Introduction
The repair of peripheral nervous system (PNS) damage
resulting from trauma, excessive stretching and even iatrogenic
injury is a tremendous challenge for clinical medicine as the
recovery of nerve function may be poor due to broken end distortion
following nerve anastomosis and scar ingrowth, ineffective nerve
regeneration and slow regeneration. Among several current therapies
for PNS damage, for example, neurosuture and nerve grafting, the
combined application of nerve regeneration-promoting drugs is a
promising technology that has few adverse reactions (1). Tacrolimus (FK506) is a macrolide
immunosuppressant approved by the US Food and Drug Administration.
It plays a role not only in immunosuppression but also in the
effective promotion of nerve regeneration. However, at present, the
systemic application of FK506 has certain side-effects, such as
nephrotoxicity, gastrointestinal dysfunction and hypertension
(2–5). In current research, the local
application of FK506 is regarded as a feasible method for reducing
side-effects. However, no accurate test is available to evaluate
the effective concentration for local application (6). A newly emerging technology known as
microfluidics has been demonstrated to be very useful for screening
the dosages of drugs. A stable cell culture environment can be
constituted and maintained by a continuous medium supply and waste
removal system that resembles the human circulatory system.
Furthermore, it reduces the number of cells required and the
requirement for large volumes of culture medium and costly
reagents, which makes the microfluidic device an attractive
platform for high-throughput screening (7,8).
In the present study, an integrated microfluidic
device was designed and fabricated that may provide an ideal
platform for cell level manipulation and analysis in vitro.
Furthermore, rat Schwann cells (SCs) were loaded into the device
and the optimum concentration of FK506 was determined with the aim
of providing an experimental and theoretical reference for the
therapy of peripheral nerve injury.
Materials and methods
Materials
FK506 (molecular weight, 804.02 g/mol) was purchased
from Purac Biochem Co. (Gorkum, The Netherlands), and used without
further purification. Rat SCs (RSC96 cell line) were purchased from
ScienCell Research Laboratories (Carlsbad, CA, USA).
Design and fabrication of the
microfluidic device
The microfluidic device was designed using
computer-aided design (CAD) software, to provide a continuous
concentration gradient for the chemical stimulation of a cell
culture (9). The device included
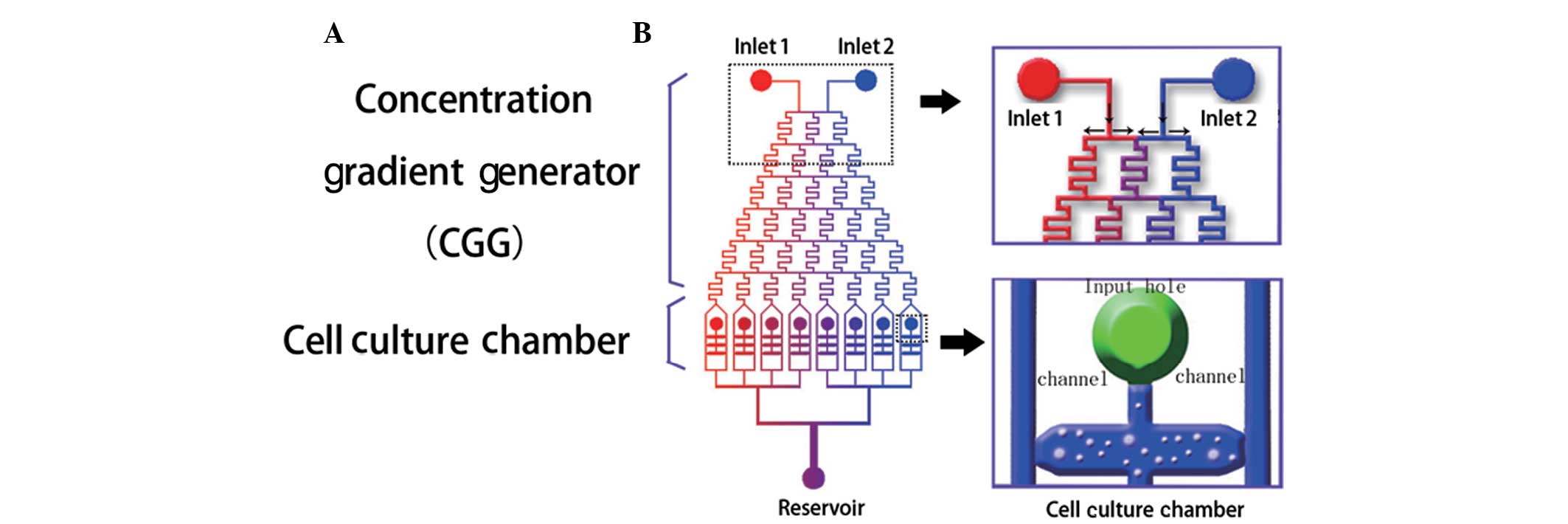
two parts (Fig. 1A), namely a
pyramid shaped concentration gradient generator (CGG) and a cell
culture chamber. In accordance with the Reynolds effect, the
Reynolds number can be very low when the capillary diameter is ~100
nm to several hundred micrometers, and liquid will show a laminar
flow when it passes through the serpentine channels of the
microfluidic chip. When using this chip, if one solution
(concentration A) and a second solution (concentration 0) are
injected into the CGG via the two inlets, they are split at the
nodes, combined with neighboring streams in a laminar fashion, and
mixed by diffusion in the serpentine channels, and the
concentrations in the eight outlets are, respectively, 0, 1/7A,
2/7A, 3/7A, 4/7A, 5/7A, 6/7A and A.
Three cell culture chambers of the same function
were connected between two parallel channels of each outlet of the
CGG. The volume of each chamber was 0.4 μl (length, 800 μm; width,
500 μm; height, 100 μm). The SCs were loaded into a chamber through
an input hole. When solutions flow down the parallel channels,
substances contained in them, such as FK506, diffuse into the
chamber by osmosis until saturation is achieved.
The microfluidic devices were fabricated in
poly-dimethylsiloxane (PDMS) using rapid prototyping and soft
lithography (11). Firstly, a
transparency mask was generated by a high resolution printer from
the CAD file. The mask was used in 1:1 contact photolithography
with an SU-8 photoresist (MicroChem, Newton, MA, USA) to generate a
negative master consisting of a patterned photoresist on a silicon
wafer. Positive replicas with embossed channels were fabricated by
molding PDMS against the master. Secondly, the inlets and outlets
(ϕ=1 mm) for the fluids were punched out of the PDMS using a
sharpened needle. Then, an ultrasonically cleaned glass substrate
and the PDMS molding were irreversibly combined together to form a
system of microfluidic channels in the PDMS-glass composite chip.
The channels were 100 μm wide and 30 μm deep.
CGG performance validation
Fluorescein isothiocyanate-dextran (FITC-dextran;
Nanocs, Boston, MA, USA) was used as an indicator for evaluating
the gradient generated by the CGG. By injecting FITC-dextran and
phosphate-buffered saline into the two inlets, respectively, the
FITC-dextran was continuously diluted. A series of concentrations
of FITC-dextran were thus acquired in the CGG and then flowed into
the corresponding downstream cell culture devices. As the intensity
of fluorescence of FITC-dextran is proportional to its
concentration, the concentration was positively associated with the
intensity of fluorescence of FITC-dextran in the CGG (10). The intensities of FITC-dextran at
the eight outlets of the CGG were imaged by confocal laser scanning
microscopy (Leica Microsystems, Wetzlar, Germany) and quantified
using Image-Pro Plus software (version 6.0 for Windows 7; Media
Cybernetics, Inc., Rockville, MD, USA). All experiments were
repeated three times. The measured values were compared with
theoretical values (9),
whereafter, their relativity was analyzed.
Screening of the effective concentration
of FK506
Culture media with and without FK506 were
simultaneously infused into the microfluidic device. The fluid was
driven by a medical syringe pump, and the flow speed was controlled
at 0.1 μl/min (Fig. 2). The
concentration gradient of FK506 was established at the eight
outlets of the CGG 30 min later following the repeated splitting,
mixing, and recombination of the fluid streams as they traveled
through the channels of the CGG. In each cell culture chamber to
which the SCs had been loaded, a culture medium with different
concentrations of FK506 was obtained when the solution flowed in
the parallel channels of each outlet (12). To prevent the washing out of cells,
a 3-h incubation step was conducted prior to infusion of the
culture media since SCs loaded inside the chambers attach to FK506
after a while and their locations remain relatively stable. The
device was then kept in an incubator at 37°C, 5% CO2 and
100% humidity for an additional 1, 3, 5, 7 and 9 days. At every
indicated time interval, the extent of the cell proliferation was
evaluated using a cell counting kit 8 (CCK-8) assay (Dojindo,
Tokyo, Japan) (13). Then, the
concentration of FK506 was analyzed by liquid chromatography-mass
spectrometry (3200QTRAP®; Applied Biosystems, Foster
City, CA, USA). All cell proliferation assays were repeated in
96-well plates with the same concentrations.
Statistical analysis
All quantitative data were analyzed and expressed as
the mean ± standard deviation. Cell proliferation assay results
were assessed by one way analysis of variance (ANOVA). The
comparison between two means was analyzed using Tukey’s test, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
CGG performance validation
Figs. 1 and
2 are images of the microfluidic
device. As streams of dye traveled down the network, they were
repeatedly split, combined with neighboring streams in laminar mode
at the nodes, and mix by diffusion in the serpentine channels. A
series of solutions with different concentrations of FITC-dextran
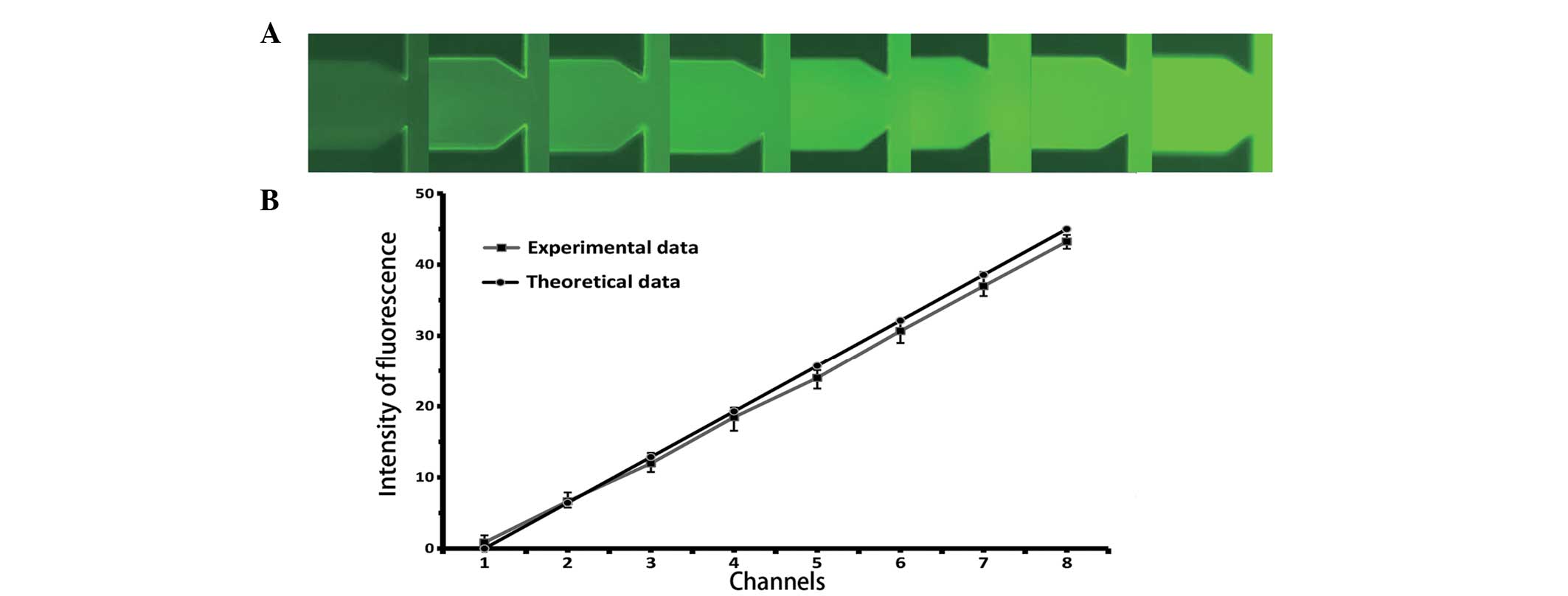
were formed at the cell culture chamber (Fig. 3A). The fluorescent intensities of
FITC-dextran in the junctions between the CGG and the cell culture
module were quantified, corrected by subtraction of the background
fluorescence, and compared with the theoretical data. As shown in
Fig. 3B, there was a good
coherence (correlation coefficient = 0.9948) between the
experimental and theoretical data.
Cell proliferation and effective
concentration of FK506
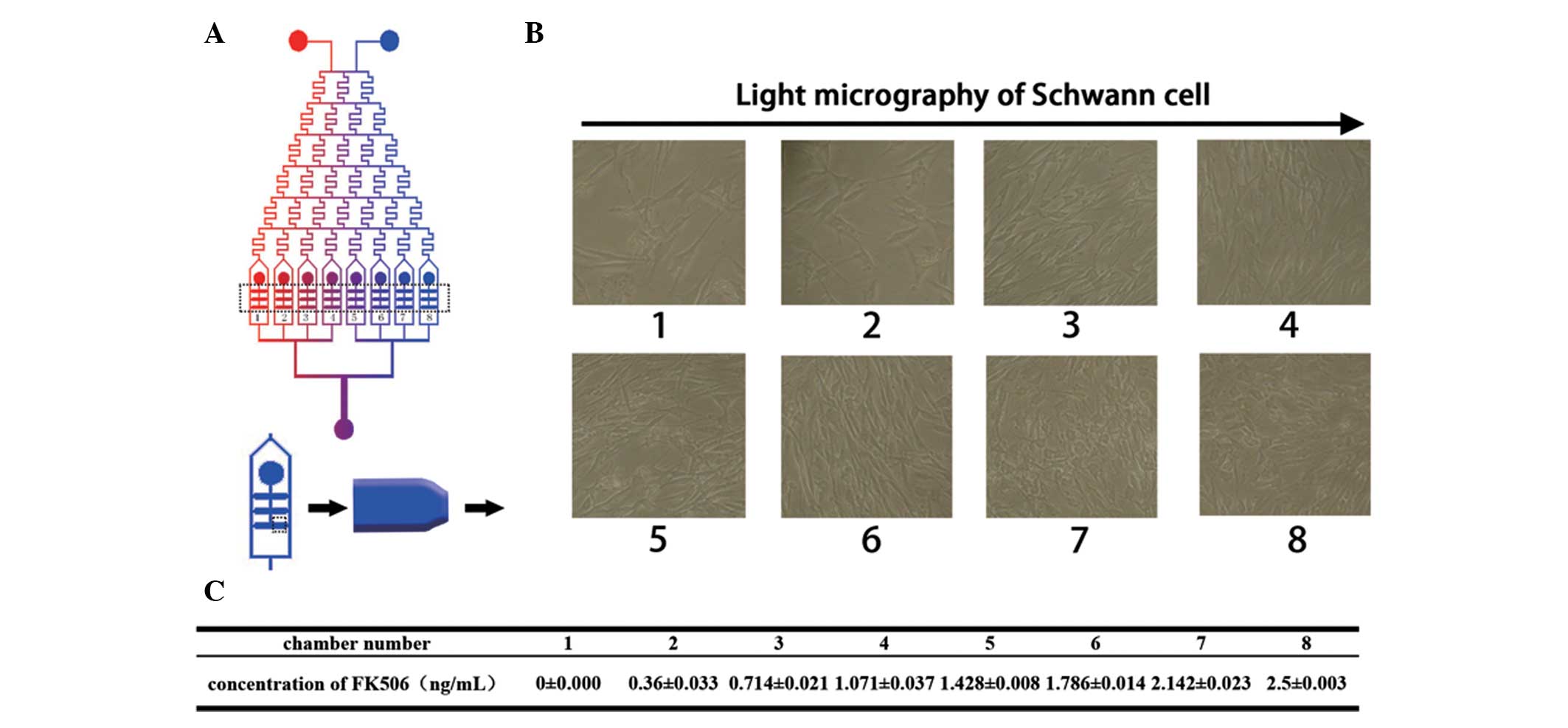
To measure the effective concentration of FK506, SCs
were loaded into the microfluidic device and exhibited a polygonal
morphology after nine days in the presence of different
concentrations of FK506 solution (Fig.
4B). The effective concentration was screened over a large
range, and it was confirmed that the highest proliferation rate was
obtained at a concentration between 1.786±0.014 and 2.5±0.003
ng/ml. The concentrations of FK506 from chamber 1 to chamber 8 are
shown in Fig. 4C. The
concentration-dependency of SC proliferation in the presence of
FK506 was apparent between 0 and 2.5±0.003 ng/ml as shown in
Fig. 4B. The proliferation rate
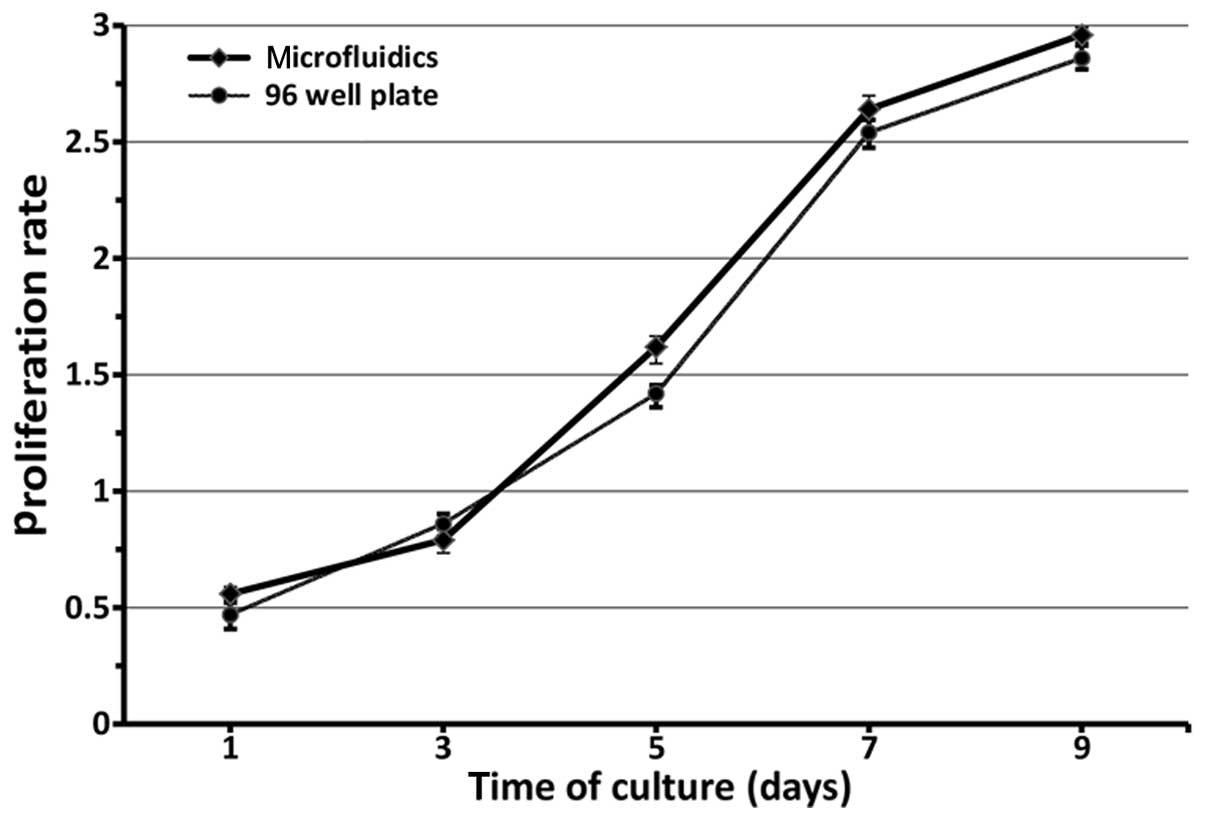
reached a maximum in the 1.786±0.014 ng/ml group (Fig. 5), which was statistically
significantly different from the proliferation rates in the groups
of lower FK506 concentrations (P<0.01). These results were
consistent with the light micrographs shown in Fig. 4B. There was no statistically
significant difference between the proliferation rate in the
1.786±0.014 ng/ml group and that in the groups of higher FK506
concentration (P>0.05). Furthermore, in Fig. 6, the proliferation of RSCs was
evaluated by CCK-8 quantitative assay after culturing for nine
days. The RSCs in the microfluidic device and 96-well plate
continued to proliferate as the culture time was prolonged. There
were no statistically significant differences between the
microfluidic chips and 96-well plate with regard to proliferation
rate in each corresponding group (F=121.366, P>0.05). Thus, it
is considered that the effective local concentration of FK506 is in
the vicinity of 1.786±0.014 ng/ml.
Discussion
Microfluidic devices integrate the preparation of
pharmaceutical compositions, separation, detection, cell culture
and other basic operations into a very small chip (14). Compared with traditional methods of
pharmaceutical analysis, microfluidic chips are characterized by
minimal sample demand and a significant improvement of detection
sensitivity. The use of such chips may reduce consumption and costs
significantly. Due to the characteristics of integration,
miniaturization and automation, it is also known as ‘lab-on-a-chip’
(15). In the present study, the
high throughput screening of the effective concentration of FK506
was determined using very small amounts of cells and drugs. The
experimental conditions were easily controlled by changing the
concentration of the FK506 solution, and the risk of contamination
was minimal. More importantly, a stable culture environment was
constituted and maintained by the continuous medium supply and
waste removal system (16).
Furthermore, FK506 diffused into the cell culture chamber, and the
metabolic waste generated by cell growth was drained away
simultaneously. This bionic design was used to test different
concentrations of FK506 for their ability to improve SC growth. The
design of parallel pipelines through from the side of the cell
culture chamber kept cells away from the direct flushing effect of
solution. Due to the low consumption of cells in microfluidic
devices, cell loss can influence the result significantly. The
small but effective design of the device in the present study may
reduce the influence of cell loss. The consistency of the cell
proliferation between the microfluidic chip and 96-well plates
(Fig. 6) demonstrates that the
microfluidic device performs excellently in the high throughput
screening of effective drug concentrations. Due to the
aforementioned advantages, microfluidic devices are ideal platforms
for cell level manipulation and analysis in vitro.
FK506 is widely used clinically as an
immunosuppressant following liver and kidney transplantations. By
combining with FK506 binding protein-1 and suppressing the immune
responses induced by nerve injury, it creates a suitable
microenvironment for nerve regeneration (17,18).
Although the effectiveness of FK506 for the promotion of nerve
regeneration is known (19), at
present, due to the fact that systemic application may lead to
opportunistic infections and tumors and is not generally accepted
for the treatment of peripheral nerve injury, FK506 has not been
widely used for the treatment of simple peripheral nerve injury.
The systemic application of FK506 has certain side-effects such as
nephrotoxicity, gastrointestinal dysfunction and hypertension.
These factors restrict the application of FK506. The method of
local application of FK506 to avoid side-effects from systemic
application has been widely approved by clinics (20). However, no accurate test is
available to determine the effective concentration for local
application. For the purpose of testing the effective
concentration, a series of FK506 concentrations were established
and their effects on rat SCs were investigated using the
microfluidic device in the present study. It was shown that the
cell proliferation rate increased as the FK506 concentration
increased and reached a peak at 1.786±0.014 ng/ml. As the
concentration of FK506 increased from 1.786±0.014 to 2.5±0.003
ng/ml, no further increase occurred, which indicates that higher
concentrations have no additional promoting effect. Accordingly, it
is considered that FK506 shows a maximum capacity for stimulating
peripheral nerve regeneration at a concentration of 1.786±0.014
ng/ml. The risk of adverse effects is likely to be greatly
increased at higher dosages.
In the present study, FK506 synergistically promoted
SC proliferation. The effective FK506 concentration for local
application was determined to be 1.786±0.014 ng/ml. A microfluidic
device was fabricated from PDMS. The device design was developed in
order to adapt to the cell culture and the effectiveness for
high-throughput screening was demonstrated by the intensity of
fluorescence of FITC-dextran. The data obtained revealed that the
microfluidic device described in the present study is a candidate
for cell level manipulation and analysis in vitro. The
advantages of miniaturization and high integration indicate that
microfluidic technology holds great promise for use in high-through
screening at the cellular level.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (81171464).
References
|
1
|
Terzis JK, Sun DD and Thanos PK:
Historical and basic science review: past, present, and future of
nerve repair. J Reconstr Microsurg. 13:215–225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varghese J, Reddy MS, Venugopal K, et al:
Tacrolimus-related adverse effects in liver transplant recipients:
its association with trough concentrations. Indian J Gastroenterol.
33:219–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamazoe K, Yamazoe K, Yamaguchi T, Omoto M
and Shimazaki J: Efficacy and safety of systemic tacrolimus in
high-risk penetrating keratoplasty after graft failure with
systemic cyclosporine. Cornea. 33:1157–1163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters DH, Fitton A, Plosker GL and Faulds
D: Tacrolimus. A review of its pharmacology, and therapeutic
potential in hepaticand renal transplantation. Drugs. 46:746–794.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Dieren JM, van Bodegraven AA, Kuipers
EJ, Bakker EN, et al: Local application of tacrolimus in distal
colitis: feasible and safe. Inflamm Bowel Dis. 15:193–198. 2009.
View Article : Google Scholar
|
|
6
|
Udina E, Ceballos D, Gold BG and Navarro
X: FK506 enhances reinnervation by regeneration and by collateral
sprouting of peripheral nerve fibers. Exp Neurol. 183:220–231.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeon JH and Park JK: Microfluidic cell
culture systems for cellular analysis. Biochip J. 1:17–27.
2007.
|
|
8
|
Sittinger M, Schultz O, Keyszer G, et al:
Artificial tissues in perfusion culture. Int J Artif Organs.
20:57–62. 1997.PubMed/NCBI
|
|
9
|
Jeon NL, Dertinger SKW, Chiu DT, et al:
Generation of solution and surface gradients using microfluidic
systems. Langmuir. 16:8311–8316. 2000. View Article : Google Scholar
|
|
10
|
Xia YN: Soft lithography and the art of
patterning - A tribute to Professor George M. Whitesides. Advanced
Materials. 16:1245–1246. 2004. View Article : Google Scholar
|
|
11
|
Li Y, Qin J, Lin B, et al: The effects of
insulin-like growth factor-1 and basic fibroblast growth factor on
the proliferation of chondrocytes embedded in the collagen gel
using an integrated microfluidic device. Tissue Eng Part C Methods.
16:1267–1275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Y, Zhou J, Zheng Z, et al: An improved
method for isolating Schwann cells from postnatal rat sciatic
nerves. Cell Tissue Res. 337:361–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng XF, Lu SB, Zhang WG, et al:
Mesenchymal stem cells on a decellularized cartilage matrix for
cartilage tissue engineering. Biotechnol Bioprocess Eng.
16:593–602. 2011. View Article : Google Scholar
|
|
14
|
Li Y, Yang M, Huang Z, et al: AxonQuant: A
microfluidic chamber culture-coupled algorithm that allows
high-throughput quantification of axonal damage. Neurosignals.
22:14–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whitesides GM: The origins and the future
of microfluidics. Nature. 442:368–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kane BJ, Zinner MJ, Yarmush ML and Toner
M: Liver-specific functional studies in a microfluidic array of
primary mammalian hepatocytes. Anal Chem. 78:4291–4298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang RK, Lowe JB III, Sobol JB, et al:
Dose-dependent effects of FK506 on neuroregeneration in a rat
model. Plast Reconstr Surg. 112:1832–1840. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mackinnon SE, Doolabh VB, Novak CB and
Trulock EP: Clinical outcome following nerve allograft
transplantation. Plast Reconstr Surg. 107:1419–1429. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bavetta S, Hamlyn PJ, Burnstock G, et al:
The effects of FK506 on dorsal column axons following spinal cord
injury in adult rats: neuroprotection and local regeneration. Exp
Neurol. 158:382–393. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konofaos P and Terzis JK: FK506 and nerve
regeneration: past, present, and future. J Reconstr Microsurg.
29:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|