Introduction
Coronary atherosclerosis, the hardening of arteries
due to a build-up of lipoproteins, is one of the leading causes of
mortality in many countries (1,2).
Since percutaneous transluminal angioplasty (PTCA) was introduced
by Grüntzig et al (3), it
has rapidly become the most frequently applied interventional
therapy for heart attacks and has been recommended as the standard
of care (4). Following the
introduction of PTCA, the combinatorial method of metallic stent
implantation along with angioplasty was developed, in order to
improve the efficacy of the coronary angioplasty and to ensure that
the treatment provided a permanent solution for an occluded artery.
However, restenosis following angioplasty, with or without stent
implantation, occurs in 35–45% of patients at 6 months, and
represents one of the most critical problems with this technique
(5). Although the molecular
mechanisms of restenosis are poorly understood, and numerous
factors, including inflammation, granulation and extracellular
matrix remodeling, may be involved in the process of restenosis, it
has been elucidated that the abnormal proliferation and migration
of vascular smooth muscle cells (VSMCs) in the neointima is one of
the main causes of restenosis following angioplasty and stent
implantation (6).
In order to prevent and treat restenosis, novel
devices and protocols, such as drug-eluting stents (DESs), gene
therapy, brachytherapy and laser treatment, have been developed
(7–10). The stent-based local release of
antiproliferative agents, such as sirolimus or paclitaxel, at the
site of vascular injury via polymer-coated stents has been shown to
result in effective local drug concentrations for a designated
period, and to avoid systemic toxicity (11). Advances in DESs have substantially
reduced the incidence of restenosis; however, there has been little
impact on the long-term prognosis as compared with bare metal
stents (BMSs) (12). Furthermore,
there are considerations regarding the incidence of instent
thrombosis and safety concerns with DESs. Therefore, alternative
medical treatments for the inhibition of VSMC proliferation are
required.
Recently, the advantages of thermal treatment have
been recognized. As an effective, safe and environmentally sound
approach, thermal treatment or hyperthermia has been applied as a
monotherapy and as an adjunctive therapy (13). Although hyperthermia has
predominantly been utilized for the treatment of cancer, the
clinical applications of hyperthermia are gradually being extended,
indicating that the physiological effects of heating treatments may
be wide-ranging. It has been revealed that repeated whole-body
hyperthermia may improve vascular endothelial and cardiac functions
in patients with chronic heart failure (14). Furthermore, Orihara et al
showed that hyperthermia treatment (43°C, 2 h) was capable of
inhibiting the proliferation of the dividing VSMCs without damaging
the quiescent VSMCs (15).
However, with regard to restenosis, which normally occurs at the
site of the stent in the coronary artery, there is a requirement
for localized or targeted heating. The coronary stents developed
for clinical application are most commonly made of a metal alloy,
such as 316L stainless steel, nickel-titanium (Ni-Ti) or
cobalt-chrome (Co-Cr), which demonstrate the desired inductive
heating characteristics under an alternative magnetic field (AMF).
It is thus feasible that localized heating through magnetic stent
hyperthermia (MSH) may be possible.
In this study, we investigated the inductive heating
characteristics of the stents that are currently utilized in
clinical application under AMF exposure. Rabbit VSMCs were used to
study the effect of MSH on the cell cycle, cell apoptosis and the
expression of proliferating cell nuclear antigen (PCNA). The
results are likely provide useful information to aid the
understanding of the mechanisms behind the effects of heat
treatments and AMF exposure on smooth muscle cells. The deductions
from the experimental conclusions may have significance with regard
to the use of MSH as an alternative approach for the treatment of
restenosis.
Materials and methods
Cell culture
In the present study, rabbit VSMCs were provided by
the Cell Center of the Institute of Basic Medical Sciences, Chinese
Academy of Medical Sciences and Peking Union Medical College
(Beijing, China). Cells between passages four and ten were used.
The VSMCs were cultured in Dulbecco’s modified Eagle’s Medium
(DMEM), supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin solution. Cells were supplied with fresh
medium three times a week and passaged at 80% confluence.
Coronary stents, application of an AMF
and temperature measurement
Stainless steel stents (316L) with typical coronary
stent dimensions of a diameter of 3.5 mm and a length of 14.5 mm
were provided by Beijing MED Zenith Medical Scientific Co., Ltd.
(Beijing, China), and were used in the present study in an expanded
form.
A portable inductive heating device with a frequency
of 300 kHz and an adjustable field intensity was provided by
Shuangping Instrument Technology, Co., Ltd. (Shenzhen, China). The
field generator consisted of an alternating current generator
feeding the coil inductor. A copper-constantan thermocouple
temperature probe (model IT-18; Physitemp Instruments, Inc.,
Clifton, NJ, USA) was utilized for the temperature measurements.
The probe fibers were connected to a four-channel millivoltmeter
(model XSOL-4; Beijing Kunlun Tianchen Instrument Technology, Co.,
Ltd., Beijing, China) and the data were collected every 6 sec by a
personal computer (PC) with home-written software. Prior to each
experiment, the thermocouple temperature probe was calibrated at 0
and 100°C.
Inductive heating properties of the
coronary stent under AMF
The thermocouple probe was fixed at the surface of
the stent in an expanded form by insertion into the mesh of the
stent. Following this, the thermocouple-loaded stent was wrapped
carefully in thermal insulation materials, such as asbestos fibers,
and then placed into a water jacket incubator, which was designed
for temperature maintenance. The double-layer jacket was connected
to a water bath, so that it was possible to adjust and maintain the
temperature inside the jacket at ∼37°C. The jacket was made of
glass to prevent the device itself inducing heating when exposed to
the AMF. Fig. 1 shows the
experiment set-up for the evaluation of the inductive heating
properties of the coronary stent.
Microscopic observation of the cellular
morphology of the VSMCs under MSH
Rabbit VSMCs were routinely cultured in the culture
dish. When the cells reached 80% confluence, the stent was
carefully attached to the cell monolayer with 2% agarose gel. The
stent was co-incubated with the VSMCs for 3 to 5 days prior to the
initiation of MSH treatment for 10 min at different treating
temperatures. Following the MSH treatment, the cellular morphology
of the VSMCs was observed under an inverted microscope. In order to
examine the local heating effects produced by the MSH, two fields
of view were selected for microscopic observation. These comprised
one within the area of the stent location and one outside the stent
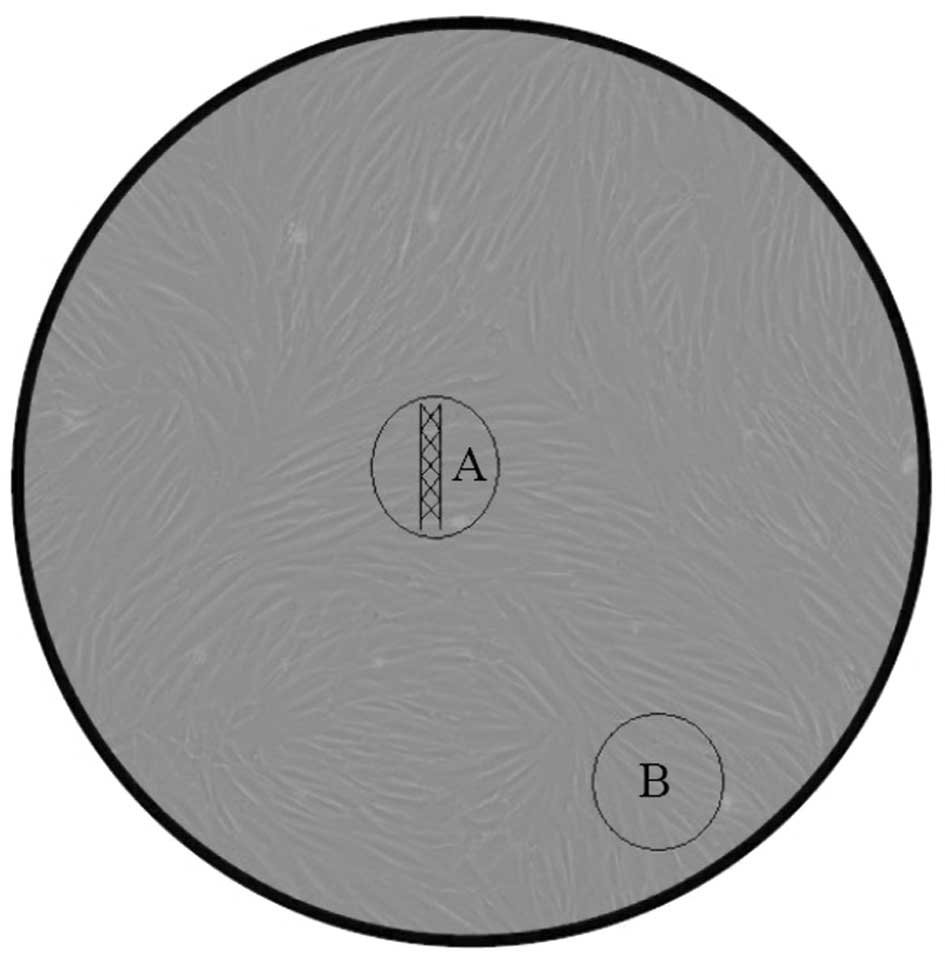
area (at the edge of the culture dish). Fig. 2 illustrates the co-incubation of
the stent and the VSMCs, and the two fields of view for microscopic
observation. Subsequently, the cells were further cultured for up
to 72 h to enable the analysis of cell proliferation, apoptosis and
cell cycle, as well as to perform an immunohistochemical assay for
PCNA expression.
MTT assay for cell proliferation
The effect of the heat treatment at different
temperatures was assessed using a colorimetric MTT assay, and
compared with that in the control area. Following treatment, the
cells were harvested with trypsin-ethylenediaminetetraacetic acid,
seeded in 96-well microtiter plates at a density of 5,000 cells per
well and incubated at 37°C in a humidified atmosphere with 5%
CO2 for different durations. Subsequent to incubation,
20 μl 10 mg/ml MTT solution was added to each well and the
plates were incubated for 4 h, allowing the viable cells to reduce
the yellow MTT into dark blue formazan crystals, which were
dissolved in 150 μl dimethyl sulfoxide (DMSO). The
absorbance of the individual wells was measured at 490 nm using an
automated microplate reader (Bio-Rad, Hercules, CA, USA).
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double-staining assays of the
apoptotic cells
The occurrence of apoptosis and/or necrosis was
evaluated by Annexin-V binding and PI uptake. Annexin-V binding was
performed using an Annexin-V-FITC kit (Kaiji Co., Ltd., Nanjing,
China), in accordance with the manufacturer’s instructions. Cells
were plated at a density of 1×106 cells/well into
24-well plates for 24 h and were pretreated with various
concentrations of methanol extract (25 and 50 μg/ml) of
binding buffer. After 24 h, deoxyribose (dRib; 50 mM) was added to
the plates, which were incubated at 37°C for an additional 24 h.
The cells were then harvested, washed with phosphate-buffered
saline (PBS) and suspended in 100 μl Annexin-V binding
buffer [containing 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)/NaOH (pH
7.4), 140 mM NaCl and 2.5 mM CaCl2]. Following this, the
cells were double stained with 10 μl FITC-labeled Annexin-V
and 10 μl PI solution (containing 50 μg/ml PBS). The
samples were then incubated for 20 min at room temperature and
analyzed using flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA).
Cell cycle analysis
The cells were harvested, washed with PBS,
resuspended in 200 μl PBS and fixed in 800 μl iced
100% ethanol at −20°C. Having been left to stand overnight, cell
pellets were collected by centrifugation, re-suspended in 1 ml
hypotonic buffer (0.5% Triton X-100 in PBS and 0.5 μg/ml
RNase), and incubated at 37°C for 30 min. Following this, 1 ml PI
solution (50 μg/ml) was added, and the mixture was allowed
to stand for ≥30 min at 37°C in the dark, prior to being filtered
through a nylon mesh of 400 screen meshes. A total of
1×106 cells were analyzed by a fluorescence-activated
cell sorter caliber II (FACSCaliber II) cell sorter and the Cell
Quest FACS system (BD Biosciences). The experiment was repeated
three times and an average was taken from the three results. No
less than 10,000 cells were analyzed in each sample. The
percentages of cells in the G0/G1, S and G2/M phases were
determined by the FACSCalibur II (BD Biosciences).
Immunohistochemical localization of PCNA
protein
PCNA immunodetection was conducted using a PCNA
staining kit (ZSGB-Bio, Beijing, China) and the procedure was
performed according to the protocol described by Raucci and Di
Fiore (16). An anti-PCNA mouse
antibody in a dilution of 1:100 and a goat anti-mouse
immunoglobulin (Ig) G-horseradish peroxidase (HRP) antibody in a
dilution of 1:200 were used. Cells were stained with the
diaminobenzidine (DAB) substrate and visualized using a light
microscope.
Scratch wound healing assay
The effect of the heat treatment on the migration
activities of VSMCs was assessed using a scratch healing assay,
according to the protocol of Liang et al (17). Briefly, cells were seeded into
12-well cell culture plates for routine culture to produce a
nearly-confluent cell monolayer. A linear wound was subsequently
generated in the monolayer using a sterile 200 ml plastic pipette
tip to produce an ∼1 mm-wide scratch. Any cellular debris was
removed by washing the wells with PBS. Cells were then treated with
different thermal doses by water-bath heating. Digital images were
captured at 0, 4, 8, 18 and 24 h subsequent to the creation of the
scratches to document the results.
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD). Statistical analyses were performed using SPSS
statistical software (SPSS, Inc., Chicago, IL, USA). Group
comparisons were performed using the Student’s t-test followed by
the least significant difference t-test (LSD-t). P<0.05 was
considered to indicate a statistically significant difference.
Results
Inductive heating properties of the 316L
stainless stent under an AMF
Fig. 3 demonstrates
the inductive heating profile of the 316L stainless stent under AMF
exposure. Fig. 3A shows that the
316L stainless steel stent possessed ideal inductive heating
properties under AMF exposure with a frequency of 300 kHz. Rapid
temperature increases, as shown by the initial slopes of the
curves, were observed and the equilibrium temperature was reached
within 100 sec. The equilibrium temperature was then maintained
stably throughout the observation period. Fig. 3A also shows that field strength was
directly correlated with the inductive heating characteristics of
the stent, with a higher field strength resulting in a higher
equilibrium temperature.
Fig. 3B shows the
effect of the orientation of the stent axis on the inductive
heating of the stent. The results demonstrate that a maximal
temperature increase in the stent was achieved when the stent was
positioned parallel to the field direction, i.e. with 0° angle
between the stent axis and the direction of the AMF. In addition,
the temperature increase was compromised by the angle increase,
with a minimal temperature increase obtained at an angle of
90°.
Microscopic observation of VSMC
morphology under MSH
Fig. 4 shows the
microscopic observations of the VSMC morphology subsequent to MSH
treatment for 10 min at different temperatures. Prior to MSH
treatment, the cells were co-incubated with the stent for 3–5 days.
As shown in Fig. 4A and C, no
difference was observed between the VSMC cultures with or without
the stent, indicating the biocompatibility of the stent. Fig. 4 also demonstrates that the shape
and living status of VSMCs were markedly influenced by the MSH
treatment, depending on the temperature and the location of the
VSMCs. Although in the same culture dish as the stent, the
morphology of the cells outside the stent area remained unaffected,
and consistent with the cells in the control group, indicating that
MSH produced a localized and specific effect. With regard to the
cells cultured within the stent area, there were no marked changes
in cell morphology following a 43°C MSH treatment, as Fig. 4 shows that the cells were
spindle-shaped and well-arranged, indicating a good growth.
However, following MSH treatments at ≥47°C, typical apoptotic
morphological changes were observed. The VSMCs demonstrated a
tendency to grow in a rounded shape, and adhered poorly to the
culture dish. In addition, there were increases in the space
between cells and in the numbers of free cell, as shown in Fig. 4E and F. It was also notable that
the cells under AMF exposure only (without the stent) maintained
the same morphology as those in the control group, suggesting that
AMF exposure contributed little to the effect of MSH on cell
morphology (Fig. 4B).
Effect of MSH on cell proliferation
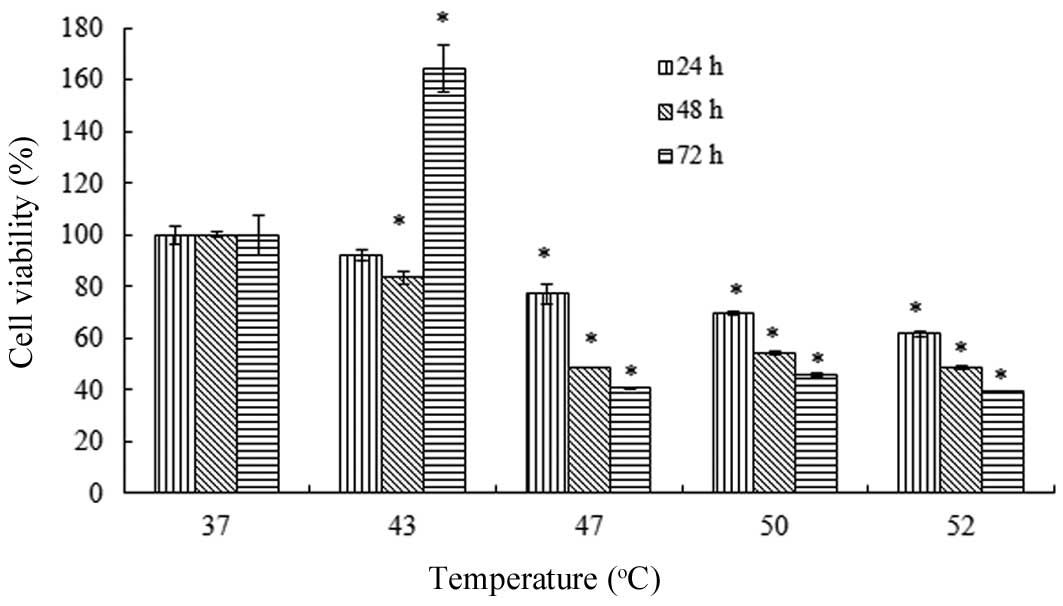
Fig. 5 shows the
dual effect of the heat treatment on the viability of the VSMCs.
The effect was temperature- and incubation period-dependent. For 24
and 48 h incubation periods following heating, a 43°C treatment
demonstrated almost no effect on the cell viability. However,
treatments at ≥47°C resulted in reductions in cell viability to
significantly greater extents. By contrast, there was a marked
change in the antiproliferative effect of the 43°C heat treatment
following a 72 h incubation period, as an increased viability was
observed. This phenomenon only occurred with the 43°C heat
treatment, and in the groups treated with temperatures ≥47°C there
was a reduction in cell viability compared with the control
group.
Effect of MSH on cell apoptosis
As described in previously, Annexin V-FITC/PI
double-staining assays were performed for the analysis of cell
apoptosis. As shown in Fig. 6,
following MSH treatments, the VSMCs exhibited a
temperature-dependent increase in apoptosis. The increases in
apoptosis with MSH treatments at ≥47°C were statistically
significant when compared with control group. However, the 43°C MSH
treatment was observed to have a negligible effect on the apoptosis
of the VSMCs. These results indicated that MSH at ≥47°C effectively
led to the apoptosis of the VSMCs. It was noted that AMF exposure
demonstrated little effect on cell apoptosis. The results regarding
the effect of MSH on apoptosis were consistent with the
morphological observations, as well as the results from the cell
proliferation analysis.
Effect of MSH on the cell cycle
FACS analysis was used to investigate the effect of
temperature on the cell cycle of the VSMCs. The percentages of the
cell populations in the G0/G1, S and G2/M phases were 86.60, 2.10
and 9.76%, respectively, in the control group. Following MSH
treatment at different temperatures, as shown in Table I, the cell populations in G0/G1
phase decreased, indicating S and G2/M phase arrest. There were
statistically significant differences between the experimental and
control groups, with the exception of MSH treatment at 43°C.
 | Table I.Effect of MSH on the cell cycle of
VSMCs (n=3). |
Table I.
Effect of MSH on the cell cycle of
VSMCs (n=3).
| Treatment | Cell cycle phase |
|---|
|
|---|
| G0/G1 | S | G2/M |
|---|
| Control | 86.607±0.246 | 2.100±0.148 | 9.760±0.056 |
| 43°C | 85.813±0.831a | 2.730±0.380 | 9.987±0.100 |
| 47°C | 83.880±0.203b | 2.803±0.188a | 12.310±0.182b |
| 50°C | 71.620±0.726b | 6.857±0.455b | 16.397±0.430b |
| 52°C | 66.573±0.514b | 8.650±0.128b |
19.807±0.585b |
Effect of MSH on PCNA expression
The reduction in the proliferation rate of VSMCs
following MSH treatment at higher temperatures was further
evaluated using immunohistochemistry to examine the expression
levels of PCNA. Fig. 7
demonstrates that the expression level of PCNA was markedly
inhibited by the MSH treatment, with more significant changes
observed when the temperature was above 47°C. Following MSH
treatment at 50°C, the expression of PCNA was reduced markedly,
indicating that MSH treatment produced an anti-proliferative effect
at higher temperatures. The results also demonstrated that,
regardless of the temperature, the effect of MSH on PCNA expression
was limited to the VSMCs cultured within the stent area, since the
PCNA expression level in the cells outside the stent area was not
markedly affected by the MSH, despite the cells being in the same
culture dish as the stent. This result was consistent with the
microscopic observations of the cellular morphology of the VSMCs,
and further demonstrated the localized effect of the heat
treatment.
Effect of heat treatment on the migration
activities of VSMCs
Fig. 8 shows the
effect of the heat treatment at various temperatures on the
migration activities of VSMCs, as observed using an in vitro
scratch assay. In the control group, the cells gradually
repopulated the cell-void area following scratching. A complete
recovery of the scratch was obtained within 48 h. A 43°C heat
treatment demonstrated similar results to those of the control
group. Moreover, the cell density of the scratched area appeared to
be higher than that of the control treatment area. However, the
47°C heat treatment resulted in a significant and long-term effect
on VSMC migration. It was demonstrated that 48 h following the
scratching, almost no cells had migrated to the void area.
Discussion
Restenosis, or renarrowing or the arteries, is
largely a result of the body’s own wound healing response to the
mechanical injury that occurs with stent implantation. A greater
understanding of the cellular processes involved in restenosis is
required, although abnormal proliferation and migration have been
suggested to be predominant features. At present, DES implantation
is the most frequently adopted approach in the treatment of
restenosis. DES implantation implements a controlled delivery
system for a local, site-specific drug release for neointima
formation; however, as mentioned previously, although the clinical
and long-term outcomes of DES implantation have justified the role
of DESs in curbing undesirable neointimal hyperplasia, risks
concerning instent thrombosis have been identified. Therefore, an
effective and safe technology based on BMSs is highly desired.
Although the beneficial effects of hyperthermia on
the cardiovascular system have been gradually revealed, an
unequivocal identification of the mechanisms leading to the
favorable clinical results of hyperthermia have not yet been
elucidated. The technical limitations of the locoregional delivery
of heat and the poor control of the thermal dosage are possible
factors impeding the successful application or translation of the
research into clinical practice. The recent breakthrough in
magnetic-mediated hyperthermia (MMH) may lead to the development of
a novel alternative for locoregional hyperthermia, as it couples
the heat magnetically to the mediators or agents within the target
site only. Since the concept of MMH was first proposed by Gilchrist
et al (18) in the 1960s,
following years of investigation, the developments in MMH have been
successfully applied in clinical oncology with desirable results
(18,19). However, little attempt has been
made to expand the novel hyperthermia approach to other diseases,
particularly cardiovascular disease (20). In the current study, the inductive
heating property of a 316L stainless steel stent revealed that the
expanded stent possessed ideal inductive heating characteristics
upon exposure to the AMF. The parameters of the AMF, as well as the
orientation of the stent inside the AMF, demonstrated significant
effects on the stent’s heating characteristics. The ideal inductive
heating properties of the coronary stent indicate the feasibility
of the use of MSH in the treatment or prevention of restenosis.
As the mediator of MMH, the magnetic agents are
critical in the hyperthermia treatment. Although the desirable
inductive heating properties of the stent were demonstrated in the
present study, it is necessary to note that the analysis presented
in the study was a conservative one, due to the fact that it was in
the absence of blood perfusion. In order to further improve the
heating properties of the stent, Oya et al (21) developed a stent of magnetic shunt
steel, excited by AMF, for thermo-therapeutic applications
(21). Floren et al
(20) proposed that it was
possible to obtain enhanced heating results by coating the stent
surface with ferromagnetic nanoparticles (20). Moreover, in recognition of the fact
that temperature self-regulation has been fully achieved in MMH for
cancer treatment by alloy thermoseeds with specific Curie-points, a
coronary stent with the appropriate Curie point is highly desired
for MSH.
For hyperthermia treatment, the therapeutic
effectiveness is closely associated with the temperature during the
treatment. The current study demonstrated that heat treatment
exhibited an effect on the proliferation and migration of VSMCs,
and that such effect was temperature-dependent. In general, a 43°C
treatment demonstrated little effect on cell morphology, and did
not effect cell proliferation or migration. However, an abnormal
induction of VSMC proliferation was observed following 72 h
incubation with 43°C heat treatment. Higher temperatures were
demonstrated to exert a significant antiproliferative effect on the
VSMCs and a marked inhibitory effect on cell migration. Brasselet
et al (22) studied the
effect of localized heating by in situ heated water on
restenosis in a rabbit model. The results demonstrated that
treatment at 50°C reduced instent neointimal hyperplasia, without
the induction of thrombosis (22).
The conclusions of the present study were consistent with the
observations made by Li et al, in a study investigating the
effect of heat treatment on the shape and living status of VSMCs
(23). The results were
categorized into three stages: i) At temperatures <44°C, the
living status was not changed; ii) between 44 and 50°C, the cells
demonstrated shrinkage, but remained alive; iii) at temperatures
>50°C, all cells died. Although the present results showed that
some VSMCs remained alive following MSH treatment at 50°C, the
present study and that by Li et al concurred with regard to
the conclusions that the effect of hyperthermia was
temperature-dependent, and that temperatures <43°C demonstrated
no effect on cell growth. The difference between the two
investigations may be due to the difference between the two heating
approaches, as the present study used MSH while the study by Li
et al used water bath heating. Since the proliferation and
migration of VSMCs mainly account for the restenosis, there is a
requirement for higher temperatures to be considered for MSH in the
treatment of restenosis.
The current study investigated the possible
mechanisms behind the VSMC proliferation following MSH treatment
through the measurements of PCNA expression and the inhibition of
cell cycle progression. The results from the cell cycle analysis
demonstrated that there was significantly less progression to the
G0/G1 phase in MSH-treated cells than in the control group 24 h
following the heat treatment, suggesting S and G2/M phase arrest.
Orihara et al (15)
evaluated the effect of heating on VSMCs from the rat thoracic
aorta and revealed that the results indicated G1 arrest (15). Two plausible explanations may
account for the difference between the two observations: There was
an interspecies difference between the two studies and the two
heating approaches, i.e. MSH or water bath heating, may have had an
effect. Immunochemistry was used to study the effect of MSH on PCNA
expression, with the results clearly demonstrating that MSH exerted
a significant effect on the PCNA expression of the VSMCs. The
higher the temperature, the lower the level of PCNA expression
observed. PCNA is a protein that acts as a processivity factor for
DNA polymerase δ in eukaryotic cells, and was originally identified
as an antigen expressed in the nuclei of cells during the DNA
synthesis phase of the cell cycle. PCNA is important for DNA
synthesis and repair. The reduced expression of PCNA following MSH
treatment may explain the inhibition of the VSMC proliferation.
An important issue to be considered with magnetic
hyperthermia is the contribution of the AMF exposure to
hyperthermic cytotoxicity. At present, the field frequency of AMF
for magnetic hyperthermia is of an intermediate frequency range.
The field effects of frequencies ≤1 kHz and >1 MHz are well
known; however, the intermediate frequency range lacks intensive
investigation and, to date, has received little attention. The
current investigation showed that AMF exposure had a negligible
effect on the growth status, proliferation or apoptosis of the
VSMCs. This observation was consistent with our previous study, in
which we demonstrated that 100 kHz AMF exposure had little effect
on the proliferation and apoptosis of human esophageal cancer cells
(24). The present results
suggested that AMF exposure (in the intermediate frequency range)
contributed little to the effect of magnetic hyperthermia.
One unique favorable feature of MSH is that it
specifically heats only the target site loaded or infused with
magnetic mediator. The present study provided results that
demonstrated the localized heating effect of MSH. Even in the same
culture dish, the apoptotic morphological changes and the
inhibition of the PCNA expression only occurred to the cells
cultured in the proximity of the stent, while cells outside the
stent area were not affected. Therefore, it may be concluded that
MSH heating is restricted within the stent location and thus only
induces a small or even negligible injury to the nearby tissues
during the treatment.
From the previously mentioned discussions, it may be
concluded that the thermal effect on the growth of VSMCs was
temperature-, cell line- and heating approach-dependent. In order
to provide a more direct insight into the effect of hyperthermia on
the prevention and treatment of restenosis for clinical
application, investigations involving human VSMCs (hVSMCs) are
required. In addition, further systematic studies are required to
address the different effects of the two different heating
approaches on cell growth and to optimize the treatment time and
temperatures. Such studies are under close investigation in our
laboratory.
In conclusion, the results of the present study
demonstrate that 316L stainless steel stents possess ideal
inductive heating characteristics under AMF exposure for clinical
application. MSH treatment has significant effects on the
proliferation, apoptosis and migration of VSMCs, and the effects of
MSH are temperature-dependent. MSH treatment at temperatures
>47°C effectively inhibits the proliferation and migration of
VSMCs. The possible mechanisms behind the inhibition of
proliferation by MSH may be due to a reduction in the progression
to the G0/G1 phase of the cell cycle, in addition to the inhibition
of PCNA expression.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant no. 81070175),
the China Postdoctoral Foundation (special grade no. 200801091) and
the State Science and Technology Support Plan of the Ministry of
Science and Technology (grant no. 2012BAI15B04).
References
|
1.
|
Gryn SE and Hackam DG: Lifetime risk
prediction in cardiovascular prevention: wave of the future? Can J
Cardiol. 29:142–143. 2013.PubMed/NCBI
|
|
2.
|
Riegler J, Liew A, Hynes SO, et al:
Superparamagnetic iron oxide nanoparticle targeting of MSCs in
vascular injury. Biomaterials. 34:1987–1994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Grüntzig AR, Senning Å and Siegenthaler
WE: Nonoperative dilatation of coronary-artery stenosis -
percutaneous transluminal coronary angioplasty. N Engl J Med.
301:61–68. 1979.PubMed/NCBI
|
|
4.
|
Puranik AS, Dawson ER and Peppas NA:
Recent advances in drug eluting stents. Int J Pharm. 441:665–679.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wei Y, Ji Y, Xiao LL, Lin QK, Xu JP, Ren
KF and Ji J: Surface engineering of cardiovascular stent with
endothelial cell selectivity for in vivo re-endothelialisation.
Biomaterials. 34:2588–2599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Chen X and Fujise K: Restenosis: Emerging
molecular targets: Going beyond drug-eluting stents. Drug Discov
Today: Dis Mech. 2:1–9. 2005. View Article : Google Scholar
|
|
7.
|
Latib A, Mussardo M, Ielasi A, et al:
Long-term outcomes after the percutaneous treatment of drug-eluting
stent restenosis. JACC Cardiovasc Interv. 4:155–164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kishore R and Losordo DW: Gene therapy for
restenosis: Biological solution to a biological problem. J Mol Cell
Cardiol. 42:461–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Movahed MR: Brachytherapy with gamma
radiation of a coronary artery for instent restenosis may induce
the regression of in-stent restenosis of an adjacent coronary
artery without angioplasty. First case report and review of the
literature. Cardiovasc Radiat Med. 5:166–170. 2004. View Article : Google Scholar
|
|
10.
|
Shammas NW, Shammas GA, Hafez A, Kelly R,
Reynolds E and Shammas AN: Safety and one-year revascularization
outcome of excimer laser ablation therapy in treating in-stent
restenosis of femoropopliteal arteries: A retrospective review from
a single center. Cardiovasc Revasc Med. 13:341–344. 2012.
|
|
11.
|
Acharya G and Park K: Mechanisms of
controlled drug release from drug-eluting stents. Adv Drug Deliv
Rev. 58:387–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Stettler C, Wandel S, Allemann S, et al:
Outcomes associated with drug-eluting and bare-metal stents: a
collaborative network meta-analysis. Lancet. 370:937–948. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chicheł A, Skowronek J, Kubaszewska M and
Kanikowski M: Hyperthermia - description of a method and a review
of clinical applications. Rep Pract Oncol Radiother. 12:267–275.
2007.
|
|
14.
|
Atienza JM, Guinea GV, Rojo FJ, et al: The
influence of pressure and temperature on the behavior of the human
aorta and carotid arteries. Rev Esp Cardiol. 60:259–267. 2007.(In
Spanish).
|
|
15.
|
Orihara K, Biro S, Hamasaki S, Eto H,
Miyata M, Ikeda Y and Tei C: Hyperthermia at 43 degrees C for 2h
inhibits the proliferation of vascular smooth muscle cells, but not
endothelial cells. J Mol Cell Cardiol. 34:1205–1215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Raucci F and Di Fiore MM: The reproductive
activity in the testis of Podarcis s. sicula involves
D-aspartic acid: A study on c-kit receptor protein, tyrosine kinase
activity and PCNA protein during annual sexual cycle. Gen Comp
Endocrinol. 161:373–383. 2009.PubMed/NCBI
|
|
17.
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gilchrist RK, Medal R, Shorey WD,
Hanselman RC, Parrott JC and Taylor CB: Selective inductive heating
of lymph nodes. Ann Surg. 146:596–606. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Thiesen B and Jordan A: Clinical
applications of magnetic nanoparticles for hyperthermia. Int J
Hyperthermia. 24:467–474. 2008. View Article : Google Scholar
|
|
20.
|
Floren MG, Günther RW and Schmitz-Rode T:
Noninvasive inductive stent heating: alternative approach to
prevent instent restenosis? Invest Radiol. 39:264–270. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Oya J, Shoji H, Sato F, et al:
Thermotherapy with metallic stent excited by the magnetic field.
IEEE T Magn. 42:3593–3595. 2006. View Article : Google Scholar
|
|
22.
|
Brasselet C, Durand E, Addad F, et al:
Effect of local heating on restenosis and in-stent neointimal
hyperplasia in the atherosclerotic rabbit model: a dose-ranging
study. Eur Heart J. 29:402–412. 2008. View Article : Google Scholar
|
|
23.
|
Li CJ, Zheng YF and Zhao LC: Heating NiTi
stent in magnetic fields and the thermal effect on smooth muscle
cells. Key Eng Mater. 288:579–582. 2005.
|
|
24.
|
Liu JY, Zhao LY, Wang YY, Li DY, Tao D, Li
LY and Tang JT: Magnetic stent hyperthermia for esophageal cancer:
an in vitro investigation in the ECA-109 cell line. Oncol Rep.
27:791–797. 2012.PubMed/NCBI
|