Introduction
Neoadjuvant chemotherapy (NAC) has become a standard
therapy for patients (pts) with locally advanced breast cancer.
Despite the initial good responses of pts with stage III breast
cancer to NAC, these pts tend to relapse earlier and have worse
prognoses than pts with stage I/II breast cancer. According to the
databases of the American Cancer Society (1), the five-year overall survival (OS)
rates for stage III breast cancer are 67% in stage IIIA and 41–49%
in stage IIIB–IIIC. A number of prognostic factors for NAC have
been correlated with OS and disease free survival (DFS) in locally
advanced breast cancer, such as the triple-negative type, the human
epidermal growth factor receptor 2 (HER2)-enriched type (hormone
receptor negative/HER2 positive type) (2,3), a
pathological complete response (pCR) (4,5) and
the number of involved axillary lymph nodes (ALNs) at surgical
staging (6,7). However, only a small number of
studies have investigated the prognostic indicators that are
associated with long-term survival in pts with stage III breast
cancer treated with NAC. The aim of this small-scale study was to
investigate the prognostic indicators in pts with stage III breast
cancer who have been treated with NAC.
Patients and methods
Study design and approval
This study was designed as an analysis of
retrospective data in a single-facility, the Kurume University
School of Medicine (Kurume, Japan). This observational study was
approved by the Ethics Committee of Kurume University and all pts
provided written informed consent for the treatment and publication
of the data.
Eligibility criteria
Women under the age of 75 years with previously
untreated clinical stage III breast cancer, who were diagnosed by
mammography, ultrasonography, breast magnetic resonance imaging
(MRI), core needle biopsy and positron emission tomography-computed
tomography (PET/CT), were eligible for this study. Each pt had a
locally advanced breast cancer with ALN involvement. The
eligibility criteria also included adequate performance status
[Eastern Cooperative Oncology Group (ECOG) performance 0–1],
adequate hematology, renal and liver function and an ejection
fraction ≥60%, confirmed by ultrasonic cardiography. Pts who had an
otherwise adverse medical history, another malignancy,
contralateral breast cancer and/or a severe systemic condition were
excluded.
NAC regimens and surgical methods
Three different NAC regimens were used: ED (60
mg/m2 epirubicin and 60 mg/m2 docetaxel), FEC
(500 mg/m2 fluorouracil, 75–100 mg/m2
epirubicin, and 500 mg/m2 cyclophosphamide) and EC (60
mg/m2 epirubicin and 600 mg/m2
cyclophosphamide). For the pts who underwent EC and most of the pts
who underwent FEC, a further four cycles of D (docetaxel 70
mg/m2) were then administered. Each chemotherapy regimen
was administered every three weeks for four cycles; however, this
interval was prolonged by at least one week if the pt did not
recover from the adverse effects. Subsequent to the completion of
the four cycles of NAC, we evaluated the clinical responses and
performed surgery within 2–3 weeks. The surgical methods included
Patey’s procedure in three pts, mastectomy in 16 pts and lumpectomy
in three pts. All pts underwent level I+II ALN dissection. In
addition, the three pts who received Patey’s procedure underwent
level III lymph node dissection.
Adjuvant therapy after surgery
Following surgery, extensional adjuvant chemotherapy
was administered to 13/22 pts (59%) who had numerous ALN metastases
(≥4 positive nodes) and/or poor pathological responses to NAC. Each
regimen of extensional chemotherapy was selected by the clinician.
Nine of the 22 pts (41%), who had a positive HER2 status, were
treated with adjuvant trastuzumab (initially 8 mg/kg, followed by 6
mg/kg) for 12 months. Subsequent to the completion of adjuvant
chemotherapy, whole breast irradiation of 50 Gy was performed for
the pts who underwent a lumpectomy, while chest wall and regional
lymph node irradiation of 50–60 Gy was performed for the majority
of the pts. In addition, postmenopausal pts were treated with
aromatase inhibitors for ≥5 years, whereas premenopausal pts were
given tamoxifen until menopause, prior to being switched to
aromatase inhibitors.
Assessment of NAC in stage III breat
cancer
To compare the efficacy of NAC using epirubicin
and/or docetaxel in stage III breast cancer, we investigated 31 pts
with stage III breast cancer who were treated with adjuvant
chemotherapy between 1996 and 2005.
Evaluation of chemotherapy responses and
toxicities
The clinical response was assessed based on a
physical examination, mammography, ultrasonography, MRI and CT
according to the Response Evaluation Criteria In Solid Tumors
(RECIST) version 1.1 criteria (8).
A clinically complete response (cCR) was defined as the
disappearance of all known lesions; a clinically partial response
(cPR) was defined as a ≥30% reduction in the sum of the longest
diameter (LD) of the primary lesion; progressive disease (PD) was
defined as a ≥20% increase in the sum of the LD of the primary
lesion and stable disease (SD) was defined as neither sufficient
shrinkage to qualify for cPR nor sufficient increase to qualify for
PD. The efficacy of NAC was examined in the surgical specimens,
while the Ki-67 labeling index was examined in the pre-treatment
biopsy specimens. The pathological response was assessed based on
the histological changes in the invasive area by the Japanese
Breast Cancer Society criteria (9). A pCR was defined as no residual
invasive cancer in the breast tissue, regardless of the ALN status,
while the grade 0 response indicated no cancerous degeneration. A
grade 2 response was defined as ≥2/3 cancerous degeneration or a
small amount of invasive cancer in the specimen, while a grade 1
response was defined as <2/3 cancerous degeneration in the
specimen. The number of involved ALNs was confirmed in the
dissected ALN specimen by the pathologist. In addition, toxicities
of the NAC were graded by the ECOG common toxicity criteria.
Statistical analysis
OS and DFS were considered from the onset of NAC and
from the day of breast surgery, respectively. The statistical
analysis was conducted using JMP version 9.0 statistical software
(SAS Institute, Inc., Cary, NC, USA). The correlation analysis was
performed to compare two variables, including categorical and
continuous variables. Univariate survival analyses to investigate
predictive factors for OS and DFS were performed with a Cox
proportional hazard model. The significant factors (P<0.05) were
entered into a Cox multivariate regression model to analyze the
potential simultaneous effects of the predictors of OS and DFS
identified by univariate analyses. The survival analysis of the
most significant factor was performed with the Kaplan-Meier method,
and comparisons between the survival curves were performed with the
log-rank test. Pt follow-up was performed in our hospital, from the
beginning of chemotherapy either until mortality or the last visit
of the pt.
Results
Pt characteristics
A total of 22 women with stage III breast cancer
underwent NAC between January 2005 and May 2011. The median age was
55 years (range, 33–72 years) and the median follow-up period was
66 months (range, 9.3–90.0 months). The clinicopathological
characteristics of the pts are shown in Table I. The observed intrinsic subtypes
were as follows: six luminal, nine HER2-positive and seven
triple-negative types. The pts’ histological types showed invasive
ductal carcinoma in 19 pts, mucinous carcinoma in two pts and
invasive lobular carcinoma in one pt. There were no significant
differences between the pts who underwent the FEC regimen and those
who underwent a non-FEC regimen (ED, EC followed by D).
 | Table I.Characteristics of the patients
treated with NAC. |
Table I.
Characteristics of the patients
treated with NAC.
| Characteristic | n (%)a |
|---|
| Median age [years
(range)] | 55 (33–72) |
| Menopausal
status | |
| Premenopause | 9 (41) |
| Postmenopause | 13 (59) |
| Histological
type | |
| Invasive ductal
carcinoma | 19 (86) |
| Mucinous
carcinoma | 2 (9) |
| Invasive lobular
carcinoma | 1 (5) |
| Nuclear grade | |
| I–II | 14 (64) |
| III | 8 (36) |
| Intrinsic
subtype | |
| Luminal | 6 (27) |
| HER2-positive | 9 (41) |
| Triple
negative | 7 (32) |
| NAC regimen | |
| FEC (+D) | 10 (45) |
| ED | 10 (45) |
| EC+D | 2 (10) |
| Initial tumor size
(cm) | |
| ≤2 | 3 (13) |
| >2, ≤5 | 9 (41) |
| >5 | 10 (46) |
| Initial axillary
nodal status | |
| N1 | 6 (27) |
| N2 | 11 (50) |
| N3 | 5 (23) |
| Clinical stage | |
| Stage IIIA | 10 (45) |
| Stage IIIB | 7 (32) |
| Stage IIIC | 5 (23) |
| Surgical method | |
| Lumpectomy | 3 (13) |
| Mastectomy | 16 (73) |
| Patey’s
procedure | 3 (13) |
| Radiotherapy | |
| Yes | 16 (73) |
| No | 6 (27) |
| Clinical
response | |
| CR/PR | 1/13 (5/59) |
| SD/PD | 6/2 (27/9) |
| Pathological
response | |
| pCR/grade 2 | 4/4 (18/18) |
| grade 0/grade
1 | 3/11 (14/50) |
Clinical/pathological responses and
toxicities
Clinical efficacy of NAC was observed in 64% (cCR,
5%; cPR, 59%) of all pts (Table
I). The remaining 36% had SD (27%) and PD (9%). A younger age
(P=0.010) and non-FEC regimen (P=0.005) were correlated with poor
clinical responses. Pathological efficacy was observed in 36% (pCR,
18%; grade 2, 18%) of all pts (Table
I). The remaining 64% showed grade 1 (50%) and grade 0 (14%)
responses. There was no significant factor predicting whether the
pathological response was likely to be good or poor.
Based on the ECOG common toxicity criteria, the most
common toxicities were grade 1 and 2 neutropenia (n=12, Table II). One pt required an admission
for grade 4 neutropenia, and six pts were classified as grade 3.
The other grade 3 toxicities are shown in Table II. Additional common toxicities
were anorexia (n=7) and fever (n=6). The majority of the pts
experienced alopecia.
 | Table II.Adverse events of NAC. |
Table II.
Adverse events of NAC.
| Adverse event | Grade 1–2 | Grade 3 | Grade 4 |
|---|
| Constitutinal
symptom | | | |
| Fever | 5 | 1 | |
| Malaise | 2 | | |
| Gastrointestinal | | | |
| Anorexia | 7 | | |
| Nausea | 4 | | |
| Diarrhea | 3 | | |
| Oral mucositis | 3 | | |
| Neurological | | | |
| Dysgeusia | 3 | | |
| Stroke | 1 | | |
| Blood/bone
marrow | | | |
| Anemia | 3 | 1 | |
| Neutropenia | 12 | 6 | 1 |
|
Thrombocytopenia | | 1 | |
| Laboratory | | | |
| AST/ALT
elevation | 2 | | |
Prognostic factors associated with OS and
DFS
The median survival time was 66 months, and 11/22
pts (50%) succumbed to refractory breast cancer. There were 12
distant metastases (five brain metastases, five lung metastases,
one bone metastasis and one subclavian lymph nodes metastasis) and
two local recurrences. pCR was not a prognostic factor for the
success of NAC in this study. Univariate analyses showed that the
triple-negative type, positive status of estrogen receptor and the
number of involved ALNs were correlated with DFS, while the
triple-negative type, Ki-67 labeling index (%), pathological tumor
size (cm) and the number of involved ALNs were correlated with OS
(Table III). Multivariate analyses
showed that the number of involved ALNs [hazard ratio (HR), 1.079;
95% confidence interval (CI), 1.011–1.155; P= 0.023] was correlated
with DFS, while the Ki-67 labeling index (HR, 1.109; 95% CI,
1.004–1.265; P=0.042) and the number of involved ALNs (HR, 1.087;
95% CI, 1.012–1.180; P=0.023) were correlated with OS (Table III).
 | Table III.Uni- and multivariate analyses of the
clinicopathological factors associated with overall and
disease-free survival. |
Table III.
Uni- and multivariate analyses of the
clinicopathological factors associated with overall and
disease-free survival.
| Factor | Overall survival
| Disease-free
survival
|
|---|
Univariate
| Multivariate
| Univariate
| Multivariate
|
|---|
| HR | P-value | HR (95% CI) | P-value | HR | P-value | HR (95% CI) | P-value |
|---|
| Menopausal
status | | | | | | | | |
|
Post-/premenopause | 0.400 | 0.135 | | | 0.466 | 0.161 | | |
| NAC regimen | | | | | | | | |
| FEC/non-FEC
regimen | 1.081 | 0.903 | | | 0.595 | 0.344 | | |
| Initial stage | | | | | | | | |
|
IIIB-IIIC/IIIA | 0.835 | 0.769 | | | 1.343 | 0.584 | | |
| Nuclear grade | | | | | | | | |
| III/I-II | 1.591 | 0.450 | | | 1.412 | 0.528 | | |
| Triple-negative
type | | | | | | | | |
| Yes/no | 27.99 | <0.001 | 9.905
(0.692–274.6) | 0.091 | 3.329 | 0.047 | 1.206
(0.111–7.789) | 0.857 |
| Estrogen
receptor | | | | | | | | |
|
Positive/negative | 0.336 | 0.123 | | | 0.167 | 0.006 | 0.273
(0.037–1.388) | 0.118 |
| HER2 status | | | | | | | | |
|
Positive/negative | 0.366 | 0.113 | | | 1.310 | 0.615 | | |
| Ki-67 labeling
index (%) | 1.120 | <0.001 | 1.109
(1.004–1.265) | 0.042 | 1.048 | 0.057 | | |
| Pathological tumor
size (cm) | 1.605 | 0.006 | 1.242
(0.895–1.826) | 0.194 | 1.350 | 0.057 | | |
| Involved ALNs
(number) | 1.061 | 0.037 | 1.087
(1.012–1.180) | 0.023 | 1.068 | 0.027 | 1.079
(1.011–1.155) | 0.023 |
| Pathological
responses | | | | | | | | |
| pCR, grade
2/grade 1, grade 0 | 0.348 | 0.139 | | | 0.383 | 0.112 | | |
Treatment outcome of the pts with
confirmed pCR
We assessed the outcome of four pts with observed
pCR subsequent to surgery (Table
IV). The five-year survival rate of these pts was 75% (3/4 pts)
and 2/4 pts (50%) suffered from a relapse of the breast cancer.
These two pts relapsed with brain metastasis, having had a short
DFS (2.9 and 7.9 months). For the four pts with observed pCR
subsequent to surgery, initial staging, intrinsic subtype and Ki-67
labeling index were suggested as prognostic indicators.
 | Table IV.Outcomes of the patients with
confirmed pCR. |
Table IV.
Outcomes of the patients with
confirmed pCR.
| Case no. | Outcome | DFS (months) | Relapse site | Adjuvant
chemotherapy | Stage | Involved ALNs | Subtype | Ki-67 (%) |
|---|
| 1 | Alive | 34.0 | - | - | IIIB | 0 | Luminala | 14.7 |
| 2 | Alive | 2.9 | Brain | Docetaxel,
trastuzumab | IIIC | 0 | HER2b | 7.1 |
| 3 | Dead | 7.9 | Brain | Tegafur | IIIC | 1 | TNc | 55.2 |
| 4 | Alive | 8.4 | - | - | IIIA | 1 | TNc | 42.0 |
Feasibility of NAC in stage III breast
cancer
Comparisons of the characteristics for the pts
treated with NAC and those treated with adjuvant therapy (AT) are
shown in Table V. The pts treated
with AT included a higher proportion of stage IIIA disease and a
smaller proportion of stage IIIC disease (P=0.040). The majority of
the pts treated with AT underwent four cycles of anthracycline-
and/or taxane-based regimens using doxorubicin, epirubicin and/or
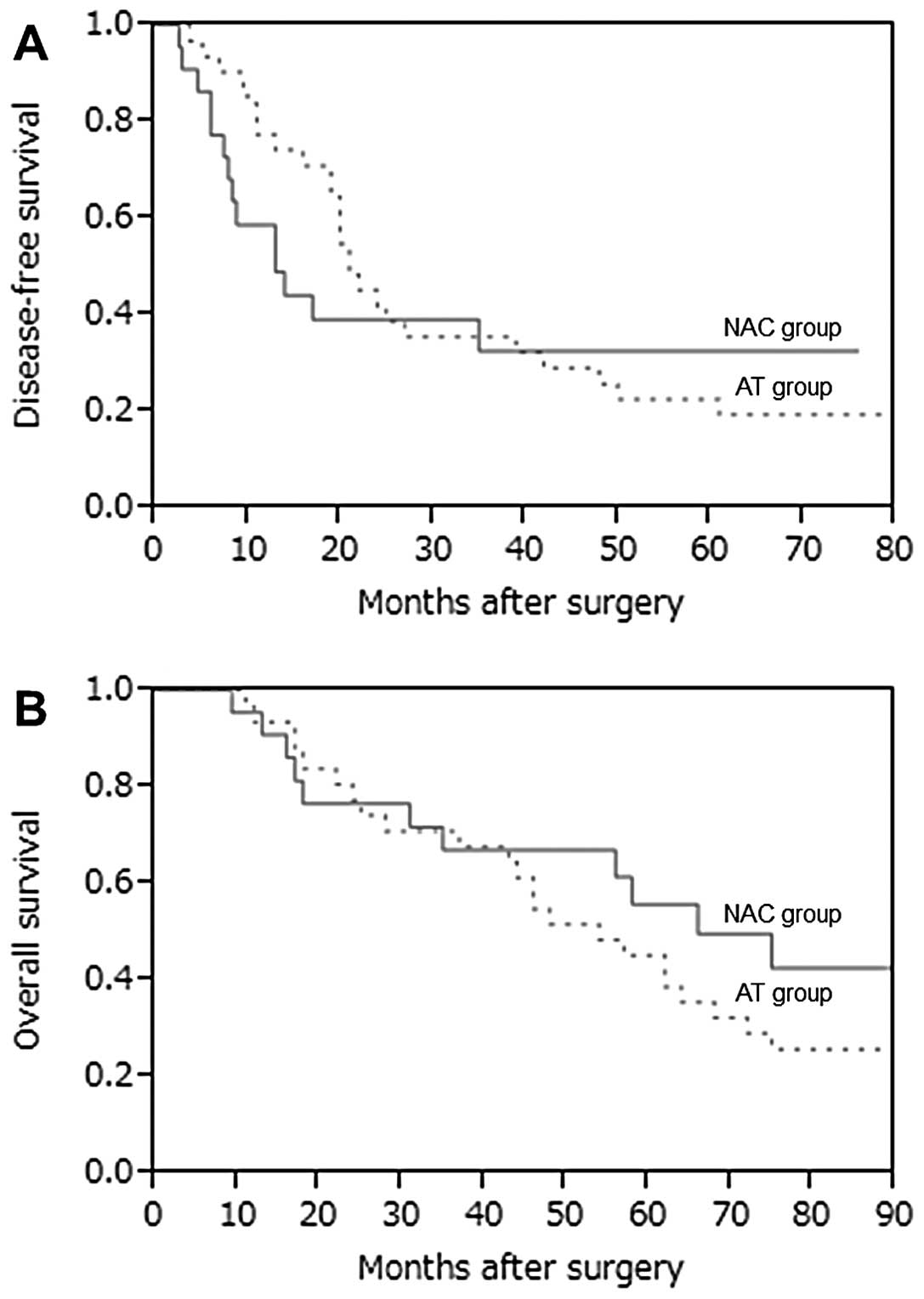
paclitaxel. DFS and OS curves for the NAC and AT groups are shown
in Fig. 1. In our hospital,
epirubicin and/or docetaxel-based NAC was found not contribute to
enhanced survival in stage III breast cancer.
 | Table V.Characteristics of the NAC and AT
groups. |
Table V.
Characteristics of the NAC and AT
groups.
| Characteristic | NAC group
(n=22) | AT group
(n=31) | P-value |
|---|
| Age [years
(range)] | 55 (33–72) | 52 (37–77) | 0.564 |
| Histological
type | | | 0.904 |
| Invasive ductal
carcinoma | 19 | 27 | |
| Invasive lobular
carcinoma | 1 | 2 | |
| Mucinous
carcinoma | 2 | | |
| Metaplastic
carcinoma | | 2 | |
| Intrinsic
subtype | | | 0.409 |
| Luminal type | 6 | 14 | |
| HER2-positive
type | 9 | 9 | |
| Triple-negative
type | 7 | 8 | |
| Initial stage | | | 0.040 |
| Stage IIIA | 10 | 23 | |
| Stage IIIB | 7 | 7 | |
| Stage IIIC | 5 | 1 | |
| Events of
recurrence | | | 0.693 |
| Local
recurrence | 2 | 6 | |
| Distant
metastasis | 12 | 21 | |
Prognostic indicators in stage III breast
cancer pts treated with NAC
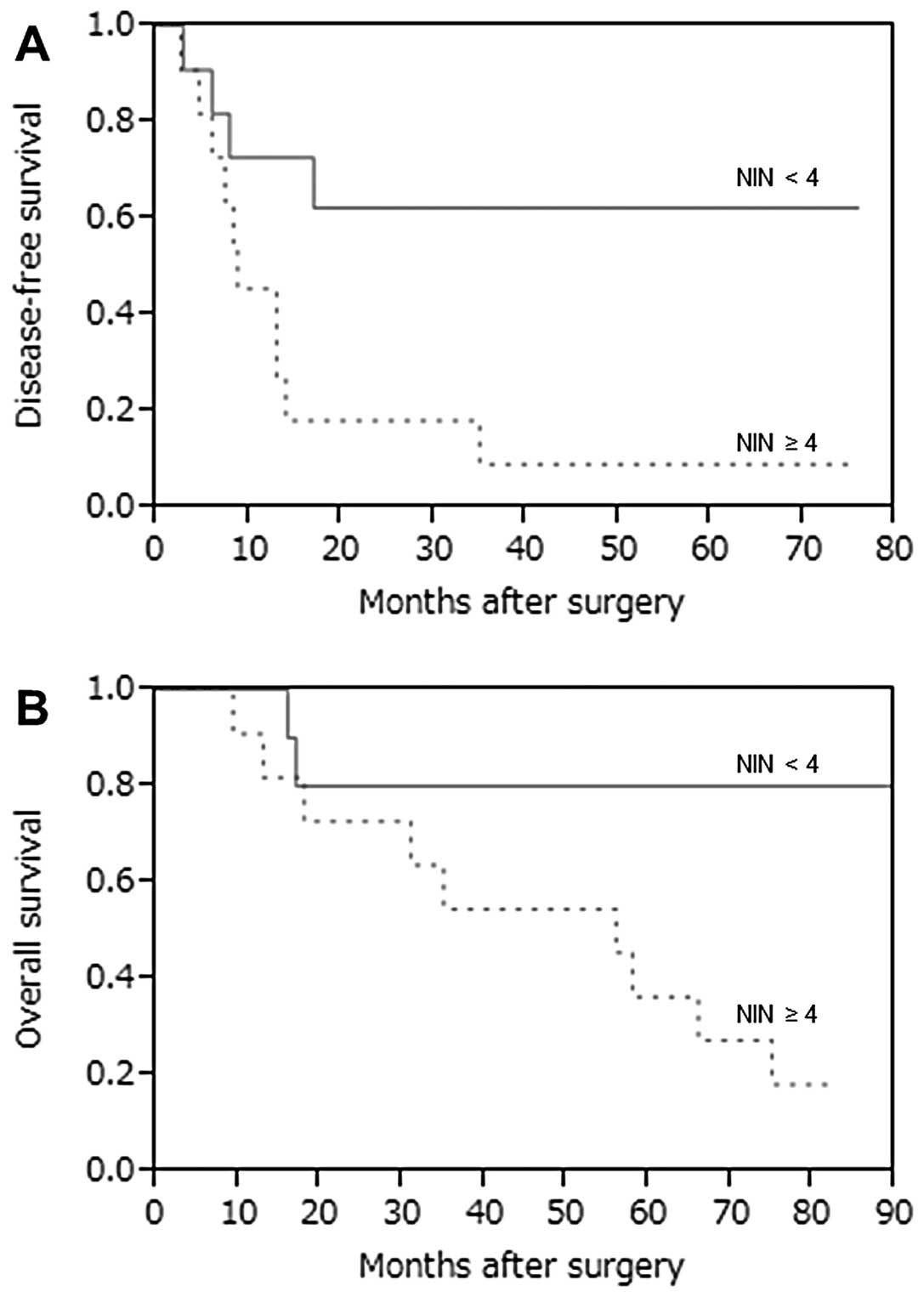
We compared DFS and OS curves between pts with NIN
<4 and pts with NIN ≥4, and a greater number of NIN (≥4) was
significantly correlated with poor prognoses. Tausch et al
(10) observed that an increased
number of involved nodes (NIN) and an increased ratio of involved
to removed nodes (LNR) were significantly correlated with worse DFS
and OS in univariate and multivariate analyses (P<0.001). We
compared DFS and OS curves between pts with NIN <4 and pts with
NIN ≥4 (Fig. 2). A high number of
NINs (≥4) was a significant prognostic indicator correlated with
DFS and OS (P=0.025 and P=0.024, respectively). In the current
study, the Ki-67 index was indicated to be as an independent
prognostic factor for OS (HR, 1.109; 95% CI, 1.004–1.265; P=0.042).
However, achieving pCR subsequent to NAC was not associated with
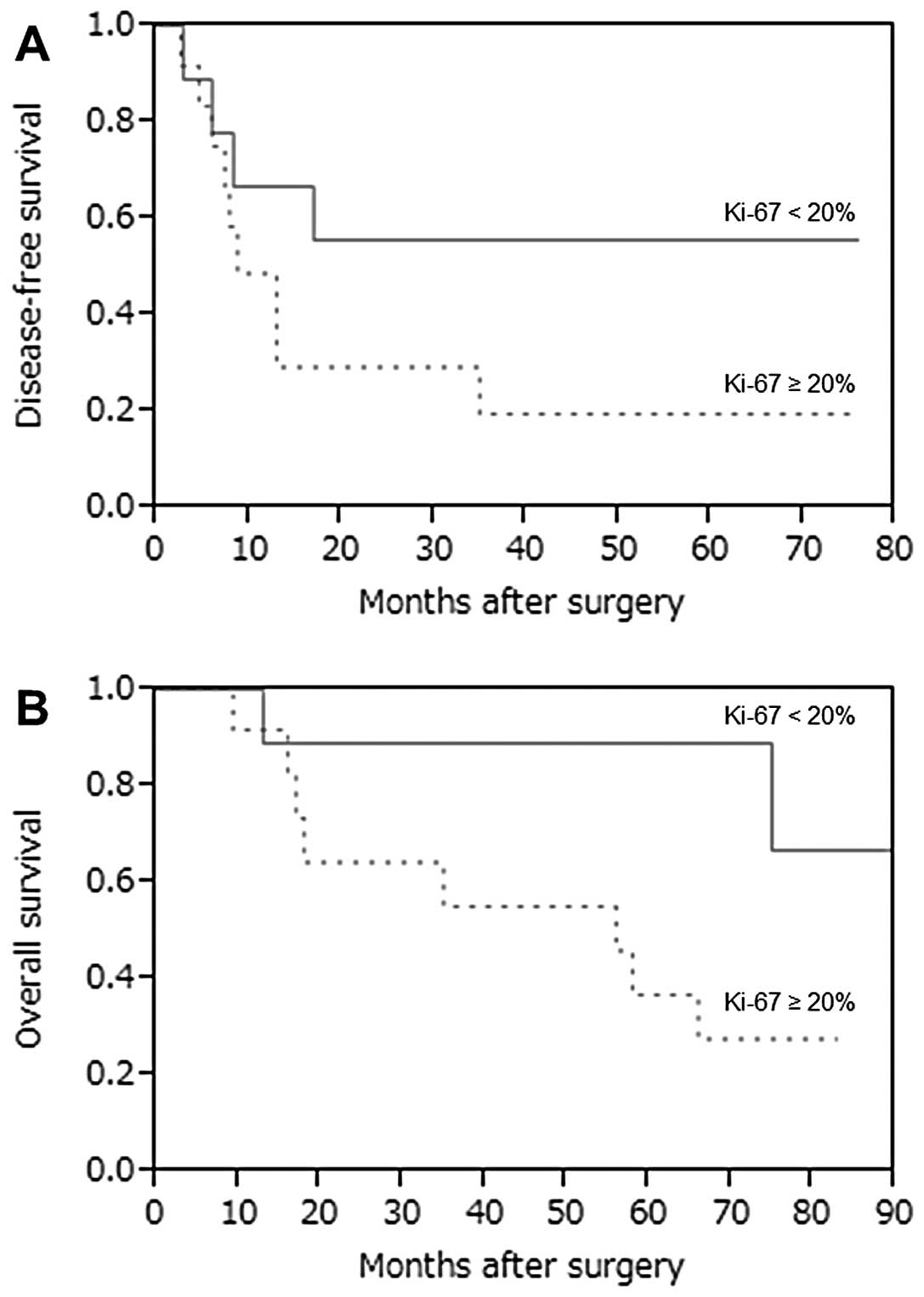
the Ki-67 labeling index (P=0.654, Wilcoxon test). Since the median
percentage of Ki-67 was 21.3% (range, 7.1–55.2%), we compared the
DFS and OS curves with a cut-off value of Ki-67 at 20% (Fig. 3). A high percentage of Ki-67 (≥20%)
was suggested as a prognostic indicator correlated with OS
(P=0.057), while there was no significant difference between the
DFS curves (P=0.183).
Discussion
According to our five years of follow-up data for
NAC in stage III breast cancer, the five-year OS and DFS rates were
50 and 36.4%, respectively. The pCR and breast conserving rates
after NAC were 18.2 and 13.6%, respectively. Chávez-MacGregor and
González-Angulo (4) suggested that
achieving pCR after NAC correlated with improved DFS and OS and
that, therefore, the amount of residual disease was a prognostic
predictor. Ionta et al (11) analyzed 58/74 consecutive pts with
stage IIIB breast cancer, who failed to achieve pCR following up to
six cycles of a primary cisplatin, epirubicin and vinorelbine
regimen. Following a median follow-up of 99 months, the 10-year DFS
and OS rates were 37.6 and 50.3%, respectively, which were
significantly worse than those in the pCR group (n=16; P=0.003 and
P=0.008, respectively). Their results suggested that the number of
residual ALNs and being negative for hormone receptors were strong
predictors of poor outcomes, while the triple-negative type showed
a trend towards early recurrence and mortality. Our results also
suggested that the pathological tumor size subsequent to NAC and a
triple-negative type were prognostic predictors. However, no
significant difference was observed in the multivariate analysis.
It was not possible to evaluate these predictors accurately;
therefore, larger numbers of pts with stage III breast cancer
treated with NAC are required for analysis.
With regard to the validity of NAC in stage III
breast cancer, Tanioka et al (12) investigated the predictive factors
of recurrence in 88 pts achieving pCR following NAC. During a
median 46-month follow-up period, there were 12 recurrences,
including eight distant metastases. Multivariate analyses showed
that ALN metastasis (HR, 13.6; P<0.001) and HER2-positive type
(HR, 5.0; P=0.019) were significant predictors of recurrence. In
the current study, we observed two pts who experienced relapses of
breast cancer into the brain out of the four pts who achieved pCR
after NAC (Table IV). Although
these two pts had a small number of involved ALNs, they were stage
IIIC; one pt had a HER2-positive type and the other had a high
percentage of Ki-67 (55.2%). Yuan et al (13) revealed that NAC exhibited better
recurrence control and DFS and OS rates than adjuvant chemotherapy
in stage III breast cancer; however, it did not result in greater
survival in stage II disease. By contrast, our results indicated
that NAC may have no survival advantages compared with adjuvant
chemotherapy in stage III breast cancer pts. Notably, higher
frequencies of the triple-negative type and stage IIIB-IIIC breast
cancer were shown in the NAC group. However, these results indicate
the need to consider more tailored and effective NAC regimens for
pts with stage III breast cancer.
Our results suggested that the Ki-67 labeling index
and the number of involved ALNs are prognostic predictors in stage
III breast cancer. In a recent study, Zhang et al (7) investigated axillary nodal staging in
stage II/III breast cancer after NAC. The authors observed that the
ypN staging adjusted by pCR following NAC may predict differential
DFS. We showed that the ypN staging after NAC may be a prognostic
indicator among stage III breast cancer pts, although the number of
pts was too small to confirm this. A greater number of NIN (≥4)
after NAC may also be a predictive factor for recurrence or poor
prognosis in our study. Furthermore, we evaluated the Ki-67 in
pre-treatment biopsy specimens, due to the fact that tissue
degeneration following chemotherapy often makes it difficult to
identify Ki-67-positive tumor cells. In a recent review of Ki-67
data (14), Ki-67 was an
independent prognostic factor for DFS (HR, 1.05-1.72) in
multivariate analyses in seven randomized trials (level of
evidence, I-B) and for OS (HR, 1.11–1.83) in univariate analyses in
five trials. In addition, a high Ki-67 was observed to be
correlated with immediate pCR with neoadjuvant therapy (level of
evidence, II-B). I-B and II-B levels of evidence may be defined as
follows: The I-B level of evidence applies to instances where a
randomized controlled trial (RCT) was not specifically performed to
assess the utility of the biomarker. The samples were stored during
the study and analyzed when the study had finished, following a
protocol. Only one validation study, or several studies with
inconsistent results were desirable. For a II-B level of evidence,
an RCT was not specifically performed to assess the utility of the
biomarker. The samples were stored during the study and analyzed
once the study had finished, following a protocol. One or more
validation studies with consistent results were desirable. In the
present study, the Ki-67 was indicated to be an independent
prognostic factor for OS, and a high percentage of Ki-67 (≥20%) was
correlated with poor prognosis, although there was no significant
difference between the DFS curves. These results indicate that the
Ki-67 index in pre-treatment tumor tissues may be used as a
prognostic indicator for localized advanced breast cancer pts.
In conclusion, even if pts with stage III breast
cancer show good responses to NAC using anthracycline and/or
taxanes, most eventually relapse and have a poor prognosis. The
Ki-67 labeling index and the number of involved ALNs may be
prognostic indicators in stage III breast cancer.
Acknowledgements
The authors thank Dr Tatsuyuki Kakuma
(Biostatistic Center, Kurume University, Kurume, Japan) for
supporting the data analysis in this study.
References
|
1.
|
American Cancer Society: Breast cancer
survival rates by stage. http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-survival-by-stageuri.
Accessed September 6, 2012.
|
|
2.
|
Bhargava R, Beriwal S, Dabbs DJ, Ozbek U,
Soran A, Johnson RR, et al: Immunohistochemical surrogate markers
of breast cancer molecular classes predicts response to neoadjuvant
chemotherapy: a single institutional experience with 359 cases.
Cancer. 116:1431–1439. 2010. View Article : Google Scholar
|
|
3.
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Chollichio F, et al: The triple negative paradox: primary
tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res.
13:2329–2334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chávez-MacGregor M and González-Angulo AM:
Breast cancer, neoadjuvant chemotherapy and residual disease. Clin
Transl Oncol. 12:461–467. 2010.
|
|
5.
|
Kong X, Moran MS, Zhang N, Haffy B and
Yang Q: Meta-analysis confirms achieving pathological complete
response after neoadjuvant chemotherapy predicts favourable
prognosis for breast cancer patients. Eur J Cancer. 47:2084–2090.
2011. View Article : Google Scholar
|
|
6.
|
Zhang GC, Zhang YF, Xu FP, Qian XK, Guo
ZB, Ren CY and Yao M: Axillary lymph node status, adjusted for
pathologic complete response in breast and axilla after neoadjuvant
chemotherapy, predicts differential disease-free survival in breast
cancer. Curr Oncol. 20:e180–192. 2013. View Article : Google Scholar
|
|
7.
|
Kim J, Lee J, Chang E, Suh K, Lee C, Jee J
and Shin H: Prognostic factors in patients with stage II/III breast
cancer treated with adjuvant extension of neoadjuvant chemotherapy:
a retrospective cohort study with ten-years of follow-up data. J
Breast Cancer. 14:39–45. 2011.PubMed/NCBI
|
|
8.
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, et al: New response evaluation
criteria in solid tumours: revised RECIST guideline (version 1.1).
Eur J Cancer. 45:228–247. 2009. View Article : Google Scholar
|
|
9.
|
Kurosumi M, Akashi-Tanaka S, Akiyama F,
Komoike Y, Mukai H, Nakamura S, et al Committee for Production of
Histopathological Criteria for Assessment of Therapeutic Response
of Japanese Breast Cancer Society: Histopathological criteria for
assessment of therapeutic response in breast cancer (2007 version).
Breast Cancer. 15:5–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tausch C, Taucher S, Dubsky P, Seifert M,
Reitsamer R, Kwasny W, et al: Prognostic value of number of removed
lymph nodes, number of involved lymph nodes, and lymph node ratio
in 7502 breast cancer patients enrolled onto trials of the Austrian
Breast and Colorectal Cancer Study Group (ABCSG). Ann Surg Oncol.
19:1808–1817. 2012. View Article : Google Scholar
|
|
11.
|
Ionta MT, Atzori F, Deidda MC, Pusceddu V,
Palmeri S, Frau B, et al: Long-term outcomes in stage IIIB breast
cancer patients who achieved less than a pathological complete
responses (<pCR) after primary chemotherapy. Oncologist.
14:1051–1060. 2009.PubMed/NCBI
|
|
12.
|
Tanioka M, Shimizu C, Yonemori K,
Yoshimura K, Tamura K, Kouno T, et al: Predictors of recurrence in
breast cancer patients with a pathologic complete response after
neoadjuvant chemotherapy. Br J Cancer. 103:297–302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yuan Z, Qu X, Zhang ZT and Wang Y:
Neoadjuvant chemotherapy in patients with stage II and III breast
cancer. Chin Med J (Engl). 122:2993–2997. 2009.PubMed/NCBI
|
|
14.
|
Luporsi E, André F, Spyratos F, Martin PM,
Jacqueimer J, Penault-Llorca F, et al: Ki-67: level of evidence and
methodological considerations for its role in the clinical
management of breast cancer: analytical and critical review. Breast
Cancer Res Treat. 132:895–915. 2012. View Article : Google Scholar : PubMed/NCBI
|