Introduction
Fatigue may be defined as a situation in which the
capacity for work is diminished and efficiency of accomplishment
reduced (1), and it can be
classified as physical or mental, depending on its cause. As
examples, physical fatigue is caused by excessive exercise and
mental fatigue is caused by sleep deprivation (2). Physical fatigue may be accompanied by
deterioration in performance. Several factors have been identified
to contribute to physical fatigue. First, exercise promotes
consumption of energy sources including glycogen by mobilizing the
internal energy metabolism to the maximum and using and depleting
the energy source (3). Second,
exercise causes the production and accumulation of metabolic
products including lactic acid and ammonia in the body (4). Third, intense exercise produces a
large quantity of reactive oxygen species (ROS) due to increased
oxygen consumption. The superoxide anion radical
(O2−) and hydrogen peroxide are generated as
metabolic intermediates in the presence of oxygen. These may lead
to a disturbance in the homeostasis of the endogenous antioxidative
defense systems in the body, resulting in the development of
fatigue (5). To date,
pharmacological drugs or therapies used for treating fatigue have
not been effective. Recently, interest has increased in the use of
natural substance supplements for the attenuation of
exercise-induced physical fatigue.
Hericium erinaceus (HEP) belongs to the
Aphyllophorales, Hydnaecae and Hericium families and its fruiting
body is called ‘Houtou’ in Chinese. It has been used as an edible
and medicinal fungus in China and other oriental countries and
areas for a number of years (6).
The fungus contains essential constituents, including
polysaccharides, lectins, proteins, lipids, hericenone, erinacol,
erinacine and terpenoids (7).
Polysaccharides from HEP have attracted considerable attention due
to their numerous physiological activities, including
immunomodulatory, hepatoprotective, antitumor, anti-aging,
antioxidant and hypoglycemic activities (8–11).
However, the anti-fatigue activity of HEP has not been investigated
until now. The present study was designed to evaluate the
anti-fatigue activity of HEP in a mouse model.
Materials and methods
Material
Dried fruiting bodies of HEP were obtained from a
market in Wuhan city and identified by Professor Ming Wang from the
Hubei Society for Microbiology (Wuhan, China). Voucher specimens
(EH-SCN1391) were preserved in Hubei Natural Product Research
Institute (Wuhan, China).
Reagents and kits
Glucose was purchased from Guoyao Chemical Reagent
Factory (Shanghai, China). The diagnostic kits for blood lactic
acid (BLA), tissue glycogen and malondialdehyde (MDA) were
purchased from Jiancheng Bioengineering Institute (Nanjing, China).
The diagnostic kits for serum urea nitrogen (SUN) were purchased
from Biosino Biotechnology and Science Inc. (Beijing, China). The
diagnostic kits for superoxide dismutase (SOD) and glutathione
peroxidase (GPx) were purchased from Comin Biotechnology Co., Ltd.
(Suzhou, China). Other commercial chemicals used in the experiments
were of analytical grade and were purchased from the Hongshan
Reagent Company (Wuhan, China).
Preparation of polysaccharides from
HEP
Polysaccharides from HEP were prepared using the
ethanol precipitation method as described by Li et al
(12) and modified by Hui et
al (13). Briefly, dried
fruits of HEP were ground and extracted with petroleum ether at
60°C for 4 h to remove colored materials, oligosaccharides and
small molecule materials under reflux in the apparatus. The organic
solvent was separated by centrifugation (4390 × g, 20 min) and
pretreated powder was obtained.
Next, the dried pretreated powder was extracted with
boiling water (at a ratio of 1:30 w/v) for 4 h. The mixture was
centrifuged (4390 × g, 20 min) and filtered, and the insoluble
residue was treated again as mentioned above. The supernatant was
incorporated and concentrated using a rotary evaporator at 50°C
under a vacuum. The concentrated extract was precipitated by the
addition of 95% (v/v) ethanol to a final concentration of 80% (v/v)
and incubated for 12 h at 4°C. The precipitate was collected by
centrifugation (4390 × g, 20 min) and then vacuum-dried at 40°C to
afford crude polysaccharides from HEP. The polysaccharide content
was measured by the phenol-sulfuric acid method using glucose as
standard.
Experiment animals
Male ICR mice, weighing 18–20 g at the beginning of
the study, were purchased from Wanqian Jiaxing Biotechnology Co,
Ltd. (Wuhan, China). They were fed under controlled environmental
conditions of temperature (22±2°C) and a 12-h light/dark cycle, and
maintained on a standard rodent diet and tap water ad
libitum unless otherwise stated. All animals received
professional humane care in compliance with the guidelines of the
Ethical Committee of Wuhan Institute of Physical Education (Wuhan,
China).
Experiment design
After one week of acclimation, the mice were
randomly divided into four groups (ten mice in each group) as
follows: i) control (C) group: the mice were allowed free access to
a standard rodent diet and treated with saline solution; ii)
low-dose HEP-treated (LHT) group: the mice were allowed free access
to a standard rodent diet and treated with 50 mg/kg bw of HEP; iii)
moderate-dose HEP-treated (MHT) group: the mice were allowed free
access to a standard rodent diet and treated with 100 mg/kg bw of
HEP; iv) High-dose HEP-treated (HHT) group: the mice were allowed
free access to a standard rodent diet and treated with 200 mg/kg bw
of HEP.
HEP was dissolved in 2.0 ml saline solution, and the
control group received the same volume of saline solution.
Treatments were administered orally by gavage using a feeding
needle, once a day for 28 consecutive days.
Forced swimming test
One hour after the final treatment, forced swimming
tests (FSTs) were conducted using the method described by Zhang
et al (14). Tests were
carried out in an acrylic plastic pool (90×45×45 cm) 35 cm deep
with water maintained at 25±2°C. A tin wire (5% of body weight) was
loaded on the tail root of each mouse. Exhaustion was determined by
observing loss of coordinated movements and failure to return to
the surface within 10 sec, and the exhaustive swimming time was
immediately recorded.
Analysis of biochemical parameters
related to fatigue
After FSTs, the animals were sacrificed immediately
by decapitation under anesthesia with sodium pentobarbital (40
mg/kg bw, ip). Blood samples of the mice were respectively
collected in heparinized tubes and tubes without anticoagulant.
Blood plasma was prepared by centrifugation at 4°C (2919 × g, 10
min) for the BLA analysis, and serum was prepared by centrifugation
at 4°C (2919 × g, 15 min) for the SUN analysis. After the blood was
collected, the gastrocnemius muscles and liver were rapidly excised
and immediately frozen in liquid nitrogen and stored at −80°C for
the tissue glycogen, SOD, GPx and MDA analysis.
Analytical methods
The BLA content was determined based on the lactate
dehydrogenase enzymatic method and the absorbance was read at 530
nm (15). SUN content was
determined by the diacetyl monoxime colorimetric method and the
absorbance was read at 520 nm (16). Glycogen content was determined by
the sulfuric anthrone method and the absorbance was read at 620 nm
(17). SOD activity was determined
by the xanthine oxidase method (hydroxylamine method) and the
absorbance was read at 550 nm (18). GPx activity was determined by the
dithio-binitrobenzoic acid method and the absorbance was read at
412 nm (19). MDA content was
determined by the thiobarbituric acid method and the absorbance was
read at 532 nm (20).
Statistical analysis
The results are expressed as the means ± standard
deviation. Comparisons between groups were made using Student’s
t-test, and P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
Effects of HEP on exhaustive swimming
times
The FST, a behavioral test for rodents, previously
used to predict the efficacy of antidepressants, has recently been
used to examine whether certain agents have anti-fatigue activities
(21). Prolonged swimming times in
an FST indicate a decrease in fatigue (22).
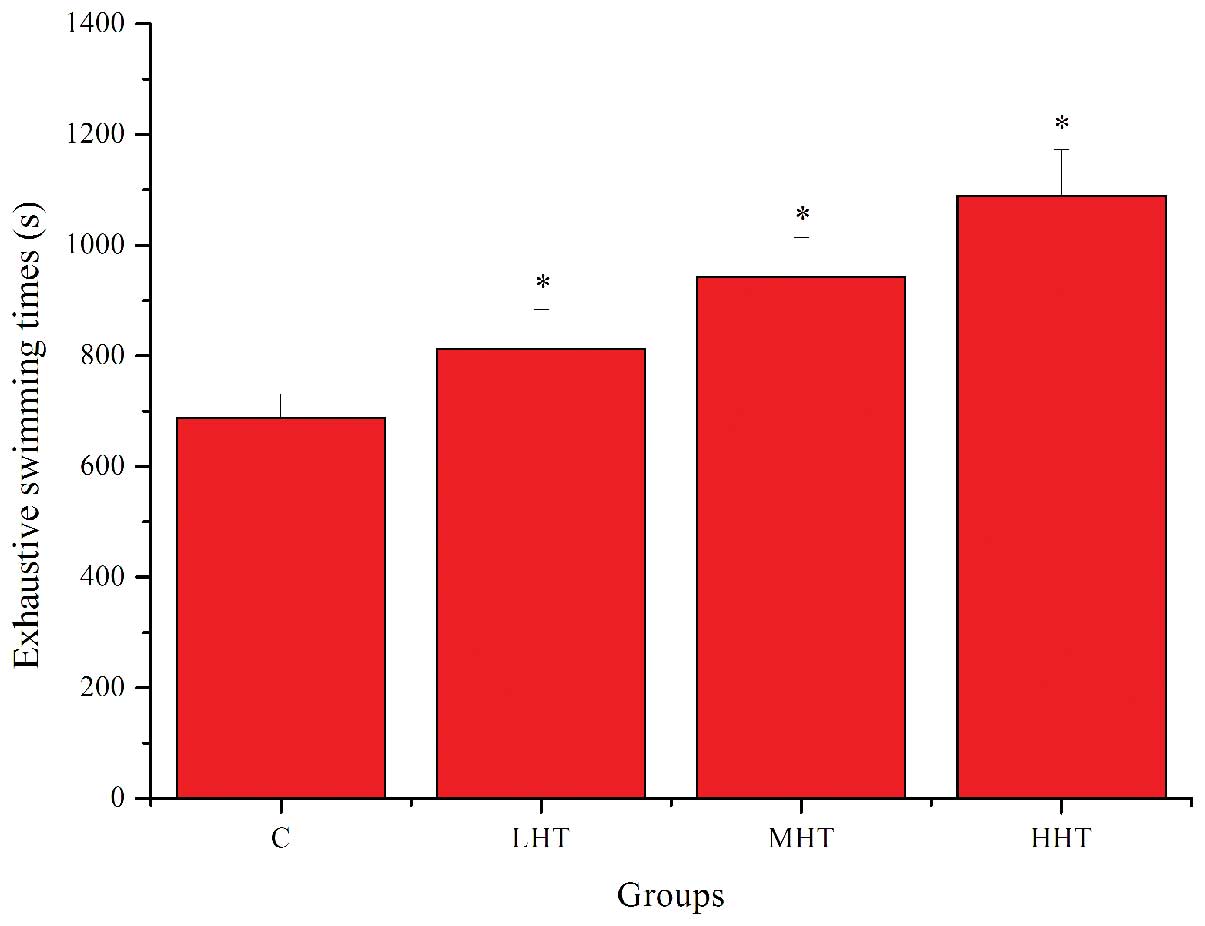
The effects of HEP on exhaustive swimming times are
shown in Fig. 1. Exhaustive
swimming times in the LHT, MHT and HHT groups were significantly
longer (P<0.05) than that in the C group, by 18.15, 37.18 and
58.46%, respectively. These results indicated that HEP had
significant anti-fatigue activity and was capable of elevating the
exercise tolerance in mice.
Effects of HEP on BLA and SUN
content
In general, the swimming exercise is known to induce
blood biochemical changes (23).
The muscle produces a considerable amount of lactic acid when it
obtains sufficient energy from anaerobic glycolysis, and the
increased concentration of lactic acid brings about a reduction in
the pH of muscle tissue and blood, which could induce various
biochemical and physiological side effects, including glycolysis
and phosphofructokinase and calciumion release, through muscular
contraction (24). Therefore, BLA
is a sensitive index of fatigue status. Urea is formed in the liver
as the end product of protein metabolism. During digestion, protein
is broken down into amino acids. Amino acids contain nitrogen,
which is removed as NH4+ (an ammonium ion),
while the remainder of the molecule is used to produce energy and
other substances required by the cell (14). There is a positive correlation
between the urea nitrogen in vivo and exercise tolerance. In
other words, the worse the body is adapted for exercise tolerance,
the more significantly the urea nitrogen level increases (25). Thus, SUN is another sensitive index
of fatigue status.
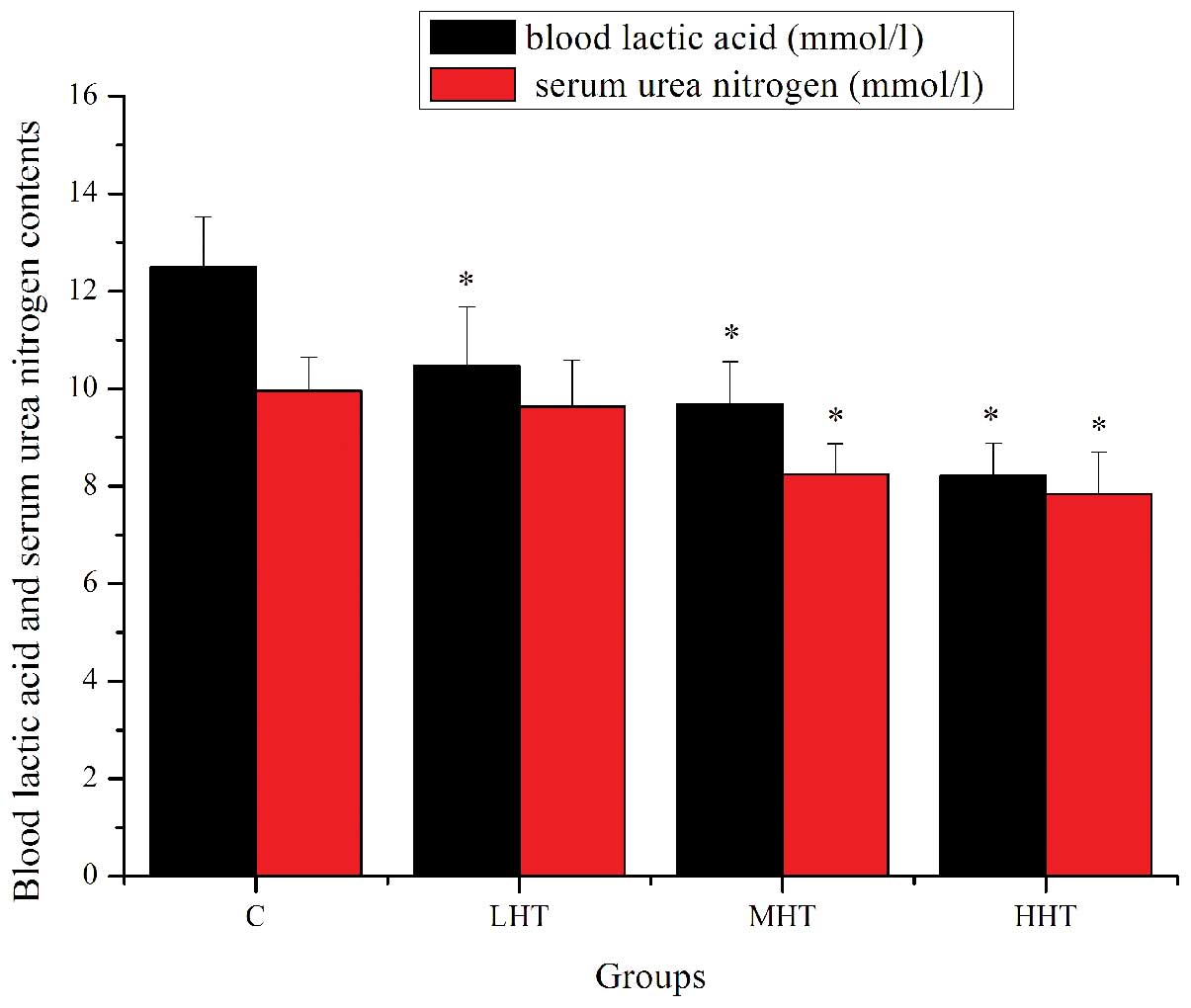
The effects of HEP on BLA and SUN content are shown
in Fig. 2. The BLA content of the
LHT, MHT and HHT groups was significantly lower (P<0.05) than
that of the C group, by 16.43, 28.90 and 52.13%, respectively. The
SUN content of the MHT and HHT groups was significantly lower
(P<0.05) than that of the C group, by 20.87 and 27.04%,
respectively. The SUN content of the LHT group was also lower, but
not significantly (P>0.05). These results indicated that HEP
effectively delayed the increase in BLA, reduced the catabolism of
protein for energy and increased the adaptive capacity to exercise
load, which ultimately postponed the appearance of physical
fatigue.
Effects of HEP on glycogen content in
liver and muscle
Energy for exercise is derived initially from the
breakdown of glycogen in muscle. Following strenuous exercise, it
may be depleted, and at later stages the energy will be derived
from hepatic glycogen (26).
Therefore, the depletion of glycogen stores may be a significant
factor in the development of fatigue.
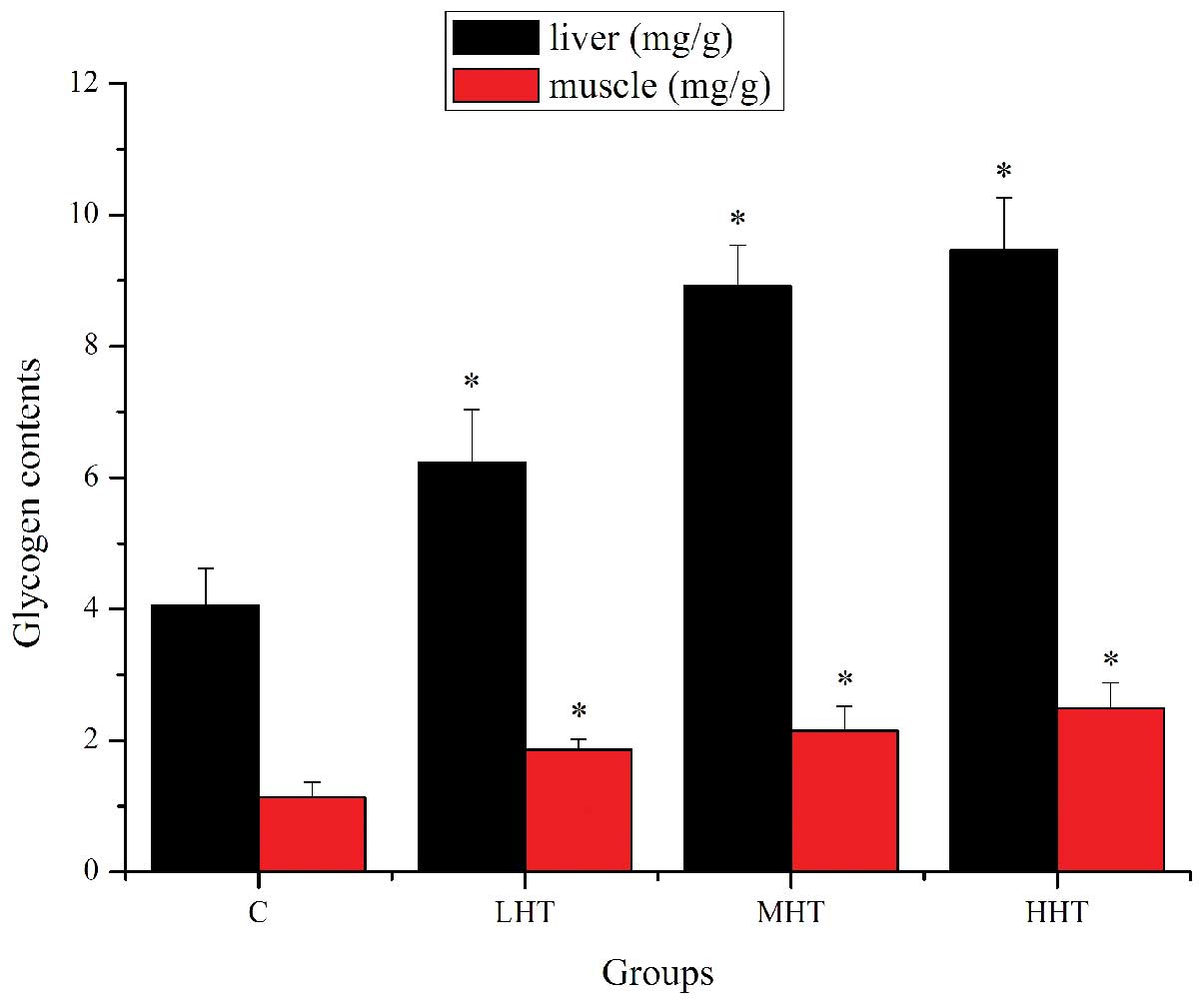
The effects of HEP on glycogen content in liver and
muscle are shown in Fig. 3. The
liver glycogen content of the LHT, MHT and HHT groups was
significantly higher (P<0.05) than that of the C group, by
53.45, 119.70 and 133.25%, respectively. The muscle glycogen
content of the LHT, MHT and HHT groups was significantly higher
(P<0.05) than that of the C group, by 64.60, 90.27 and 120.35%,
respectively. These results indicated that HEP may contribute to
the improvement of metabolic control of exercise and the activation
of energy metabolism (27), which
could ameliorate physical fatigue by increasing the storage of
glycogen in liver and muscle.
Effects of HEP on SOD and GPx activity in
liver and muscle
Previous studies have reported that ROS are
responsible for exercise-induced protein oxidation, and contribute
significantly to muscle fatigue (28). Two major classes of endogenous
protective mechanisms, enzymatic and non-enzymatic antioxidants,
work to reduce the harmful effects of ROS in cells (29). SOD and GPx constitute the principal
components of the enzymatic antioxidant defense systems. There is
growing evidence indicating that the improvement in the activity of
SOD and GPx help fight against fatigue and protect cells from
oxidative damage (30,31).
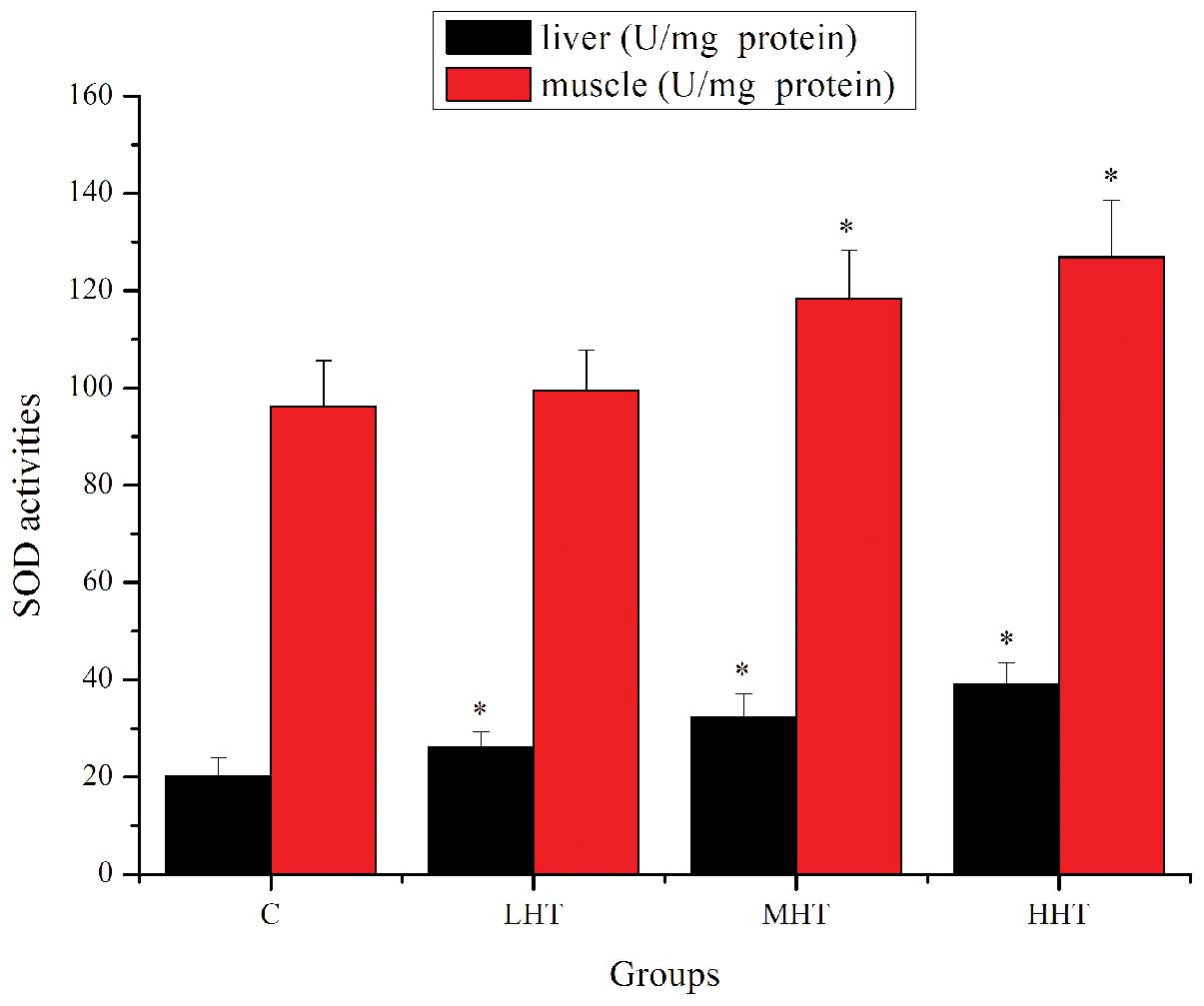
The effects of HEP on SOD activity are shown in
Fig. 4. The SOD activity in the
liver of the LHT, MHT and HHT groups was significantly higher
(P<0.05) than in that of the C group, by 29.96, 60.66 and
94.39%, respectively. SOD activity in the muscle of the MHT and HHT
group was significantly higher (P<0.05) than in that of the C
group, by 22.96 and 31.81%, respectively. SOD activity in the
muscle of the LHT group was also higher but not significantly
(P>0.05). The effects of HEP on GPx activity are shown in
Fig. 5. GPx activity in the liver
of the LHT, MHT and HHT groups was significantly higher (P<0.05)
than in that of the C group, by 25.50, 35.11 and 59.39%,
respectively. GPx activity in the muscle of the LHT, MHT and HHT
groups was significantly higher (P<0.05) than in that of the C
group, by 37.74, 78.77 and 130.35%, respectively. These results
indicated that HEP was able to upregulate antioxidant enzymes
activity to ameliorate physical fatigue.
Effects of HEP on MDA content in muscle
and liver
It is generally accepted that fatigue causes the
release of ROS, which leads to lipid peroxidation of the membrane
structure and causes oxidative damage to cellular macromolecules
(32). MDA is the breakdown
product of the major chain reactions leading to the oxidation of
polyunsaturated fatty acids and thus serves as an indicator of
lipid peroxidation (33). A number
of studies have reported that exhaustive exercise increased the MDA
content in liver and muscle tissues in rats and mice (34,35).
The effects of HEP on MDA content are shown in
Fig. 6. The MDA content in the
liver of the LHT, MHT and HHT groups was significantly lower
(P<0.05) than in that of the C group, by 16.71, 33.62 and
52.84%, respectively. The MDA content in the muscle of the LHT, MHT
and HHT groups was significantly lower (P<0.05) than that of the
C group, by 26.92, 54.69 and 40.93%, respectively. These results
indicated that HEP reduced lipid peroxidation and prevented
exercise-induced oxidative damage.
The results of the present study suggest that HEP
possesses significant anti-fatigue activity by decreasing BLA, SUN
and MDA content, and increasing tissue glycogen content and
antioxidant enzyme activity. Based on these results, this study
provides theoretical support for the application of HEP in the
field of sports nutrition.
Acknowledgements
This study was supported by the Department of
Education of Hubei Province, China (grant no. 20120484).
References
|
1
|
Zhang G, Zhou SM, Tian JH, Huang QY and
Gao YQ: Anti-fatigue effects of methazolamide in high-altitude
hypoxic mice. Trop J Pharm Res. 11:209–215. 2012. View Article : Google Scholar
|
|
2
|
Yan F, Zhang Y and Wang BB: Effects of
polysaccharides from Cordyceps sinensis mycelium on physical
fatigue in mice. Bangladesh J Pharmacol. 7:217–221. 2012.
View Article : Google Scholar
|
|
3
|
Wang L, Zhang HL, Lu R, Zhou YJ, Ma R, Lv
JQ, Li XL, Chen LJ and Yao Z: The decapeptide CMS001 enhances
swimming endurance in mice. Peptides. 29:1176–1182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikeuchi M, Koyama T, Takei S, Kino T and
Yazawa K: Effects of Benzylglucosinolate on endurance capacity in
mice. J Health Sci. 55:178–182. 2009. View Article : Google Scholar
|
|
5
|
Blokhina O, Virolainen E and Fagerstedt
KV: Antioxidants, oxidative damage and oxygen deprivation stress: a
review. Ann Bot. 91(Spec No): 179–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JS, Min KM, Cho JY and Hong EK: Study
of macrophage activation and structural characteristics of purified
polysaccharides from the fruiting body of Hericium erinaceus. J
Microbiol Biotechnol. 19:951–959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Li Q, Mao G, Zou Y, Feng W, Zheng
D, Wang W, Zhou L, Zhang T, Yang J, Yang L and Wu X: Optimization
of enzyme-assisted extraction and characterization of
polysaccharides from Hericium erinaceus. Carbohydr Polym.
101:606–613. 2014. View Article : Google Scholar
|
|
8
|
Zhang Z, Lv G, Pan H, Pandey A, He W and
Fan L: Antioxidant and hepatoprotective potential of
endo-polysaccharides from Hericium erinaceus grown on tofu whey.
Int J Biol Macromol. 51:1140–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JC, Hu SH, Su CH and Lee TM:
Antitumor and immunoenhancing activities of polysaccharide from
culture broth of Hericium spp. Kaohsiung J Med Sci. 17:461–467.
2001.
|
|
10
|
Khan MA, Tania M, Liu R and Rahman MM:
Hericium erinaceus: an edible mushroom with medicinal values. J
Complement Integr Med. 10:1–6. 2013.
|
|
11
|
Han ZH, Ye JM and Wang GF: Evaluation of
in vivo antioxidant activity of Hericium erinaceus polysaccharides.
Int J Biol Macromol. 52:66–71. 2013. View Article : Google Scholar
|
|
12
|
Li FL, Li QW, Gao DW, Peng Y and Feng CN:
Preparation and antidiabetic activity of polysaccharide from
Portulaca oleracea L. Afr J Biotechnol. 8:569–573. 2009.
|
|
13
|
Hui MK, Wu WK, Shin VY, So WH and Cho CH:
Polysaccharides from the root of Angelica sinensis protect bone
marrow and gastrointestinal tissues against the cytotoxicity of
cyclophosphamide in mice. Int J Med Sci. 3:1–6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang XL, Ren F, Huang W, Ding RT, Zhou QS
and Liu XW: Anti-fatigue activity of extracts of stem bark from
Acanthopanax senticosus. Molecules. 16:28–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shang Y, Cheng J, Qi J and Miao H:
Scutellaria flavonoid reduced memory dysfunction and neuronal
injury caused by permanent global ischemia in rat. Pharmacol
Biochem Behav. 82:67–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wybenga DR, Di Giorgio J and Pileggi VJ:
Manual and automated methods for urea nitrogen measurement in whole
serum. Clin Chem. 17:891–895. 1971.PubMed/NCBI
|
|
17
|
Sotelo-Félix JI, Martinez-Fong D, Muriel
P, Santillán RL, Castillo D and Yahuaca P: Evaluation of the
effectiveness of Rosmarinus officinalis (Lamiaceae) in the
alleviation of carbon tetrachloride-induced acute hepatotoxicity in
the rat. J Ethnopharmacol. 81:145–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang H, Shan J, Pan XH, Wang HP and Qian
LB: Carvedilol protected diabetic rat hearts via reducing oxidative
stress. J Zhejiang Univ Sci B. 7:725–731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang QH, Wu CF, Yang JY, Mu YH, Chen XX
and Zhao YQ: Reduction of cyclophosphamide-induced DNA damage and
apoptosis effects of ginsenoside Rb (1) on mouse bone marrow cells
and peripheral blood leukocytes. Environ Toxicol Pharmacol.
27:384–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Sun YP, Li Y, Liu WW, Xiang HG,
Fan LY, Sun Q, Xu XY, Cai JM, Ruan CP, et al: Hydrogen-rich saline
ameliorates the severity of l-arginine-induced acute pancreatitis
in rats. Biochem Biophys Res Commun. 393:308–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koo HN, Lee JK, Hong SH and Kim HM:
Herbkines increases physical stamina in mice. Biol Pharm Bull.
27:117–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan W, Yu KQ, Liu YY, Ouyang MZ, Yan MH,
Luo R and Zhao XS: Anti-fatigue activity of polysaccharides extract
from Radix Rehmanniae Preparata. Int J Biol Macromol. 50:59–62.
2012. View Article : Google Scholar
|
|
23
|
An HJ, Choi HM, Park HS, Han JG, Lee EH,
Park YS, Um JY, Hong SH and Kim HM: Oral administration of hot
water extracts of Chlorella vulgaris increases physical stamina in
mice. Ann Nutr Metab. 50:380–386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang CC, Hsu MC, Huang WC, Yang HR and
Hou CC: Triterpenoid-rich extract from antrodia camphorata improves
physical fatigue and exercise performance in mice. Evid Based
Complement Alternat Med. 2012:3647412012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang W, Zhang Y, Gao J, Ding X and Gao S:
The anti-fatigue effect of 20 (R)-ginsenoside Rg3 in mice by
intranasally administration. Biol Pharm Bull. 31:2024–2027. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anand T, Phani Kumar G, Pandareesh MD,
Swamy MS, Khanum F and Bawa AS: Effect of bacoside extract from
Bacopa monniera on physical fatigue induced by forced swimming.
Phytother Res. 26:587–593. 2012. View
Article : Google Scholar
|
|
27
|
Xu C, Lv J, Lo YM, Cui SW, Hu X and Fan M:
Effects of oat β-glucan on endurance exercise and its anti-fatigue
properties in trained rats. Carbohydr Polym. 26:1159–1165. 2013.
View Article : Google Scholar
|
|
28
|
Powers SK and Jackson MJ: Exercise-induced
oxidative stress: cellular mechanisms and impact on muscle force
production. Physiol Rev. 88:1243–1276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Powers SK, DeRuisseau KC, Quindry J and
Hamilton KL: Dietary antioxidants and exercise. J Sports Sci.
22:81–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Li S, Wang X and Zhang CL:
Protective effects of Radix Pseudostellariae polysaccharides
against exercise-induced oxidative stress in male rats. Exp Ther
Med. 5:1089–1092. 2013.PubMed/NCBI
|
|
31
|
Yan F, Wang B and Zhang Y: Polysaccharides
from Cordyceps sinensis mycelium ameliorate exhaustive swimming
exercise-induced oxidative stress. Pharm Biol. 52:157–161. 2014.
View Article : Google Scholar
|
|
32
|
Prasad VMP and Khanum F: Antifatigue
activity of ethanolic extract of Ocimum sanctum in rats. Res J Med
Plant. 6:37–46. 2012. View Article : Google Scholar
|
|
33
|
Zhonghui Z, Xiaowei Z and Fang F:
Ganoderma lucidum polysaccharides supplementation attenuates
exercise-induced oxidative stress in skeletal muscle of mice. Saudi
J Biol Sci. 21:119–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen QP and Wei P: Icariin supplementation
protects mice from exercise-induced oxidant stress in liver. Food
Sci Biotech. 22:1405–1413. 2013. View Article : Google Scholar
|
|
35
|
Shan X, Zhou J, Ma T and Chai Q: Lycium
barbarum polysaccharides reduce exercise-induced oxidative stress.
Int J Mol Sci. 12:1081–1088. 2011. View Article : Google Scholar : PubMed/NCBI
|