:1142–1148.
2010. View Article : Google Scholar
|
|
29.
|
Khachik F, Carvalho L, Bernstein PS, Muir
GJ, Zhao DY and Katz NB: Chemistry, distribution, and metabolism of
tomato carotenoids and their impact on human health. Exp Biol Med
(Maywood). 227:845–851. 2002.PubMed/NCBI
|
|
30.
|
Qu M, Zhou Z, Chen C, et al: Lycopene
protects against trimethyltin-induced neurotoxicity in primary
cultured rat hippocampal neurons by inhibiting the mitochondrial
apoptotic pathway. Neurochem Int. 59:1095–1103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Maeda K, Maeda T and Qi Y: In vitro
and in vivo induction of human LoVo cells into apoptotic
process by non-invasive microwave treatment: A potentially novel
approach for physical therapy of human colorectal cancer. Oncol
Rep. 11:771–775. 2004.
|
|
32.
|
Caraglia M, Marra M, Mancinelli F, et al:
Electromagnetic fields at mobile phone frequency induce apoptosis
and inactivation of the multi-chaperone complex in human epidermoid
cancer cells. J Cell Physiol. 204:539–548. 2005. View Article : Google Scholar
|
|
33.
|
Capri M, Scarcella E, Bianchi E, et al:
1800 MHz radiofrequency (mobile phones, different Global System for
Mobile communication modulations) does not affect apoptosis and
heat shock protein 70 level in peripheral blood mononuclear cells
from young and old donors. Int J Radiat Biol. 80:389–397. 2004.
View Article : Google Scholar
|
|
34.
|
Capri M, Scarcella E, Fumelli C, et al: In
vitro exposure of human lymphocytes to 900 MHz CW and GSM modulated
radio-frequency: studies of proliferation, apoptosis and
mitochondrial membrane potential. Radiat Res. 162:211–218. 2004.
View Article : Google Scholar
|
|
35.
|
Aydın S, Tokaç M, Taner G, et al:
Antioxidant and antigenotoxic effects of lycopene in obstructive
jaundice. J Surg Res. Nov 7–2012.(Epub ahead of print). View Article : Google Scholar
|
|
36.
|
Saada HN, Rezk RG and Eltahawy NA:
Lycopene protects the structure of the small intestine against
gamma-radiation-induced oxidative stress. Phytother Res. 24(Suppl
2): S204–S208. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Tang Y, Parmakhtiar B, Simoneau AR, Xie J,
Fruehauf J, Lilly M and Zi X: Lycopene enhances docetaxel’s effect
in castration-resistant prostate cancer associated with
insulin-like growth factor I receptor levels. Neoplasia.
13:108–119. 2011.PubMed/NCBI
|
|
38.
|
Gervais FG, Xu D, Robertson GS, et al:
Involvement of caspases in proteolytic cleavage of Alzheimer’s
amyloid-beta precursor protein and amyloidogenic A beta peptide
formation. Cell. 97:395–406. 1999.
|
|
39.
|
Türk G, Ceribaşi AO, Sakin F, Sönmez M and
Ateşşahin A: Antiperoxidative and anti-apoptotic effects of
lycopene and ellagic acid on cyclophosphamide-induced testicular
lipid peroxidation and apoptosis. Reprod Fertil Dev. 22:587–596.
2010.PubMed/NCBI
|