Introduction
Coronary heart disease (CHD), also known as coronary
artery disease, is the result of the accumulation of atheromatous
plaques within the walls of the coronary arteries that supply the
myocardium. It is the leading cause of mortality worldwide
(1). The preliminary number of
mortalities due to CHD in the USA for 2010 was 595,444, making it
the leading cause of mortality (2). In China, a population-based survey of
residents in the city of Beijing revealed that the standardized
prevalence of 10.9% for CHD among participants ≥65 years old was
much higher than the 4.1% for CHD among individuals <65 years
old (3). Although there have been
advances in treatment, CHD remains the leading cause of mortality
worldwide (4).
One symptom of CHD is angina pectoris, a type of
chest discomfort caused by poor blood flow through the coronary
vessels in the myocardium. The most common cause of angina is CHD
(5) and ~50% of patients with the
disease exhibit chronic stable angina as their initial symptom
(6). Recent studies have indicated
that angina is one of the disease states that may modify the
pharmacokinetics of a drug (7–9).
However, the pathway by which angina is regulated by the
pharmacokinetics of traditional Chinese medicine is unknown.
The Chinese medicinal formula Guanxin II (Japanese
name Kan-shin No. 2) is widely used in China, Japan and Korea for
the treatment of CHD and exhibits a beneficial effect in the
attenuation of angina (10–15).
Guanxin II contains Salvia miltiorrhiza Bge., Carthamus
tinctorius L., Paeonia lactiflora Pall., Ligusticum
wallichii Hort. and Dalbergia odorifera T. Chen. in
ratio of 2:1:1:1:1 dry weight. To date, 57 compounds have been
identified in Guanxin II (16).
Amongst these, ferulic acid (FA, chemical structure shown in
Fig. 1), from the herb
Ligusticum wallichii Hort., is the main bioactive component
of Guanxin II that, according to our previous studies, exerts a
cardioprotective effect on myocardial ischemia injuries (11,17).
It has been reported that FA is able to exert a vasorelaxant effect
on the thoracic aorta of rats and thereby attenuate angina
(18). However, studying the
effects of Guanxin II on the human aorta would provide further
evidence.
Currently, researchers studying pharmacokinetics are
focused on individual bioactive compounds rather than all of the
phytochemicals in Guanxin II (19,20).
The pharmacokinetic study of FA from Guanxin II is crucial to
aiding the understanding of the conditions that affect its
absorption, distribution, metabolism and excretion in humans.
Despite the fact that recent studies have focused on the
pharmacokinetics of FA (20–25),
there have been no studies regarding the pharmacokinetics of FA
with respect to its vasorelaxant effect on patients diagnosed with
angina pectoris.
The aim of this study was to investigate the
vasorelaxant effect of FA on the human internal mammary artery
(IMA) to provide evidence that it is a bioactive component of
Guanxin II and to explore its effect on angina pectoris by
comparing the pharmacokinetics of FA in healthy volunteers with
those of patients with CHD following the oral administration of
Guanxin II. The information obtained may be useful for the clinical
application of Guanxin II in angina pectoris patients.
Materials and methods
Crude drugs, chemicals and reagents
Guanxin II consists of five herbal components:
Salvia miltiorrhiza Bge., Carthamus tinctorius L.,
Paeonia lactiflora Pall., Ligusticum wallichii Hort.
and Dalbergia odorifera T. Chen. The constitutive ratio of
these five herbs is 2:1:1:1:1 dry weight. All herbs were purchased
from the traditional Chinese medicine dispensary at the West China
Hospital (Chengdu, China). The plants were authenticated by the
herbal medicine botanist Professor ZH Hu of the Department of
Botanical Anatomy, Northwest University (Xi’an, China). The voucher
specimen was deposited at the Laboratory of Ethnopharmacology at
Xiangya Hospital, Central South University (Changsha, China).
The Guanxin II mixture was soaked in distilled water
(1:12, w/v) for 0.5 h at room temperature with occasional stirring.
Following soaking, the herbs were boiled for 0.5 h, and the cooled
decoction was filtered through two layers of cotton gauze. The
residue was boiled again with distilled water (1:6, w/v) by the
procedure mentioned previously, and the decoctions obtained from
the two successive extractions were mixed. The decoctions were
concentrated using a rotary evaporator at 65°C (Büchi Labortechnik
AG, Flawil, Switzerland) and subsequently lyophilized and stored at
4°C. The lyophilized powder was resolved to scale using distilled
water according to the standard of 1 g/ml (w/v) prior to
experimentation (12).
Authentic standards of FA and benzoic acid were
purchased from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China).
High-performance liquid chromatography (HPLC)-grade methanol was
purchased from Tedia Company Inc. (Fairfield, OH, USA). In-house
triple-distilled water from silica glass equipment was used for all
solutions and the other reagents were of analytical grade. For the
ex vivo experiments, the FA was solubilized in dimethyl
sulfoxide (DMSO) at a concentration of 1 mol/l and diluted to the
desired concentration prior to testing (26). A control group was also included,
in which the same volume of DMSO was used as a vehicle control.
Instrumentation and determination of the
FA content of Guanxin II
The Waters 2690 HPLC system (Waters Corporation,
Milford, MA, USA) included a gradient controller, an automatic
sample injector and a 996-photodiode array detector. Separation was
performed on a Capcell Pak C18 ACR (2.0×50.0 mm)
(Shiseido Co. Ltd., Tokyo, Japan). The mobile phase was methanol/1%
aqueous acetic acid with gradient elution (0.01 min, 5:95; 0.3 min,
5:95; 2 min, 100:0 and 3 min, 100:0), and the flow rate was 0.8
ml/min. The column temperature was set at 40°C with an injection
volume of 40 μl. Mass spectrometry was performed on a
Finnigan™ TSQ® mass spectrometer equipped with an
atmospheric-pressure chemical ionization (APCI) interface (Thermo
Scientific, San Jose, CA, USA). The conditions for mass
spectrometry were optimized in order to achieve maximum
sensitivity. The APCI conditions were as follows: corona discharge
voltage, 4.5 kV; heated capillary temperature, 330°C; nebulization
temperature, 450°C; sheath gas, nitrogen (70 psi, 1 psi = 6,894.76
Pa); and auxiliary gas, nitrogen (25 a.u.). Argon with a collision
energy of 35 V was used as the collision gas. The quantification of
FA in Guanxin II was performed by the instrumentation described
previously. The yield of lyophilized powder of Guanxin II was
~24.97% (w/w). The content (mg/g) of FA in Guanxin II was
0.238±0.007. The overall intra- and inter-day variations were
<9%. These results demonstrated that the developed method is
reproducible to a high degree of precision. The accuracy tests were
performed using a recovery test. The recovery of FA was
>90%.
Vascular reactivity of FA on the IMAs of
CHD patients
The protocol for this study was approved by the
Medical Ethics Committee of Xiangya Hospital of Central South
University, (Changsha, China). The clinical trial was performed in
accordance with the Declaration of Helsinki and informed consent
was obtained from the patients and their close relatives prior to
sampling.
Samples of redundant IMAs were obtained from ten
patients undergoing coronary artery bypass graft surgery at Xiangya
Hospital (age range, 48–60 years). The patients had not received
antiplatelet drugs for 14 days or angiotensin-converting enzyme
inhibitors for three days prior to surgery.
Each IMA sample was dissected from the patient as a
pedicle with its accompanying vein from the thoracic wall using a
no-touch technique, in which the vessels remain surrounded by
internal thoracic fascia. The IMA samples were transported in cold
(4°C) oxygenated Krebs-Henseleit solution and immediately
transferred to the laboratory.
The IMA segments were cleaned in Krebs-Henseleit
solution in order to remove adherent connective tissue and then cut
into rings ~3 mm in length. The rings were carefully handled in
order to avoid damage to the inner surface and were subsequently
suspended in a 20-ml organ chamber (Radnoti LLC, Monrovia, CA, USA)
containing Krebs-Henseleit solution of the following composition:
NaCl, 118 mM; KCl, 4.7 mM; KH2PO4, 1.2 mM;
MgSO4, 1.2 mM; CaCl2, 2.9 mM;
NaHCO3, 25 mM; glucose, 11.1 mM and EDTA, 0.5 mM. The
medium was gassed with a mixture of CO2 (5%) and
O2 (95%) and maintained at 37°C (pH 7.4). The vessel
preparations were mounted with one stainless steel wire in the
organ bath and the other wire through the vessel lumen connected to
a force transducer. Isometric changes in tension were recorded by a
multichannel acquisition and analysis system (Biopac MP150; Biopac
Systems, Inc., Goleta, CA, USA). The rings were stretched
progressively to an optimal basal tension of 2.0 g and allowed to
equilibrate for 60 min. The pre-warmed and oxygenated
Krebs-Henseleit solution was changed every 20 min. Each experiment
began with repeated contraction of the rings induced by 60 mM KCl
until two consecutive contractile responses were reproducible.
Either FA (concentrations between 10−8−10−3
M) or DMSO (control) was added cumulatively to the organ baths, and
the tension was monitored for 60 min. The vasodilatory responses to
FA were expressed as the percentage of relaxation, which was
calculated from the following equation: (Maximum precontractile
force − maximum relaxation force induced by FA)/(maximum
precontractile force − baseline tension) × 100.
Pharmacokinetic comparison of FA
following the oral administration of Guanxin II
In total, 18 patients with angina pectoris (10
females and 8 males; mean age, 43.63 ± 3.42 years; age range, 40–49
years) and 18 healthy volunteers (9 females and 9 males; mean age,
42.88 ± 3.04 years; age range, 37–46 years) participated in this
study. All patients underwent a coronary angiography (CAG). The
Medical Ethics Committee of West China Hospital at Sichuan
University and Xijing Hospital of the Fourth Military Medical
University approved the study protocols since part of the
experiment was performed in Xijing Hospital of the Fourth Military
Medical University. The studies were performed according to the
Good Clinical Practice and International Conference on
Harmonization guidelines (26).
The volunteers were judged to be healthy based on
their medical history, physical examination and routine laboratory
tests (blood, urine and stool tests, hepatorenal function,
electrocardiogram, sternum and normal abdominal ultrasound
examination). Eligible subjects had a body weight within 10% of
their ideal weight for their height. Subjects were excluded if they
had participated in any investigational trial within the previous
30 days, were pregnant or lactating, had a history of substance
abuse or had consumed an excessive quantity of alcohol (>2
drinks/day).
Angina pectoris was defined by the presence of chest
pain at rest and/or upon exertion and one of the following
additional criteria: i) angiographic stenosis >50%, ii) a
positive scintigraphy (if no angiographic data available), iii) a
positive exercise stress test (if no angiographic or scintigraphic
data available), or iv) any electrocardiogram changes when at rest
(if no angiographic, scintigraphic or exercise stress test data
were available) excluding myocardial infarction and no evidence of
a non-coronary cause in the clinical history. Unstable angina was
defined as either a crescendo pain (either a change in the
frequency or severity of chest pain on exertion or the appearance
of chest pain at rest following pre-existing pain on exertion) or
chest pain at rest, with either enzyme changes or electrical
changes. In the absence of enzyme and electrical data, the
diagnosis was not upheld. The exclusion criteria were a known
contrast allergy, significant renal dysfunction (serum creatinine
>120 mmol/l); Braunwald class IA, IIA, or IIIA (unstable angina
caused by non-cardiac illness) and previous percutaneous or
surgical revascularization. All subjects provided informed
consent.
A standard stock solution was prepared by dissolving
FA in methanol to a nominal concentration of 81 μg/ml. The
stock solutions were maintained at 4°C prior to use. Standard
samples (2.11, 8.44, 33.76, 135.04, 540.16, and 2,160.64 ng/ml)
were prepared by spiking blank serum with the appropriate
quantities of the standard stock solution, which had been prepared
as described previously. Quality control (QC) samples were
independently prepared at low (8.44 ng/ml), medium (135.04 ng/ml)
and high (2,160.64 ng/ml) concentrations in order to determine the
recovery, accuracy and precision of the method. All samples were
stored at −20°C until further analysis.
Initially the FA content was calculated, and Guanxin
II was subsequently administered orally to the subjects at a dose
of 3 g/kg (equivalent to an FA dose of 0.508 mg/kg). All subjects
fasted for 12 h and had free access to water during the experiment.
Blood samples (10 ml) were collected at 0, 5, 10, 15, 30, 45, 60,
90, 120, 180 and 240 min following the oral administration. Whole
blood was centrifuged at 3000 × g for 20 min (Sigma 2–16;
Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The serum was
recovered and stored at −76°C until further analysis.
Each serum sample (1 ml) was thawed, transferred to
a 5 ml centrifuge tube and mixed with 2.4 ml 80% ethanol
[containing 1,000 ng benzoic acid as the internal standard (IS)]
for 12 h. After stirring, the resulting mixture was centrifuged at
12,000 × g for 10 min. The supernatant was transferred to a 5-ml
centrifuge tube and evaporated to dryness at 45°C under a stream of
nitrogen. The residue was dissolved in 100 μl mobile phase,
and 40 μl of this solution was injected into the HPLC
column. FA absorption in the supernatant was measured by HPLC
coupling and two stages of mass analysis (MS/MS). The same sample
preparation was used to validate the analytical method. For samples
from the healthy volunteers whose concentrations were below the
limit of quantification, samples of whole blood was centrifuged at
3000 × g for 20 min. The serum was recovered and kept at −76°C
until analysis. The serum sample was thawed and transferred to a 5
ml centrifuge tube, then mixed with 1.2 ml 80% ethanol for 12 h.
After stirring, the resulting mixture was centrifuged at 12,000 × g
for 10 min. Supernatant (0.8 ml) was transferred into a 5 ml
centrifuge tube and evaporated to dryness under a stream of
nitrogen at 45°C. The residue was dissolved in 100 μl mobile
phase and 20 μl of the solution was injected into an
UPLC-MS/MS analysis column. The calculated values were then halved
to normalize the concentration to the other samples.
Statistical analysis
All data were expressed as the mean ± SEM. A
database was created using the SPSS 15.0 software package (SPSS,
Inc., Chicago, IL, USA). Comparisons between the two groups were
made using the unpaired Student’s t-test. Comparisons between
multiple groups were performed using one-way ANOVA followed by
Tukey’s post-hoc test. The Student’s t-test was used when
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
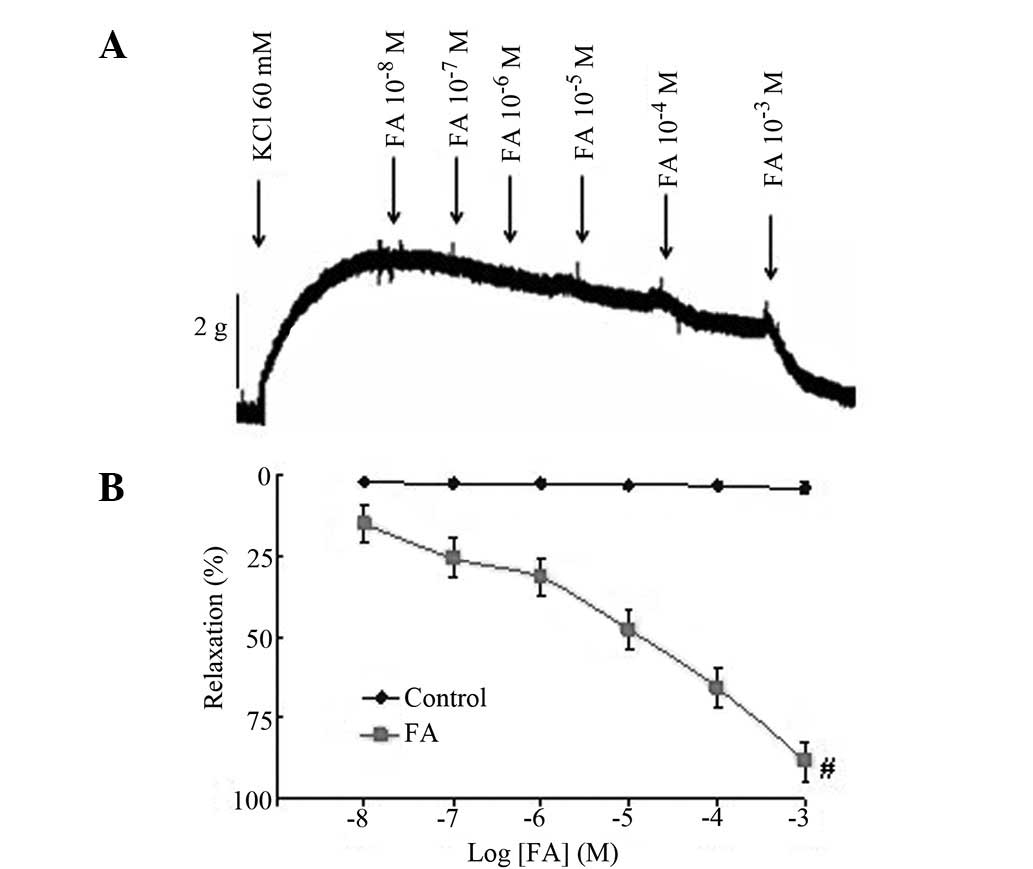
Relaxant effects of FA on human IMA
precontracted by KCl
Treatment with various doses of FA
(10−8−10−3 M) had no effect on the resting
tone (data not shown). The IMA preparations with functional
endothelia were initially contracted with 60 mM KCl. The maximum
contractile response elicited by KCl was a mean peak contraction of
3,028.64±219.70 mg for all tissues. In the endothelial IMA rings,
FA (10−8−10−3 M) relaxed the rings that had
been precontracted by KCl in a concentration-dependent manner
(Fig. 2). FA treatment resulted in
full relaxation (88.36±4.78%), suggesting that FA is effective in
inhibiting KCl-induced contraction in the IMA. The results indicate
that FA may exert a vasodilative effect on KCl-related
vasoconstriction in the human IMA.
Extraction and recovery
The method for determining the serum concentration
of FA required 1 ml of serum, and the compound was extracted using
a simple ethanol extraction procedure. The FA recovery rates from
the serum were 93.17±4.45, 95.99±3.75 and 91.47±3.30% at high,
medium, and low FA concentrations, respectively (Table I).
 | Table I.Ferulic acid recovery in human serum
(n=3). |
Table I.
Ferulic acid recovery in human serum
(n=3).
| Spiked
concentration (ng/ml) | Recovery (%) (mean
± SEM) | RSD (%) |
|---|
| 8.44 | 93.17±4.45 | 4.63 |
| 135.04 | 95.99±3.75 | 3.92 |
| 2160.64 | 91.47±3.30 | 3.61 |
Chromatographic selectivity
Fig. 3 shows the
chromatograms obtained following the analysis of drug-free serum
spiked with FA and IS, in addition to serum samples collected 30
min following the oral administration of Guanxin II. Based on the
chromatography of the pure reference standards, the peaks in
Fig. 3 were identified as FA and
IS, with retention times of 3.11 and 3.38 min, respectively. The
method used in this study is rapid, with a run-time of 4 min for
each analysis.
Calibration curves
The calibration curve for FA was linear
(r2=0.9993) over the concentration range 2.11–2,160.64
ng/ml, and a regression equation of y=248.31x + 3.22 (where x is
the peak area ratio and y is the concentration of analyte) was
obtained. The detection limit, based on a signal-to-noise ratio of
3, was 0.34 ng/ml, and the quantitation limit was 1.44 ng/ml. These
ranges were found to be adequate for the concentrations observed
from the analysis of the collected serum samples. It demonstrated
considerable linearity and a similar precision and accuracy to that
observed in previous studies (22).
Precision and accuracy
The reproducibility of the method was determined by
examining intra- and inter-day variance. The intra- and inter-day
precision assays gave satisfactory results (Table II). The mean relative standard
deviation (RSD) (<8.65%) and accuracy (>90%) for each of the
concentrations tested indicated that the method was precise and
reproducible. The results of precision and recovery rates conformed
to the principles of bio-sample analysis.
 | Table II.Precision data on the proposed HPLC
method in human serum. |
Table II.
Precision data on the proposed HPLC
method in human serum.
| Nominal
concentration (ng/ml) | Precision
|
|---|
Intra-day (n = 8)
| Inter-day (n = 5)
|
|---|
| Mean ± SEM
(ng/ml) | RSD (%) | Mean ± SEM
(ng/ml) | RSD (%) |
|---|
| 8.44 | 8.14±0.42 | 5.16 | 7.99±0.69 | 8.64 |
| 135.04 | 131.47±5.87 | 4.46 | 130.36±10.29 | 7.89 |
| 2160.64 | 2098.8±130.55 | 6.22 | 2018.2±161.66 | 8.01 |
Pharmacokinetic parameters
The concentration-time curves of serum FA were
analyzed using the DAS program (Drug and Statistics program; The
Chinese Society of Mathematical Pharmacology, The Clinical Drug
Evaluation Center of Anhui Province, China) on a personal computer
to determine the compartment model and the pharmacokinetic
parameters were calculated. The serum FA concentration-time curve
conformed to the two-compartment model, and the FA concentration
profiles vs. time for the serum from patients and healthy
volunteers treated with Guanxin II are presented in Fig. 4. The FA pharmacokinetic parameters
derived from the serum following the oral administration of the
decoctions of Guanxin II are presented in Table III.
 | Table III.Pharmacokinetic parameters of ferulic
acid in serum following the oral administration of Guanxin II. |
Table III.
Pharmacokinetic parameters of ferulic
acid in serum following the oral administration of Guanxin II.
| Parameter | Angina pectoris
patients (n=18) | Healthy volunteers
(n=18) |
|---|
| Tmax
(min) | 23.95±7.96 | 30.00±0.00 |
| Cmax
(ng/ml) | 26.20±4.45 | 33.50±3.83a |
| AUC0–240
(μg/ml/min) | 2.72±0.83 | 3.56±0.70 |
| t1/2α
(min) | 15.02±3.62 | 20.77±3.58a |
| t1/2β
(min) | 106.23±40.72 |
772.36±199.04b |
| t1/2Ka
(min) | 9.89±4.23 | 15.20±3.12a |
Discussion
In this study, it was demonstrated that FA is a
potent agent that is able to produce a vasodilative effect in human
IMA rings and potentially attenuate angina pectoris. This, to the
best of our knowledge, has not been reported previously. Although
additional studies concerning the underlying mechanisms by which FA
causes vasorelaxation are necessary, this study provides evidence
for the continued focus on the pharmacokinetics of FA originating
from Guanxin II in the treatment of patients with angina
pectoris.
The analysis of FA in animal (21–24)
and human (20,25) serum using HPLC based on
liquid-liquid extraction has been reported, but these methods were
not considered to be sufficiently sensitive in the human study
following the oral administration of Guanxin II. In the current
study, we report the development of a reversed-phase HPLC method in
combination with boiling waterbath extraction that exhibits
sufficient specificity, sensitivity and simplicity for the
measurement of FA in human serum. Compared with a previous study
that focused on human serum (25),
the t1/2Ka of FA was diminished following the oral
administration of Guanxin II to healthy volunteers. This previous
study mainly focused on a single component (the oral administration
of sodium ferulate) instead of complicated prescriptions based on
the theory of traditional Chinese medicine and its traditional use
(25). Guanxin II is a complicated
prescription; the presence of the other four components within
Guanxin II may affect the pharmacokinetics of FA (27), which may also improve the
hemodynamics.
Although the doses of Guanxin II administered to the
two groups were identical (3 g/kg), the estimated pharmacokinetic
parameters of FA in the patients and healthy volunteers differed.
Following the oral administration of Guanxin II to healthy
volunteers, FA was absorbed at a fast rate and achieved a maximum
serum concentration (Cmax) value (33.50±3.83 ng/ml)
within 23.95±7.96 min. The serum concentration of FA diminished,
with a t1/2β of 772.36±199.04 min. However, the oral
administration of Guanxin II to angina patients resulted in a
Cmax of FA of 26.20±4.45 ng/ml within 30 min, and the
serum concentration of FA decreased with a t1/2β of
106.23±40.72 min. Compared with the AUC0–240 value
(3.56±0.70 μg/ml/min) calculated following the oral
administration of Guanxin II to healthy volunteers, the
AUC0–240 value (2.72±0.83 μg/ml/min, P<0.05)
calculated in angina pectoris patients was reduced.
Angina pectoris is a symptom of myocardial ischemia,
which occurs when the myocardium receives an insufficient supply of
blood (and therefore, less oxygen) during diastole (28). Myocardial ischemia results in poor
heart function, including reduced microcirculation blood flow.
Local blood flow is a strong determinant of absorption,
distribution and metabolism rates due to the fact that it
continuously maintains the concentration gradient necessary for
passive diffusion to occur (8,27).
Therefore, this study provides evidence that angina pectoris may
modify the pharmacokinetics of a drug.
In conclusion, to the best of our knowledge, this is
the first study to explore the relationship between angina pectoris
and the administration of traditional Chinese medicine by comparing
the pharmacokinetics of the vasorelaxant compound FA in healthy
volunteers and patients with angina pectoris following the oral
administration of Guanxin II. The pharmacokinetic parameters
indicate that angina pectoris may modify the pharmacokinetics of
FA. The pharmacokinetic parameters may not only direct the clinical
use of Guanxin II, but they may also be useful for exploring the
pathology of angina pectoris.
Abbreviations:
|
RSD
|
relative standard deviation;
|
|
CHD
|
coronary heart disease;
|
|
FA
|
ferulic acid;
|
|
IMA
|
internal mammary artery;
|
|
DMSO
|
dimethyl sulfoxide;
|
|
CAG
|
coronary angiography;
|
|
QC
|
quality control;
|
|
HPLC
|
high-performance liquid
chromatography;
|
|
AUC
|
area under the curve
|
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 81173591; No.
81202781), the Doctoral Fund of the Ministry of Education of China
(No. 20100162110033) and the Administration of Traditional Chinese
Medicine of Hunan Province, China (No. 201152; No. 201141; No.
201246). In addition, this study was partly supported by the
Program for Changjiang Scholars and the Innovative Research Team of
the University of the Ministry of Education of China (No.
IRT0946).
References
|
1.
|
Thom T, Haase N, Rosamond W, et al
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics - 2006
update: a report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
113:e85–e151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Murphy SL, Xu JQ and Kochanek MA; Division
of Vital Statistics: Deaths: Preliminary data for 2010. National
Vital Statistics Reports. 60:1–68. 2012.
|
|
3.
|
He L, Tang X, Song Y, et al: Prevalence of
cardiovascular disease and risk factors in a rural district of
Beijing, China: a population-based survey of 58,308 residents. BMC
Public Health. 12:342012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nabel EG and Braunwald E: A tale of
coronary artery disease and myocardial infarction. N Eng J Med.
366:54–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lenfant C: Chest pain of cardiac and
noncardiac origin. Metabolism. 59(Suppl 1): S41–S46. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Abrams J: Clinical practice. Chronic
stable angina. N Engl J Med. 352:2524–2533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Barre J, Houin G, Brunner F, et al:
Disease-induced modifications of drug pharmacokinetics. Int J Clin
Pharmacol Res. 3:215–226. 1983.PubMed/NCBI
|
|
8.
|
Ventresca GP and Mariani L: Disease states
and changes in drug pharmacokinetics. Clin Ter. 47:131–144.
1996.(In Italian).
|
|
9.
|
Qin F, Huang X, Zhang HM and Ren P:
Pharmacokinetic comparison of puerarin after oral administration of
Jiawei-Xiaoyao-San in healthy volunteers and patients with
functional dyspepsia: influence of disease state. J Pharm
Pharmacol. 61:125–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cho EJ, Yokozawa T and Okamoto T:
Protective effect of Chinese prescription Kangen-karyu and its
crude drug Tanjin against age-related lipidosis in rats. J Pharm
Pharmacol. 59:687–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Huang X, Qin F, Zhang HM, et al:
Cardioprotection by Guanxin II in rats with acute myocardial
infarction is related to its three compounds. J Ethnopharmacol.
121:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Qin F, Liu YX, Zhao HW, et al: Chinese
medicinal formula Guan-Xin-Er-Hao protects the heart against
oxidative stress induced by acute ischemic myocardial injury in
rats. Phytomedicine. 61:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Xu R, Huang X, Li Y, et al: Clinical
observation of treating coronary heart disease with
Guan-xin-er-hao. Journal of Chengdu University of Traditional
Chinese Medicine. 24(4): 17–19. 2001.(In Chinese).
|
|
14.
|
Zhao HW, Qin F, Liu YX, et al:
Antiapoptotic mechanisms of Chinese medicine formula
Guan-Xin-Er-Hao in the rat ischemic heart. Tohoku J Exp Med.
216:309–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhao J, Huang X, Tang W, et al: Effect of
oriental herbal prescription Guan-Xin-Er-Hao on coronary flow in
healthy volunteers and antiapoptosis on myocardial
ischemia-reperfusion in rat models. Phytother Res. 21:926–931.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Qiao X, Han J, Xu M, et al:
Characterization of chemical constituents in Guan Xin II decoction
by liquid chromatography coupled with electrospray ionization-mass
spectrometry. Planta Med. 74:1720–1729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wang Y, Huang X, Qin F, et al: A strategy
for detecting optimal ratio of cardioprotection-dependent three
compounds as quality control of guan-xin-er-hao formula. J
Ethnopharmacol. 133:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Guo GP, Ye Y, Li L, et al:
Endothelium-independent vasorelaxant effect of sodium ferulate on
rat thoracic aorta. Life Sci. 84:81–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang L, Wang Z, Wo S, et al: A
bio-activity guided in vitro pharmacokinetic method to improve the
quality control of Chinese medicines, application to Si Wu Tang.
Int J Pharm. 406:99–105. 2011. View Article : Google Scholar
|
|
20.
|
Qiu XJ, Huang X, Chen ZQ, et al:
Pharmacokinetic study of the prokinetic compounds meranzin hydrate
and ferulic acid following oral administration of Chaihu-Shugan-San
to patients with functional dyspepsia. J Ethnopharmacol.
137:205–213. 2011. View Article : Google Scholar
|
|
21.
|
Li Y, Liu C, Zhang Y, et al:
Pharmacokinetics of ferulic acid and potential interactions with
Honghua and clopidogrel in rats. J Ethnopharmacol. 137:562–567.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Li FQ, Xu S, Su H, et al: Development of a
gradient reversed-phase HPLC method for the determination of sodium
ferulate in beagle dog plasma. J Chromatogr B Analyt Technol Biomed
Life Sci. 846:319–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li Y and Bi K: HPLC determination of
ferulic acid in rat plasma after oral administration of Rhizoma
Chuanxiong and its compound preparation. Biomed Chromatogr.
17:543–546. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Qi J, Jin X, Huang L and Ping Q:
Simultaneous determination of hydroxysafflor yellow A and ferulic
acid in rat plasma after oral administration of the co-extractum of
Rhizoma chuanxiong and Flos Carthami by HPLC-diode array
detector. Biomed Chromatogr. 21:816–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yang C, Tian Y, Zhang Z, et al:
High-performance liquid chromatography-electrospray ionization mass
spectrometry determination of sodium ferulate in human plasma. J
Pharm Biomed Anal. 43:945–950. 2007. View Article : Google Scholar
|
|
26.
|
Kern P: Medical treatment of
echinococcosis under the guidance of Good Clinical Practice
(GCP/ICH). Parasitol Int. 55:S273–S282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Suzuki A, Yamamoto M, Jokura H, et al:
Ferulic acid restores endothelium-dependent vasodilation in aortas
of spontaneously hypertensive rats. Am J Hypertens. 20:508–513.
2007. View Article : Google Scholar
|
|
28.
|
Sullivan JM and Lobo RA: Considerations
for contraception in women with cardiovascular disorders. Am J
Obstet Gynecol. 168:2006–2011. 1993. View Article : Google Scholar : PubMed/NCBI
|