Introduction
Microbial natural products have been the source of
the majority of antibiotics that are currently used for the
treatment of various infectious diseases. Since penicillin was
identified in 1928, studies on bacteria and fungi have revealed
that microorganisms are a rich source of structurally unique
bioactive substances (1).
Following penicillin, numerous other drugs, including
chlortetracycline, chloramphenicol, streptomycin, erythromycin,
rifamycin, lincomycin, cephalosporin C, vancomycin, nalidixic acid,
amphotericin B, nystatin and daunorubicin, the antitumor agent,
were identified from microorganisms (2).
At present, a number of the pathogens involved in
infectious disease are rapidly developing resistance to the
available antibiotics (3), making
treatment of these infections challenging (4). Therefore, research into more
effective antibiotics is required.
Pseudomonads represent the major group of
non-differentiating microorganisms that produce antibiotics. The
antibiotic substances produced by this group of organisms are
pyocyanin, pyrrolnitrin and pseudomonic acid (5,6).
Previous studies have reported that Pseudomonas aeruginosa
(PA) in clinical strains exhibit antifungal activity. In addition,
in cystic fibrosis (CF) patients infected with PA, the occurrence
of fungal infections is rare (7–9).
These phenomena demonstrate that PA may exhibit antifungal
activity. In the present study, the association between specific
pathogenic fungi, including Candida albicans (CA), and PA
was described, with the aim of investigating the mechanism behind
the lethal and inhibitory effects that PA exhibits on fungi in
vitro and in vivo.
Materials and methods
Strains
In total, 24 non-repetitive strains of PA (from
various specimens: tracheal aspirate 20.8% (5/24); urine 16.6%
(4/24); wound 25% (6/24); sputum 25 % (6/24); blood 12.5% (3/24);
were obtained from various specimens at the in-patient department
at Tongji Hospital (Wuhan, China) between May and September 2012.
The strains were identified by Gram staining, the oxidase test and
a Vitek-2 automated microbial identification system (bioMérieux,
Inc., Craponne, France), or by the API 20NE system (bioMérieux,
Inc.). All PA strains produce pyocyanin pigment. Escherichia
coli [23922; American Type Culture Collection (ATCC), Manassas,
VA, USA], Klebsiella pneumoniae (ATCC 700603) and PA (ATCC
25923) were maintained as quality control strains. The five strains
of candida were collected from clinical specimens (Candida
albicans, Candida tropicalis, Candida glabrata
and Candida krusei were from sputum, Candida
parapsilosis from urine) and were identified by CHROMagar
Candida plates (CHROMagarCompany, Paris, France) and API-20C AUX
yeast-like fungus identification strips (bioMérieux, Inc.). CA
(ATCC 90028) was preserved as the control strain. The isolated PA
strains were stored at 4°C on nutrient agar slopes, while the
Candida isolates were stored on Sabouraud dextrose agar
(SDA; Oxoid Ltd., Basingstoke, UK) plates until required for
study.
Animals
BALB/c mice were purchased and maintained separately
at the Animal Facility of Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China) under
controlled conditions (specific pathogen free, 22°C, 55% humidity,
and 12 h light/dark cycle). All experimental procedures on animals
used in this study were performed under a protocol approved by the
Institutional Animal Care and Use Committee at the Tongji Medical
College. Thirty mice were purchased and were used in this
experiment. Male mice (age, 8–10 weeks; weight, 25–30 g) were
selected for the study. All experimental procedures on animals used
in the study were performed under a protocol approved by the
Institutional Animal Care and Use Committee of Tongji Medical
College.
Disk diffusion method
Fungi (400 μl; 1×108 cells/ml) were
spread on SDA plates (one plate was used for each fungi species)
using a glass spreader and sterile filter paper disks were placed
on the plates. Each PA strain was grown overnight at 37°C in
Luria-Bertani (LB) media. Next, 3-μl specimens of the PA cultures
(8×109 CFU/ml) were spotted on the filter disks, which
was followed by incubation at 30°C for up to 48 h. Cultures of same
volume and concentration of Escherichia coli (ATCC 23922),
Klebsiella pneumoniae (ATCC 700603), Pseudomonas
aeruginosa (ATCC 25923) and sterile water were respectively
spotted on the central filter disks as a control.
Cross-streak method
Cross-streaking was performed according to the
method described by Kerr (10). A
fresh 24-h plate culture of each PA strain (1×108
CFU/ml) was prepared as an inoculum in 0.9% NaCl to be tested for
antifungal activity. The inoculum (30 μl) was streaked
diametrically at a width of 1 cm across the SDA and blood agar
(BA). SDA was used since it enhances fungal growth and all the
tested strains of Pseudomonas were shown to grow well on the
substance. The plates were incubated at 30°C for 24 h and the
macroscopic growth was then removed from the plate using a sterile
glass slide.
Sterile filter paper disks (diameter, 5 cm) were
soaked in chloroform and laid on a metal tray in a safety cabinet.
Each plate was then placed face down, without the lid, on top of a
chloroform-containing filter paper disk; the plates were left for
30 min in order to kill the microscopic remnants of the culture.
The plates were then removed from the cabinet and traces of
chloroform were eliminated by exposure to air for a few minutes. A
fresh 24-h plate culture of each fungal strain was used to prepare
an inoculum of 1×106 CFU/ml. This fungal suspension was
streaked onto the chloroform-treated medium at right angles to the
line of the original inoculum and the plates were incubated for 24
h at 30°C. Each of the 24 PA strains was tested against each of the
five fungal strains and total inhibition of fungal growth was
recorded as (+), partial inhibition of fungal growth was recorded
as (±) and no inhibition of fungal growth was recorded as (−).
Fungal strains co-cultured with PA
Sterile eppendorf tubes (EPs) were filled with 1 ml
LB and each aforementioned PA and fungal suspension (50 μl) was
added. Each fungal suspension (50 μl) was also added as a control.
The EPs were agitated at 100 rpm at 30°C for 48 h.
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) analysis of the differences in PA
bacterial protein
PA1206 and PA1215 strains, which exhibit a strong
inhibitory effect on fungi, as well as the PA1201 and PA1222
strains, which present no inhibitory effect, were analyzed with
SDS-PAGE. The four strains of PA were cultured in EPs containing 1
ml LB under conditions of 100 rpm at 30°C for 24 h (two replicates
for each PA strain were prepared). The two replicates were boiled
at 100°C for 10 min, then centrifuged at 14,500 × g for 1 min and
the supernatant and sediment were extracted for SDS-PAGE. The steps
of SDS-PAGE were performed according to Thermo Fisher Scientific
Inc. (Waltham, MA, USA). The results were observed following
bleaching.
Blood infection in mice
A model of blood infection was applied to evaluate
the anticandidal activity of PA in mice. PA and Candida
species are often identified on skin and in the mucosa of healthy
individuals. When the host defenses falter, PA and Candida
initiate invasive growth that leads to severe diseases. BALB/c mice
weighing 25–30 g were used in this study. All animals received
humane care. The mice were randomly assigned to the following three
groups. Group 1 was the total inhibition positive group (n=5+5),
where five mice applied with PA1206 and five mice applied with
PA1215 were tested against CA (ATCC 90028). Group two was the no
inhibition group (n=5+5), where five mice applied with PA1201 and
five mice applied with PA1222 were tested against CA (ATCC 90028).
Finally, group three was the control group (n=10) and only CA (ATCC
90028) was applied. The bacterial and yeast suspensions (0.2 ml;
1×108 CFU/ml) were injected into the caudal vein of the
mice in groups one and two, while only the yeast suspension (0.2
ml; 1×108 CFU/ml) was injected into the caudal vein of
the mice in group three. At 24 h after the injections, blood
samples were collected from the mice by the method of retro-orbital
blood collection. The blood drops were incubated in BA and SDA at
30°C for 48 h, and the growth of the PA and CA strains was
evaluated.
Results
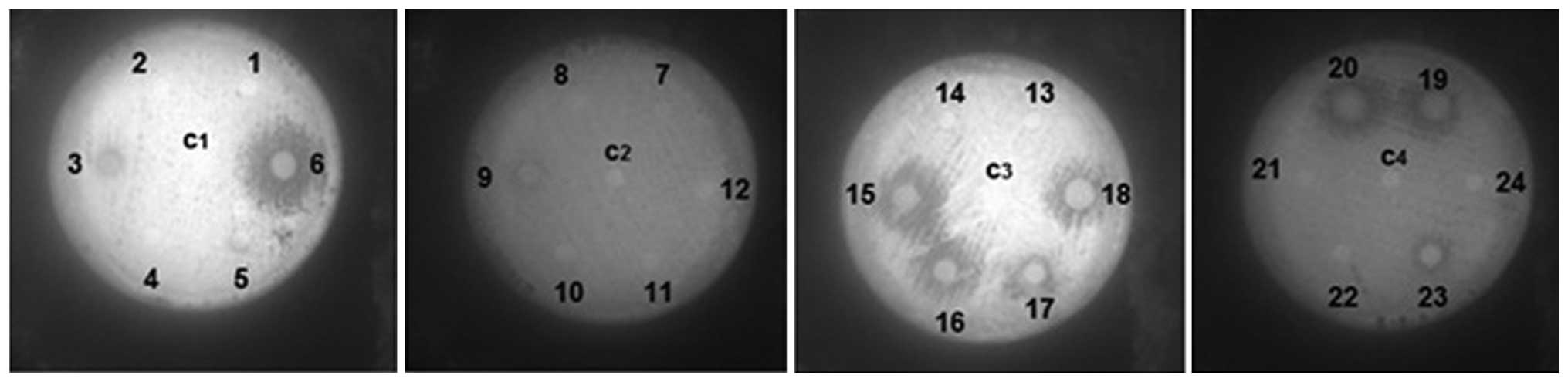
Disk diffusion method
PA strains 1206, -15, -16, -17, -18, -19 and -20
demonstrated clear zones of inhibition, while strains 1203, -09 and
-23 presented partial zones of inhibition that were not very large.
The remaining strains and the control strain exhibited no
inhibition. Diameters of the zones of inhibition were measured; (−)
no zone of inhibition; (±) zone of inhibition: 7–10 mm; (+) zone of
inhibition: >10 mm (Fig. 1 and
Table I).
 | Table IAntifungal activity of PA on
pathogenic fungi. |
Table I
Antifungal activity of PA on
pathogenic fungi.
|
PA
strains | Control strains |
|---|
|
|
|
|---|
| Fungi | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | EC | KP | PA |
|---|
| CA | − | − | ± | − | − | + | − | − | ± | − | − | − | − | − | + | + | + | + | + | + | − | − | ± | − | − | − | − |
| CT | − | − | ± | − | − | + | − | − | ± | − | − | − | − | − | + | + | + | + | + | + | − | − | ± | − | − | − | − |
| CG | − | − | ± | − | − | + | − | − | ± | − | − | − | − | − | + | + | + | + | + | + | − | − | ± | − | − | − | − |
| CP | − | − | ± | − | − | + | − | − | ± | − | − | − | − | − | + | + | + | + | + | + | − | − | ± | − | − | − | − |
| CK | − | − | ± | − | − | + | − | − | ± | − | − | − | − | − | + | + | + | + | + | + | − | − | ± | − | − | − | − |
Cross-streak method results
The cross-streak method results were consistent with
those of the disk diffusion method (Fig. 2).
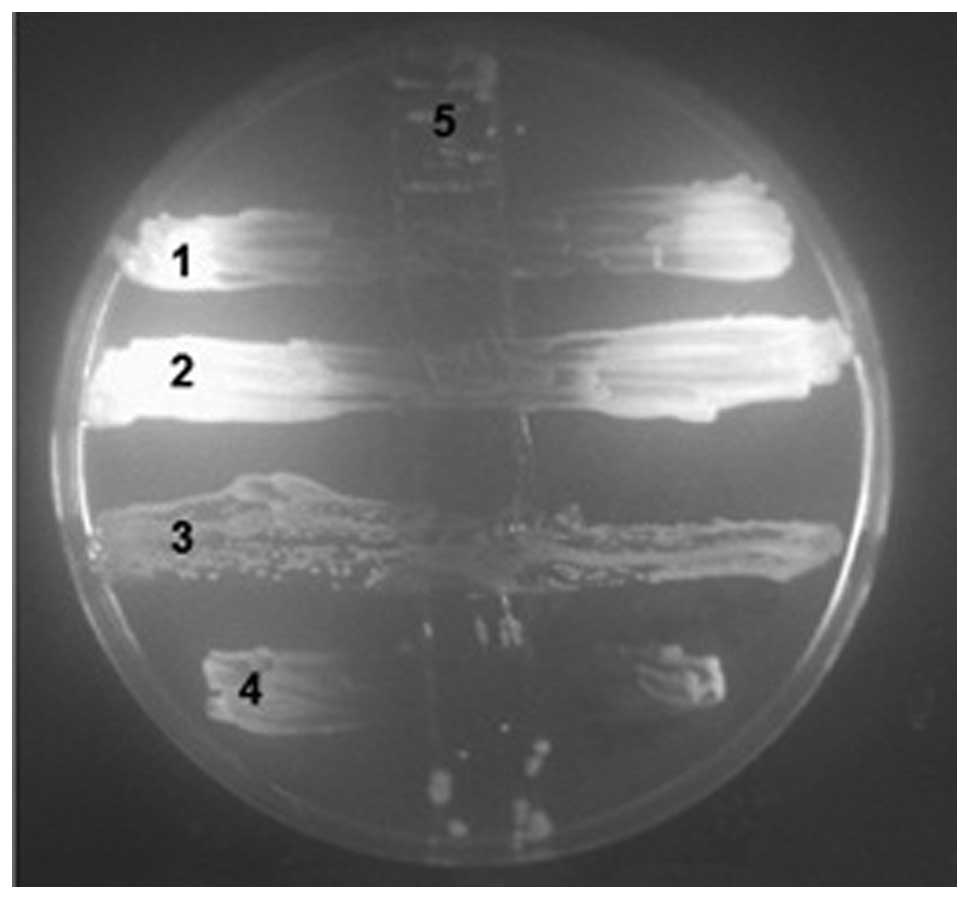 | Figure 2Cross-streak method was performed on
1, CA (ATCC 90028); 2, CT; 3, CG; 4, CP; 5, PA1206 following
killing by chloroform. CA, Candida albicans; CT, Candida
tropicalis; CG, Candida glabrata; CP, Candida
parapsilosis; PA, Pseudomonas aeruginosa; ATCC, American
Type Culture Collection. |
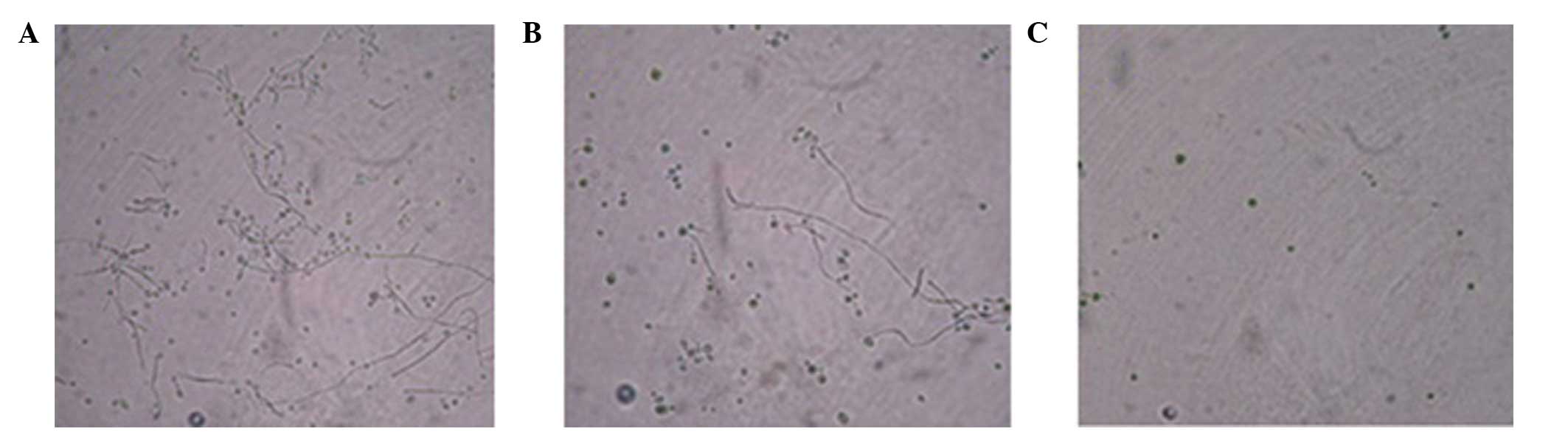
Fungi co-culture with PA
When the fungi were co-incubated with known
inhibitory PA strains, a significant reduction in the production of
fungal hyphae was observed. However, when the fungi were
co-incubated with non-inhibitory PA strains, the production of
fungal hyphae was similar to that of fungi that had been cultured
alone in LB medium (Fig. 3).
SDS-PAGE analysis of bacterial protein
differences
The two replicates of each PA strain produced the
same pattern. The supernatants produced few bands, but the
sediments presented the same bands and a number of different bands.
Almost all the strains had one band in common at ~35 kDa. However,
the inhibitory PA strains (1206 and 1215) produced distinct strips
at 38, 35, 27 and 24 kDa, while the non-inhibitory PA strains (1201
and 1222) presented no bands at those points according to the
marker. These observations indicate that the PA1206 and PA1215
strains secreted a greater variety of proteins compared with the
PA1201 and PA1222 strains. An association between these bands and
the growth inhibition effect on pathogenic fungi may exist,
however, this requires further study (Fig. 4).
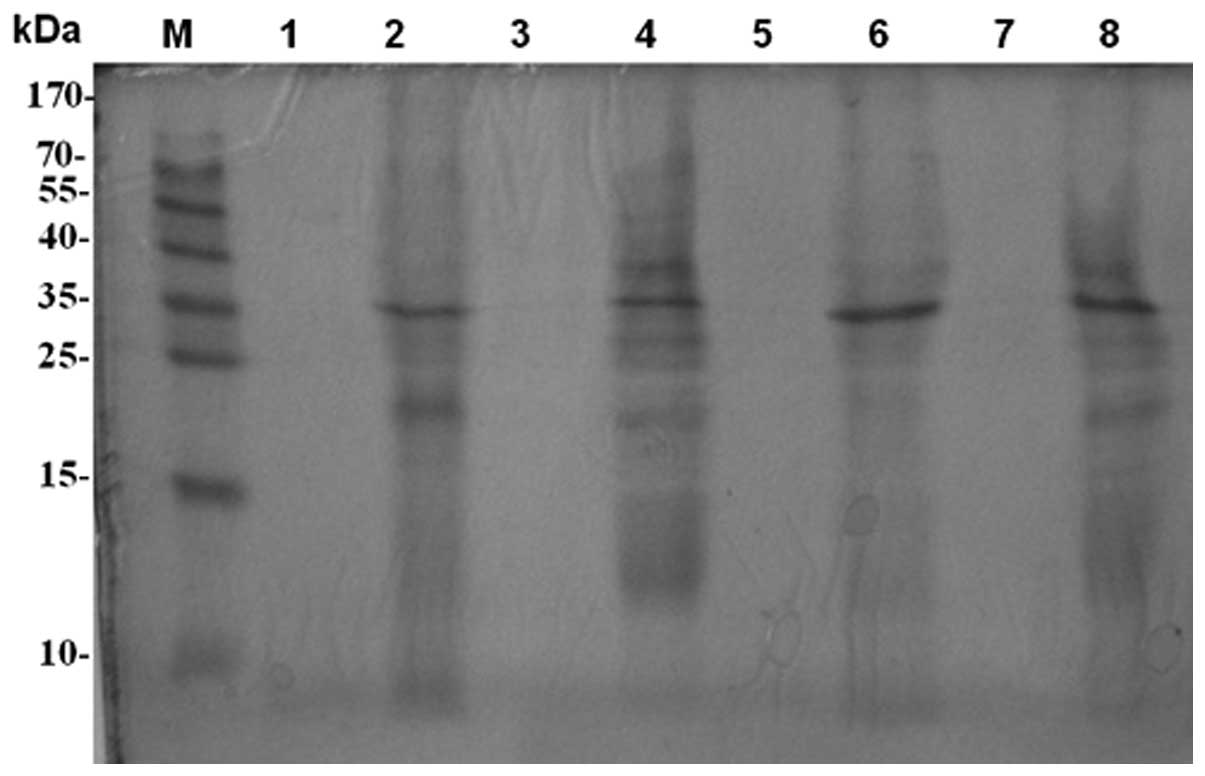 | Figure 4SDS-PAGE. M, protein marker; 1, PA1201
supernatant; 2, PA1201 sediment; 3, PA1206 supernatant; 4, PA1206
sediment; 5, PA1215 supernatant; 6, PA1215 sediment; 7, PA1222
supernatant; 8, PA1222 sediment; SDS-PAGE, sodium dodecyl
sulfate-polyacrylamide gel electrophoresis; PA, Pseudomonas
aeruginosa. |
Blood infection in mice
The mouse model of blood infection with CA and PA
revealed that in group 1, no yeast was recovered from the infected
mice, despite PA having 100% detection. In group 2, PA and yeast
were recovered on the plate, while in group 3, 100% yeast was
recovered.
Discussion
Numerous antimicrobial compounds have been isolated
from microorganisms. Microbial natural products are the source of
the majority of antibiotics that are used currently for the
treatment of various infectious diseases (2), including fungal infection. Techniques
to identify novel products against pathogenic fungi have become
increasingly important in the field of infection.
Interactions between prokaryotes and eukaryotes are
ubiquitous. Despite the fact that the pathogenic and symbiotic
associations that bacteria have with plants and animals have
garnered the most attention, the prokaryote-eukaryote encounters
that occur among microbes are likely to be far more common
(11). Bacteria and unicellular
eukaryotes, including yeasts and filamentous fungi, are found
together in a myriad of environments and exhibit synergistic and
antagonistic interactions
Previous studies have demonstrated that an
interaction exists between PA and a number of other pathogenic
fungi in the human body. Hughes and Kim (7) demonstrated that in CF patients
infected with PA, only 10% of patients produced positive CA skin
tests compared with 30% positivity in those free of PA, indicating
that the antifungal substance produced by PA prevents
Candida infections. There are also studies investigating the
growth inhibition effect of PA in Cryptococcus species
(12,13). However, to the best of our
knowledge, there have been no studies regarding the isolation of
Cryptococcus species from patients with CF. Considering that
Cryptococcus and PA are common lung pathogens, the lack of
co-colonization may result from the antifungal effect of PA on the
growth of Cryptococcus neoformans.
Grillot et al (14) investigated the interactions between
PA and yeast following incubation with a number of pure and mixed
cultures. The authors demonstrated that the growth of all the
isolates tested was inhibited by PA in blood culture medium and in
bacterial culture filtrate.
Hogan and Kolter (11) described the pathogenic interaction
between PA and CA. PA forms a dense biofilm on CA filaments and
kills the fungus. Several PA virulence factors, including type IV
pili, phospholipase C and phenazines, that are important in disease
are also involved in the killing of CA filaments. Pyocyanin and
Pseudomonas quinolone signal accumulate intensively in the
lung mucus of patients with CF (8,9);
these antifungal molecules may be important in the prevention of
pulmonary cryptococcosis in patients with CF.
Fungal-bacterial interactions also occur in skin and
nail infections. Foster et al (15) observed that dermatophyte and
non-dermatophyte fungi grew poorly in the presence of PA, and
demonstrated that large PA populations in infected nails resulted
in a lower fungal population.
In the present study, the disk diffusion and
cross-streak methods produced similar results demonstrating the
lethal and inhibitory effects of PA on Candida species. The
results revealed that certain PA strains exhibit a strong
antifungal ability, while others exhibit partial or no ability. In
the co-culture of PA with pathogenic fungi in LB medium, the
Candida species produced a markedly large number of
filaments when cultured alone or with non-inhibitory PA strains.
However, when the fungi were cultured with inhibitory PA, almost no
filaments were produced.
SDS-PAGE revealed that PA1206 and PA1215 secreted
different proteins from those of strains 1201 and 1222), yet PA1206
and 1215 exhibited similar secreted protein patterns. PA1201 and
1222 also showed a similar secretion pattern. All the strains had
one band in common at ~35 kDa. However, the inhibitory PA strains
(1206 and 1215) produced distinct strips at 38, 27 and 24 kDa,
while the non-inhibitory PA strains (1201 and 1222 ) presented no
bands at those points. PA1206 and 1215 exhibit strong inhibitory
effects on pathogenic fungi, while PA1201 and 1222 show no effects.
Therefore, an association may exist between the difference of
secreted proteins in the two types of strain and the inhibitory
effect on pathogenic fungi; the proteins may be associated with the
inhibitory effect on the growth of fungal filaments. However, the
mechanism requires further study.
In the animal model of bacteremia, a suspension of
PA and CA was injected into the caudal vein of the mice. After 24
h, blood drops were obtained using the method of orbital blood
collection and they were incubated on BA and SDA. In group 1, the
PA strains had been recovered, but no strains of CA were isolated.
In group 2, PA and CA were observed. However, in the control group
3, 100% CA was recovered from the blood. These results are in
accordance with a previous study of a rabbit model of concomitant
fungemia with CA and bacteremia with PA, in which no yeast was
recovered from the blood cultures despite 100% detection of PA
(16). Therefore, the present
study investigated the in vivo and in vitro
antifungal activity of PA strains against Candida species.
The results demonstrated that the inhibitory effect of PA exists
in vivo and in vitro. To the best of our knowledge,
the present study is the first study to use a mouse model of
co-infection with PA and Candida. The present study
investigated the in vivo anticandidal activity of PA in a
mouse model of blood infection. The results demonstrated that PA
strains inhibited the growth of Candida strains by 100%,
which is in accordance with previous studies (17,18).
In conclusion, PA strains exhibit potent antifungal
activity in vitro and in vivo. The underlying
mechanism may involve pyocyanin, biofilms and the production of a
variety of factors, including various types of proteins, a quorum
sensing system, redox phenazin and phospholipase C, which inhibit
fungal filaments and thereby inhibit fungal growth. Further study
on the separation and purification of these substances is required
to improve the treatment and prevention of fungal infections.
Acknowledgements
The study was supported by a grant from the
Infectious Diseases Control Project of the Ministry of Health of
China (no. 2012zx10004207-004).
References
|
1
|
Fenical W: Chemical studies of marine
bacteria: developing a new resource. Chem Rev. 93:1673–1683.
1993.
|
|
2
|
Tawiah AA, Gbedema SY, Adu F, et al:
Antibiotic producing microorganisms from River Wiwi, Lake Bosomtwe
and the Gulf of Guinea at Doakor Sea Beach, Ghana. BMC Microbiol.
12:2342012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singer RS, Finch R, Wegener HC, Bywater R,
Walters J and Lipsitch M: Antibiotic resistance - the interplay
between antibiotic use in animals and human beings. Lancet Infect
Dis. 3:47–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhavnani SM and Ballow CH: New agents for
Gram-positive bacteria. Curr Opin Microbiol. 3:528–534. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kandela SA, al-Shibib AS and al-Khayat BH:
A study of purified pyorubin produced by local Pseudomonas
aeruginosa. Acta Microbiol Pol. 1:37–43. 1997.PubMed/NCBI
|
|
6
|
Kerr JR, Taylor GW, Rutman A, Høiby N,
Cole PJ and Wilson R: Pseudomonas aeruginosa pyocyanin and
1-hydroxyphenazine inhibit fungal growth. J Clin Pathol.
52:385–387. 1999. View Article : Google Scholar
|
|
7
|
Hughes WT and Kim HK: Mycoflora in cystic
fibrosis: some ecologic aspects of Pseudomonas aeruginosa
and Candida albicans. Mycopathol Mycol Appl. 50:261–269.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor GW, Machan ZA, Mehmet S, Cole PJ
and Wilson R: Rapid identification of 4-hydroxy-2-alkylquinolines
produced by Pseudomonas aeruginosa using gas
chromatography-electron-capture mass spectrometry. J Chromatogr B
Biomed Appl. 664:458–462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caldwell CC, Chen Y, Goetzmann HS, Hao Y,
Borchers MT, Hassett DJ, et al: Pseudomonas aeruginosa
exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am J
Pathol. 175:2473–2488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kerr JR: Suppression of fungal growth
exhibited by Pseudomonas aeruginosa. J Clin Microbiol.
32:525–527. 1994.PubMed/NCBI
|
|
11
|
Hogan DA and Kolter R:
Pseudomonas-Candida interactions: an ecological role for
virulence factors. Science. 296:2229–2232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rella A, Yang MW, Gruber J, et al:
Pseudomonas aeruginosa inhibits the growth of
Cryptococcus species. Mycopathologia. 173:451–461. 2012.
View Article : Google Scholar
|
|
13
|
Teoh-Chan H, Chau PY, Ng MH and Wong PC:
Inhibition of Cryptococcus neoformans by Pseudomonas
aeruginosa. J Med Microbiol. 8:77–81. 1975.
|
|
14
|
Grillot R, Portmann-Coffin V and
Ambroise-Thomas P: Growth inhibition of pathogenic yeasts by
Pseudomonas aeruginosa in vitro: clinical implications in
blood cultures. Mycoses. 37:343–347. 1994.PubMed/NCBI
|
|
15
|
Foster KW, Thomas L, Warner J, Desmond R
and Elewski BE: A bipartite interaction between Pseudomonas
aeruginosa and fungi in onychomycosis. Arch Dermatol.
141:1467–1468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hockey LJ, Fujita NK, Gibson TR,
Montgomerie JZ and Edwards JE Jr: Detection of fungemia obscured by
concomitant bacteremia: in vitro and in vivo studies. J Clin
Microbiol. 16:1080–1085. 1982.PubMed/NCBI
|
|
17
|
Gupta N, Haque A, Mukhopadhyay G, Narayan
RP and Prasad R: Interactions between bacteria and Candida
in the burn wound. Burns. 31:375–378. 2005. View Article : Google Scholar
|
|
18
|
Neely AN, Law EJ and Holder IA: Increased
susceptibility to lethal Candida infections in burned mice
preinfected with Pseudomonas aeruginosa or pretreated with
proteolytic enzymes. Infect Immun. 52:200–204. 1986.
|