Introduction
Breast cancer is the most common malignancy in
females, accounting for 31% of all female cancers. Approximately
two-thirds of breast cancers exhibit high concentrations of
estrogen receptor (ER). The selective ERα modulator tamoxifen is
the most commonly prescribed endocrine therapy. A 5-year treatment
of adjuvant tamoxifen therapy has been shown to reduce the 15-year
risk for recurrence and mortality in breast cancer patients with
ERα-positive cancer (1). However,
adjuvant tamoxifen therapy fails in 30–40% of patients and nearly
all patients with metastatic disease develop tamoxifen resistance.
ERα is essential for estrogen-dependent growth and its level of
expression is a crucial determinant of the response to endocrine
therapy and the prognosis in ERα-positive breast cancer (2,3).
There is no doubt that the more ERα is present in the tumor cells,
the greater the likelihood of a favorable response to endocrine
therapy (4), but little is known
about how the expression of ERα is regulated in human breast
cancer.
MicroRNAs (miRNAs) are small (∼21 nucleotides),
noncoding RNAs that negatively regulate target genes by
predominantly binding to the 3′ untranslated region (3’UTR) of
target mRNA, resulting in either mRNA degradation or translational
repression (5). Evidence has shown
that miRNA mutations or misexpression are associated with various
types of human cancer and indicates that miRNAs are able to
function as tumor suppressors and oncogenes (6). Previously, studies have shown that
microRNA expression profiling also revealed that miRNAs are
differently expressed among the molecular subtypes of breast cancer
(7,8).
Kondo et al reported that miR-206 was
markedly decreased in ERα-positive human breast cancer tissues and
that the introduction of miR-206 into estrogen-dependent MCF-7
breast cancer cells led to the suppression of ERα expression and
growth inhibition (9). Adams et
al identified that miR-206 decreases endogenous ERα mRNA and
protein levels in MCF-7 cells by acting through two specific
miR-206 target sites within the 3’UTR of the human ERα transcript
(10). Leivonen et al
previously reported that five ERα-regulating miRNAs, miR-18a,
miR-18b, miR-193b, miR-302c and miR-206, directly targeted ERα in
3’UTR reporter assays (11).
Furthermore, other studies demonstrated that miR-22 (12,13)
and miR-221/222 (14,15) also directly interacted with the
3’UTR region of ERα and regulated ERα expression. Thus, studies
have shown critical interactions between ERα and miRNAs and
suggested that several miRNAs regulate ERα expression directly or
indirectly. It has been shown that the downregulation of miR-342 is
associated with ERα-negative breast cancer (8) and tamoxifen-resistant breast tumors
(16).
The present study was undertaken to assess the
expression of miR-342 and ERα mRNA in human breast cancer samples.
Correlations between the expression levels of miR-342 and
clinicopathological factors were analyzed. For the first time
miR-342 expression was identified as positively correlated with ERα
mRNA expression. The ectopic expression of miR-342 upregulated ERα
mRNA levels and promoted tamoxifen sensitivity in MCF-7 cells,
whereas the knockdown of miR-342 reduced ERα mRNA expression and
weakened tamoxifen sensitivity. These results indicated that
miR-342 may emerge as a significant marker for the tamoxifen
response, as well as as a potential therapeutic target.
Materials and methods
Breast cancer tissues and
immunohistochemical analysis
A total of 48 breast cancer cases and 24 normal
adjacent tissues from female patients with invasive breast
carcinoma, who were treated in the Jiangsu Province Cancer Hospital
of China between 2010 and 2012, were included in the present study.
The study protocol was approved by the institutional review board
and conformed to the guidelines of the 1975 Declaration of
Helsinki. All patients had undergone surgical treatment for primary
breast cancer (either mastectomy or lumpectomy), without previous
chemoradiotherapy and were aged between 31 and 82 years old, with a
median age of 48. The ERα, progesterone receptor (PR), human
epidermal growth factor receptor 2 (HER2) and vascular endothelial
growth factor (VEGF) expression status was confirmed by
immunohistochemistry (IHC) as follows. One 4-μm section of each
submitted paraffin block was first stained with H&E to verify
that an adequate number of invasive carcinoma cells were present
and that the fixation quality was adequate for IHC analysis. Serial
sections (4 μm) were prepared from selected blocks and float
mounted onto adhesive-coated glass slides, for staining with
monoclonal rabbit anti-human antibodies (Dako, Carpinteria, CA,
USA) at a 1:100 dilution. Any brown staining in the invasive breast
epithelium was considered a positive result. According to the
estimated proportion of tumor cells stained positive, the ER, PR,
HER2 and VEGF status was evaluated as follows: Negative (<10%),
+ (10–30%), ++ (31–50%) and +++ (>50%). HER2 gene amplification
was analyzed by fluorescence in situ hybridization (FISH)
when HER2 status + or ++, the method has been published elsewhere
(17).
Quantitative reverse transcription
(RT)-PCR detection of miRNA
Total RNA was extracted from ∼500 mg of frozen
breast cancer tissue or ∼1×106 breast cancer cells
(MCF-7, SKBR-3,MB-231) using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. cDNA was reverse transcribed from the total RNA
samples using specific miRNA primers from the TaqMan MicroRNA
Assays and reagents from the TaqMan MicroRNA Reverse Transcription
kit (Applied Biosystems, Carlsbad, CA, USA). The resulting cDNA was
amplified by PCR using TaqMan MicroRNA Assay primers with the
TaqMan Universal PCR Master Mix and analyzed with a 7500 ABI PRISM
Sequence Detector System according to the manufacturer’s
instructions (Applied Biosystems). The relative levels of miRNA
expression were calculated from the relevant signals by
normalization with the signal for U6 miRNA expression. The assay
names for miR-342 were hsa-miR-342-3p (Applied Biosystems).
Quantitative RT-PCR detection of
mRNA
The total RNA (1 μg) was subjected to reverse
transcription with random primers in a 20-μl reaction volume using
PrimeScript® RT Master Mix (Applied Takara, Dalian,
China). The ERα mRNA expression was measured by quantitative RT-PCR
with SYBR Premix Ex Taq™ (Applied Takara) and primers for ERα
(forward, 5′-TGCCCTACTACCTGGAGAAC-3′ and reverse,
5′-CCATAGCCATACTTCCCTTGTC-3′), using a 7300 ABI PRISM Sequence
Detector System according to the manufacturer’s instructions
(Applied Biosystems). The relative expression level compared with
that of β-actin was calculated using the comparative Ct method.
Cell culture and transfections
MCF-7 cells (American Type Culture Collection,
Manassas, VA, USA) were grown in DMEM (Gibco, Carlsbad, CA, USA)
containing 10% fetal bovine serum (FBS) and 2 mM/l L-glutamine and
penicillin-streptomycin (50 IU/ml and 50 mg/ml, respectively) at
37°C with 5% CO2. The transfection was performed with
Lipofectamine™ 2000 Reagent (Invitrogen Life Technologies)
according to the manufacturer’s instructions. The miR-342-3p
mimics, miR-342-3p inhibitor and the negative control (NC) were
purchased from Jima Co., Shanghai, China. The concentration of the
mimics and inhibitors were 10 and 20 nM, respectively. The
efficiency of the miR-342 transfection was measured by real-time
PCR.
Cell proliferation assay
Following transfection, the MCF-7 cells (5,000 cells
per well) were plated in 96-well plates and treated with 10 nM
17β-estradiol (E2, Sigma, St. Louis, MO, USA) alone or in
combination with 20 μM tamoxifen (Sigma) for 72 h subsequent to
overnight serum starvation. Cell proliferation was documented using
a cell counting kit-8 (CCK-8) assay kit (Dojindo Laboratories,
Kumamoto, Japan) and recording absorbance at 450 nm with a 96-well
plate reader.
Apoptosis test
Following transfection, the MCF-7 cells
(1.5×105 cells per well) were treated with 15 μM
tamoxifen for 48 h and then stained with FITC-conjugated
anti-Annexin V antibodies. The Annexin V-FITC Apoptosis Detection
kit (BD Pharmingen, San Diego, CA, USA) was used to analyze cell
apoptosis with flow cytometry (BD Aria; BD Biosciences, Franklin
Lakes, NJ, USA).
Statistical analysis
All statistical analyses were performed using SPSS
17.0. All data are expressed as the mean ± SD of at least 3
independent experiments. The differences between the groups were
analyzed using the Student’s t-test or ANOVA; P<0.05 was
considered to indicate statistically significant results.
Results
Correlations between the expression
levels of miR-342 and the clinicopathological factors
The expression levels of miR-342 in the 48 human
breast cancer tissues were examined. Quantitative RT-PCR detection
analysis showed that the expression levels of miR-342 were markedly
higher in the ERα-positive tumors (1.386±0.480) than in the
ERα-negative tumors (0.785±0.315; P= 0.000), that the miR-342
expression levels were increased in the HER2-negative tumors
(1.416±0.432) compared with the HER2-positive tumors (1.017±0.492;
P= 0.001) and that miR-342 expression was upregulated in the
VEGF-negative tumors (1.416±0.432) compared with the VEGF-positive
tumors (1.088±0.528; P= 0.031). There was no evident relevance
between the levels of miR-342 expression and PR, lymph node
metastasis status or the pathological grade (P>0.05; Table I). No discrepancy exists in the
miR-342 expression between the cancer (1.404±0.529) and cancer
adjacent (1.151±0.387; P=0.065) in this study.
 | Table I.Correlation between the miR-342
expression level and the clinicopathological characteristics of
breast cancer. |
Table I.
Correlation between the miR-342
expression level and the clinicopathological characteristics of
breast cancer.
| | Relative level of
miR-342 (log10)
|
|---|
| Variable | n | Mean ± SD | P-value |
|---|
| Age (years) | | | |
| ≥48 | 16 | 1.202±0.575 | 0.935 |
| <48 | 32 | 1.215±0.492 | |
| Pathological
grade | | | |
| I, II | 36 | 1.243±0.560 | 0.367 |
| III | 12 | 1.116±0.353 | |
| Lymph node
status | | | |
| Metastasis | 32 | 1.218±0.533 | 0.893 |
| No metastasis | 16 | 1.197±0.494 | |
| ER | | | |
| Negative | 14 | 0.785±0.315 | 0.000 |
| Positive | 34 | 1.386±0.480 | |
| PR | | | |
| Negative | 20 | 1.042±0.531 | 0.054 |
| Positive | 28 | 1.332±0.477 | |
| HER2a | | | |
| Negative | 20 | 1.482±0.423 | 0.001 |
| Positive | 28 | 1.017±0.492 | |
| VEGF | | | |
| Negative | 18 | 1.416±0.432 | 0.031 |
| Positive | 30 | 1.088±0.528 | |
| Molecular
Subtype | | | |
| Luminal A
(ER+, HER2−) | 16 | 1.624±0.333 | 0.000 |
| Luminal B
(ER+, HER2+) | 18 | 1.175±0.499 | |
| HER2 overexpression
(ER−, HER2+) | 10 | 0.732±0.340 | |
| Triple-negative
(ER−, PR−, HER2−) | 4 | 0.918±0.223 | |
| AJCC Clinical
Stage | | | |
| I | 12 | 1.150±0.562 | 0.553 |
| IIA | 30 | 1.193±0.510 | |
| IIBb | 6 | 1.423±0.480 | |
miR-342 expression is positively
correlated with ERα mRNA expression in human breast cancer and cell
lines
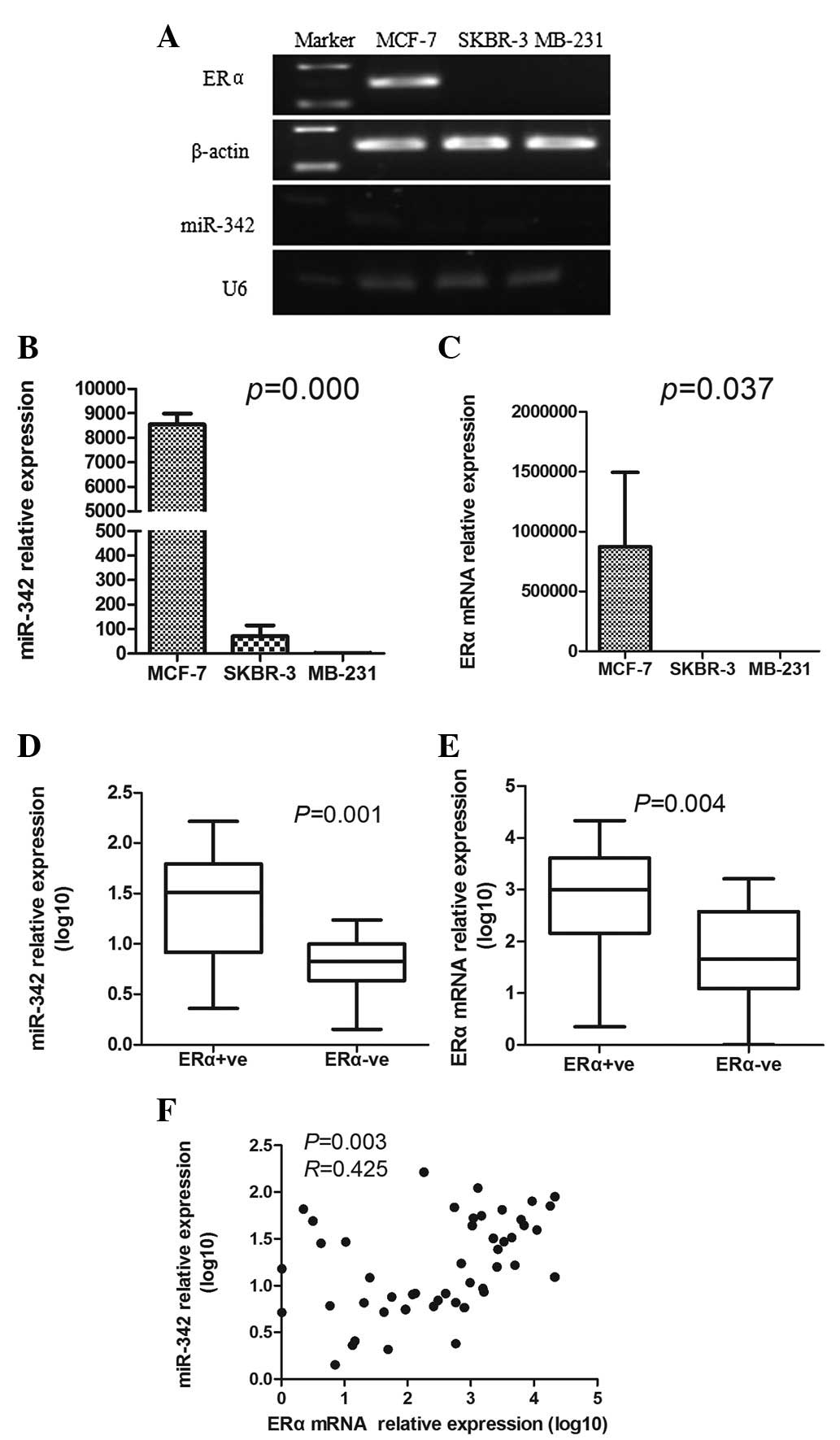
First the expression levels of ERα mRNA and miR-342
were assessed in the breast cancer cell lines and the results
showed that they were greatly increased in the ERα-positive cells
(MCF-7) compared with those in the ERα-negative cells (SKBR-3 and
MB-231; P<0.05; Fig. 1A–C).
Next the ERα mRNA and miR-342 expression levels were examined in
the human breast cancer tissues. As expected, the expression levels
of ERα mRNA were much higher in the ERα-positive tumors than in the
ERα-negative tumors (2.74±1.14 vs. 1.68±1.02; P= 0.004; Fig. 1E). To analyze the association
between the miR-342 expression and the ERα mRNA expression, the
expression levels were plotted. The scatterplots showed that
miR-342 expression was positively correlated with ERα mRNA
expression in human breast cancer (P=0.003; Fig. 1F).
miR-342 elevates ERα mRNA expression of
MCF-7 cells and promotes tamoxifen sensitivity
The MCF-7 cells were transfected with the miR-342
mimics at a concentration of 10 nM or with the miR-342 inhibitors
at a concentration of 20 nM. The control groups were transfected
with the miR-342 NCs or with the miR-342 inhibitor NCs. To examine
the efficiency of the transfection, total RNA was extracted and the
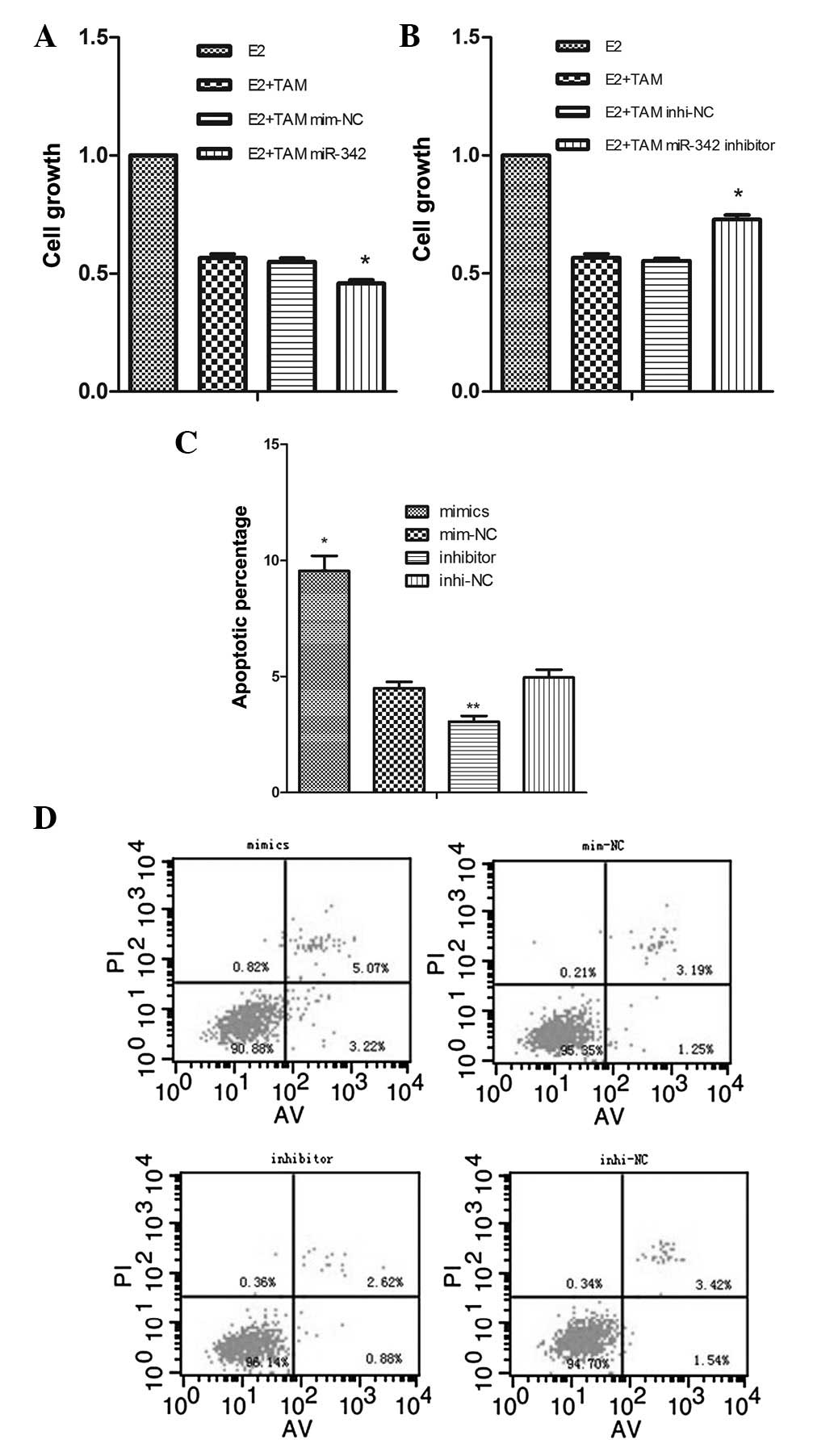
miR-342 level was measured by real-time PCR 48 h post-transfection.
The results showed that the miR-342 expression was significantly
increased in the MCF-7 cells following transfection with the
miR-342 mimics, when compared with control group treated with the
mimic NCs (P=0.000; Fig. 2A). The
miR-342 expression was markedly lower when using the miR-342
inhibitors than when using the miR-342 inhibitor NCs (P=0.000;
Fig. 2B). The ERα mRNA expression
was analyzed by RT-PCR, which showed that the levels of ERα mRNA
expression were upregulated in the group transfected with the
miR-342 mimics compared with those in the control group and
decreased in the group transfected with the miR-342 inhibitors
compared with those in the control group (Fig. 2C and D).
As miR-342 is not differently expressed between the
breast cancer and cancer adjacent tissues, we forecast that miR-342
would not play a tumor-suppressive or tumor-promotive role in
breast cancer development. To understand the functional role of
miR-342, the impact of miR-342 on cellular proliferation was
evaluated using CCK-8 in the MCF-7 cells. The results showed that
96 h after the use of miR-342 mimics or inhibition transfection,
the overexpression or suppression of miR-342 was not able to change
cellular proliferation. Transfection with the miR-342 mimics
compared with the NC, (2.460±0.036 vs. 2.517±0.050, respectively;
P=0.188). Transfection with the miR-342 inhibitors compared with
the NC, (2.363±0.1999 vs. 2.547±0.080, respectively; P=0.212).
However, in the presence of 20 μM tamoxifen for 72 h, ectopic
miR-342 expression was able to suppress cellular proliferation to a
greater extent following transfection with the miR-342 mimics than
the cells transfected with the NC (0.459±0.013 vs. 0.55±0.015,
respectively; P=0.001; Fig. 3A).
By contrast, the suppression of miR-342 is able to inhibit cellular
proliferation less following the transfection with miR-342
inhibitors than the cells with the NC (0.729±0.019 vs. 0.554±0.01,
respectively; P=0.000; Fig.
3B).
As tamoxifen is known to induce apoptosis in breast
cancer cells (18), the potential
role of miR-342 in promoting tamoxifen-mediated apoptosis was
explored. For this purpose, miR-342-overexpressing or
miR-342-suppressing MCF-7 cells were treated with 15 μM tamoxifen
for 48 h, then cell apoptosis was analyzed with flow cytometry
under the same conditions. The results showed that the apoptotic
percentage was higher in the miR-342-overexpressing cells than in
the NC (9.54±1.14 vs. 4.50±0.46%; P=0.002). Conversely, the
apoptotic percentage was lower in the miR-342-suppressing MCF-7
cells than in the NC (3.06±0.42 vs. 4.95±0.59%; P= 0.011; Fig. 3C and D). This series of analyses
demonstrated that the miR-342 indeed plays a key role in changing
the response of MCF-7 cells to tamoxifen.
Discussion
The present study demonstrated that the expression
of miR-342 in the ERα-positive breast cancer tumors and cells was
significantly greater than that in the ERα-negative breast cancer
tumors and cells. The study reported for the first time that the
levels of miR-342 expression were positively correlated with ERα
mRNA expression and also revealed a correlation between increased
tamoxifen sensitivity and the elevated levels of ERα mRNA by
augmenting the miR-342 expression.
In experimental models, a single miRNA is able to
regulate a number of genes (19).
It has been reported that miR-22 is downregulated in ERα-positive
human breast cancer cell lines and clinical samples (13). miR-22 inhibits estrogen signaling
by directly targeting the ERα mRNA (12). miR-221/222 negatively regulates ERα
and is associated with tamoxifen resistance in breast cancer
(14). Previous studies have shown
that miR-342 is an ERα-associated miRNA (8). The results of the present study show
that the expression levels of miR-342 were markedly higher in the
ERα-positive breast cancer tumors than in the ERα-negative tumors
and that the levels of miR-342 gradually increased as ERα mRNA
expression increased, suggesting that miR-342 is a key factor for
the regulation of ERα expression in the development and progression
of human breast cancer.
Endocrine therapy has become the most significant
treatment option for women with ERα-positive breast cancer, with
∼70% of primary breast cancers expressing ERα. The selective ERα
modulator tamoxifen is the most commonly prescribed endocrine
therapy. Currently there are only a few useful tumor markers to
guide management decisions for women with ERα-positive breast
tumors. Cittelly et al(16)
demonstrated that miR-342 was markedly suppressed in multiple
tamoxifen-resistant breast tumor cell lines and in primary breast
tumors of patients whose tamoxifen therapy failed. Significantly,
the reintroduction of miR-342 sensitized the refractory breast
tumor cells to tamoxifen therapy, suggesting that miR-342 is a
significant regulator of the tamoxifen response. In the present
study, miR-342 expression was shown to be positively correlated
with the expression of ERα in human breast cancer tissues and the
introduction of miR-342 into estrogen-dependent breast cancer cells
was shown to upregulate ERα expression and enhance tamoxifen
sensitivity with decreased cellular proliferation and increased
apoptosis. By contrast, inhibition of miR-342 in the MCF-7 cells
downregulated the ERα expression and weakened the response to
tamoxifen, with increased cellular proliferation and decreased
apoptosis. Based on these observations, we propose that the levels
of miR-342 expression that correspond to the ERα mRNA expression
locus may act as a biomarker for tamoxifen sensitivity in
ERα-positive breast cancer.
Cittelly et al(16) reported that there was no evident
association between the direct targets of miR-342 and the tumor
cell response to tamoxifen. Ingenuity Pathway Analysis of the
entire set of genes significantly altered by miR-342 revealed a
significant association between the miR-342-regulated genes and
cell apoptosis. This result is consistent with the observations of
the present study that showed that ectopic miR-342 expression
sensitized MCF-7 cells to tamoxifen-induced apoptosis. Similarly,
miR-342 expression in colorectal cancer cells results in tumor cell
apoptosis (20). Nevertheless, the
activity of miR-342 appears to differ functionally in colorectal
and breast tumor cells. The results of the present study indicated
that miR-342 expression alone was not sufficient to induce cell
death, but that miR-342 sensitizes cells to cellular proliferation
inhibition and apoptosis associated with tamoxifen exposure.
In addition, the results showed that the levels of
miR-342 expression increased in VEGF-negative, HER2-negative and
Luminal-A breast cancer samples. As the VEGF-negative,
HER2-negative and Luminal-A signals indicate a good prognosis,
miR-342 may be a biomarker of predicting a good prognosis for
breast cancer.
In conclusion, the present data indicated for the
first time that miR-342 expression is positively correlated with
the expression of ERα mRNA in human breast cancer tissues and that
the introduction of miR-342 into estrogen-dependent breast cancer
cells enhances tamoxifen sensitivity. miR-342 may be a novel
candidate for ERα-specific endocrine therapy in breast cancer.
References
|
1.
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG); Davies C, Godwin J, Gray R, et al:
Relevance of breast cancer hormone receptors and other factors to
the efficacy of adjuvant tamoxifen: patient-level meta-analysis of
randomised trials. Lancet. 378:771–784. 2011. View Article : Google Scholar
|
|
2.
|
Ford CH, Al-Bader M, Al-Ayadhi B and
Francis I: Reassessment of estrogen receptor expression in human
breast cancer cell lines. Anticancer Res. 31:521–527.
2011.PubMed/NCBI
|
|
3.
|
Wiechmann L, Sampson M, Stempel M, et al:
Presenting features of breast cancer differ by molecular subtype.
Ann Surg Oncol. 16:2705–2710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Yamashita H, Ando Y, Nishio M, et al:
Immunohistochemical evaluation of hormone receptor status for
predicting response to endocrine therapy in metastatic breast
cancer. Breast Cancer. 13:74–83. 2006. View Article : Google Scholar
|
|
5.
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
6.
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
7.
|
Blenkiron C, Goldstein LD, Thorne NP, et
al: MicroRNA expression profiling of human breast cancer identifies
new markers of tumor subtype. Genome Biol. 8:R2142007. View Article : Google Scholar
|
|
8.
|
Lowery AJ, Miller N, Devaney A, et al:
MicroRNA signatures predict estrogen receptor, progesterone
receptor and HER2/neu receptor status in breast cancer. Breast
Cancer Res. 11:R272009. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kondo N, Toyama T, Sugiura H, Fujii Y and
Yamashita H: miR-206 Expression is down-regulated in estrogen
receptor alpha-positive human breast cancer. Cancer Res.
68:5004–5008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Adams BD, Furneaux H and White BA: The
micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen
receptor-alpha (ERalpha) and represses ERalpha messenger RNA and
protein expression in breast cancer cell lines. Mol Endocrinol.
21:1132–1147. 2007. View Article : Google Scholar
|
|
11.
|
Leivonen SK, Mäkelä R, Ostling P, Kohonen
P, Haapa-Paananen S, et al: Protein lysate microarray analysis to
identify microRNAs regulating estrogen receptor signaling in breast
cancer cell lines. Oncogene. 28:3926–3936. 2009. View Article : Google Scholar
|
|
12.
|
Pandey DP and Picard D: miR-22 inhibits
estrogen signaling by directly targeting the estrogen receptor
alpha mRNA. Mol Cell Biol. 29:3783–3790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Xiong J, Yu D, Wei N, Fu H, Cai T, Huang
Y, et al: An estrogen receptor alpha suppressor, microRNA-22, is
downregulated in estrogen receptor alpha-positive human breast
cancer cell lines and clinical samples. FEBS J. 277:1684–1694.
2010. View Article : Google Scholar
|
|
14.
|
Zhao JJ, Lin J, Yang H, Kong W, He L, Ma
X, et al: MicroRNA-221/222 negatively regulates estrogen receptor
alpha and is associated with tamoxifen resistance in breast cancer.
J Biol Chem. 283:31079–31086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Di Leva G, Gasparini P, Piovan C, Ngankeu
A, Garofalo M, Taccioli C, et al: MicroRNA cluster 221–222 and
estrogen receptor alpha interactions in breast cancer. J Natl
Cancer Inst. 102:706–721. 2010.
|
|
16.
|
Cittelly DM, Das PM, Spoelstra NS,
Edgerton SM, Richer JK, Thor AD and Jones FE: Downregulation of
miR-342 is associated with tamoxifen resistant breast tumors. Mol
Cancer. 9:3172010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bozzetti C, Nizzoli R, Guazzi A, Flora W,
Bassano C, et al: HER2/neu amplification detected by fluorescence
in situ hybridization in fine needle aspirates from primary breast
cancer. Ann Oncol. 13:1398–1403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Obrero M, Yu DV and Shapiro DJ: Estrogen
receptor-dependent and estrogen receptor-independent pathways for
tamoxifen and 4-hydroxytamoxifen-induced programmed cell death. J
Biol Chem. 277:45695–45703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lim LP, Lau NC, Garrett-Engele P, et al:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar
|
|
20.
|
Wang H, Wu J, Meng X, et al: MicroRNA-342
inhibits colorectal cancer cell proliferation and invasion by
directly targeting DNA methyltransferase 1. Carcinogenesis.
32:1033–1042. 2011. View Article : Google Scholar : PubMed/NCBI
|