Introduction
The involvement of peripheral opioids in
inflammatory pain regulation has been well demonstrated (1–4). Under
inflammatory conditions, opioid peptides are released from local
immunocytes upon the stimulation of corticotrophin-releasing factor
(CRF) and a number of cytokines, including interleukin (IL)-1β
(5–7). Among the released opioids are
met-enkephalin (Met-Enk) and dynorphin A (DYN A); however, the
predominant opioid involved in immune cell-mediated intrinsic
antinociception is considered to be β-endorphin (β-END) (6,8). The
majority of these findings were obtained from complete Freund's
adjuvant (CFA)-induced inflammatory pain with a duration of no more
than four days. Considering that long-lasting inflammatory pain is
more often encountered in clinical practice than that with a short
duration, the changes in peripheral opioids during long-lasting
inflammatory pain may provide more valuable insight towards chronic
pain control. However, limited data in this area are available at
present. In addition, since locally infiltrating immunocyte
lineages at different inflammation stages are distinct (9), the profiles of peripheral opioids in
long-lasting inflammatory pain may differ from the aforementioned
observations.
Thus, the aim of the present study was to
systemically assess the profile of peripheral opioids in the later
stage of CFA-induced inflammatory pain on day 18 after the CFA
injection (10). The levels of
β-END, Met-Enk and DYN A, as well as their upstream inducers, CRF
and IL-1β, were analyzed, and validation experiments were performed
to confirm the intrinsic analgesic effects induced by peripheral
opioids in long-lasting inflammatory pain.
Materials and methods
Animals
Sixteen male Wistar rats (weight, 180–200 g;
six-weeks old) were obtained from Shanghai Laboratory Animal Center
(Shanghai, China). The rats were housed in a temperature-controlled
animal facility (25±1°C) under a 12-h light/dark cycle, with access
to food and water ad libitum. The experiments were conducted
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (11). The present study was approved by the
Ethics Committee of Zhejiang Chinese Medical Hospital (Hangzhou,
China).
Inflammatory pain model
Rats were randomly divided into a normal control
group (normal) and CFA-induced inflammatory pain group (CFA), with
eight rats per group. Inflammatory pain was induced by an
intraplantar injection of 100 µl CFA (Sigma-Aldrich, St. Louis, MO,
USA) into the right hind paw. The normal control rats were injected
with the same volume of saline. All the rats were sacrificed on day
18 after the injection and samples of the right hind paw
inflammatory tissue were obtained. The Wistar rats were used in the
first experiment to evaluate the profile change of the peripheral
opioids in CFA-induced long-lasting inflammatory pain.
Assessment of inflammation
Inflammation was assessed by the extent of paw
swelling. The paw volume was measured in duplicate, using a water
displacement plethysmometer (LYS-7A; Shandong Xinhua Medical
Instrument Co., Ltd., Zibo, China), prior to the injection of CFA
or saline and at the indicated time points following the injection
of CFA or saline. Paw swelling was expressed as an increase in paw
volume compared with the initial volume.
Evaluation of inflammatory pain
Inflammatory pain was evaluated by assessment of the
paw withdrawal latencies (PWLs), as described in our previous study
(12). The PWL was measured in
triplicate, using a plantar tester (Ugo Basile Srl, Varese, Italy),
prior to the CFA or saline injection and at the indicated
timepoints following the injection of CFA or saline. Briefly, the
rats were placed in a clear plastic chamber and allowed to
acclimatize for 30 min. A radiant heat stimulus (high-intensity
projector lamp bulb) was positioned under the glass floor directly
beneath the right hind paw. When the animal withdrew its hind paw,
the heat stimulus was automatically stopped, and the time recorded
was the PWL. A 20-sec cut-off was used to prevent tissue
injury.
Enzyme-linked immunosorbent assay
(ELISA) for CRF and IL-1β
Rats were anesthetized with 10% (w/v) chloral
hydrate at a dose of 3.5 ml/kg (i.p.) on day 18 after the
injection. The right hind paws were immediately removed and rinsed
in ice-cold saline. The samples were pulverized in liquid nitrogen,
resolved in cell lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China) containing protease inhibitor cocktail (10% v/v; Bio
Basic, Inc., Markham, ON, Canada), sonicated on ice (5×5 sec) and
stored for 1 h at 4°C. The samples were subsequently centrifuged at
13,201 × g for 30 min at 4°C for protein extraction. The levels of
CRF and IL-1β were measured using a rat CRF ELISA kit (Bachem
Americas, Inc., Torrance, CA, USA) and rat IL-1β ELISA kit (Enzo
Life Sciences, Inc., Farmingdale, NY, USA), according to the
manufacturers' instructions, respectively.
Radioimmunoassay (RIA) for β-END,
Mek-ENK and DYN A
Samples for the RIA were collected using the same
method as those for ELISA. A RIA was performed to measure the
levels of β-END, Mek-ENK and DYN A in the paw inflammatory tissue
at the Department of Neurobiology of the Second Military Medical
University of Chinese PLA (Shanghai, China). The levels of β-END,
Mek-ENK and DYN A were measured using a rat 125I β-END
RIA kit, rat 125I Met-ENK RIA kit and rat
125I DYN A RIA kit (Phoenix Biotechnology, Inc., San
Antonio, TX, USA), according to the manufacturer's instructions,
respectively.
Validation experiment by intraplantar
injection of δ-opioid receptor (DOR) antagonist
To determine whether the upregulation of Met-ENK
mediated analgesia, the analgesic effect of a DOR antagonist,
administered locally on normal control rats and rats with
CFA-induced inflammatory pain, was investigated. Thirty male
Sprague-Dawley rats (weight, 180–200 g; six-weeks old) were
obtained from Shanghai Laboratory Animal Center. These were
maintained in the same way as the Wistar rats and were randomly
divided into three groups, which included the CFA + saline, CFA +
DOR antagonist and normal + DOR antagonist groups (n=10 per group).
The rat model of inflammatory pain was established by the same
method as aforementioned. For the DOR antagonist groups, the rats
received an intraplantar injection of 4 µg ICI 154,129 (DOR
antagonist; Tocris Bioscience, Ellisville, MO, USA) on day 18
following the saline or CFA injection. Rats in the CFA + vehicle
group received the same volume of 0.05 ml saline. The PWLs were
measured prior to the saline or antagonist injection and 30 min
after the saline or antagonist intraplantar injection.
Validation experiment by intraplantar
injection of κ-opioid receptor (KOR) antagonist
To investigate whether the upregulation of DYN A
suppressed chronic inflammatory pain, the effect of a KOR
antagonist, administered locally on normal control rats and rats
with CFA-induced inflammatory pain, was investigated. Thirty male
Sprague-Dawley rats (weight, 180–200 g; six-weeks old) were
randomly divided into three groups: CFA + saline, CFA + KOR
antagonist and normal + KOR antagonist (n=10 per group). The rat
model of inflammatory pain was established by the same method as
aforementioned. In the KOR antagonist groups, the rats received an
intraplantar injection of 4 µg nor-Binaltorphimine dihydrochloride
(KOR antagonist; Tocris Bioscience) on day 18 after the CFA or
saline injection. Rats in the CFA + vehicle group received the same
volume of 0.05 ml saline. The PWLs were measured prior to the
saline or antagonist injection and 30 min after the saline or
antagonist intraplantar injection.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean, and were analyzed using the non-paired or paired
Student's t-test. All statistical analyses were conducted using
SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
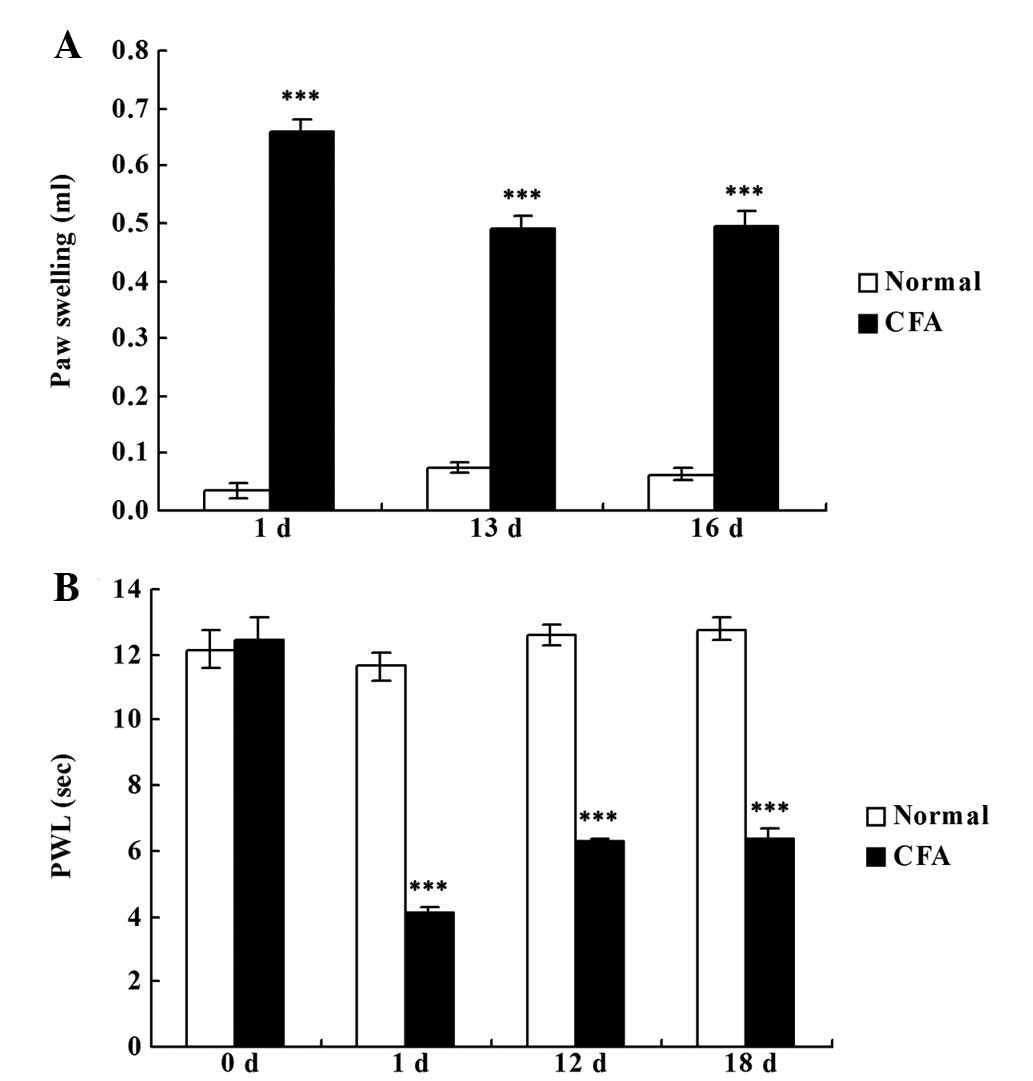
CFA successfully induces chronic
inflammatory pain
Rats that were administered an intraplantar
injection of CFA developed chronic inflammatory pain in the right
hind paw, as demonstrated by the significantly increased paw
swelling (P<0.001; Fig. 1A) and
reduced PWL (P<0.001; Fig. 1B)
when compared with the rats in the normal group.
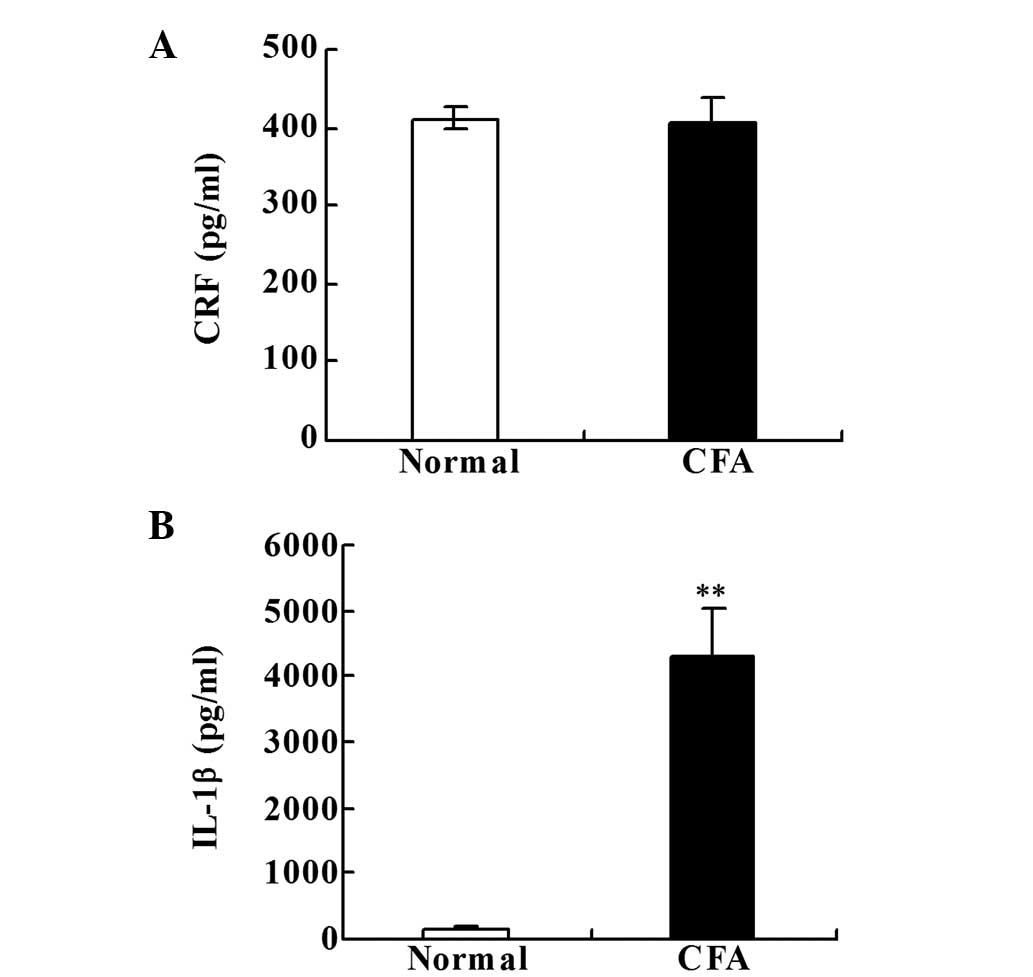
CRF and IL-1β levels in the paw
inflammatory tissue
No statistically significant difference in the CRF
level was identified between the two groups (Fig. 2A). However, the levels of IL-1β were
significantly increased in the CFA group when compared with the
normal group on day 18 after the CFA injection (P<0.001;
Fig. 2B).
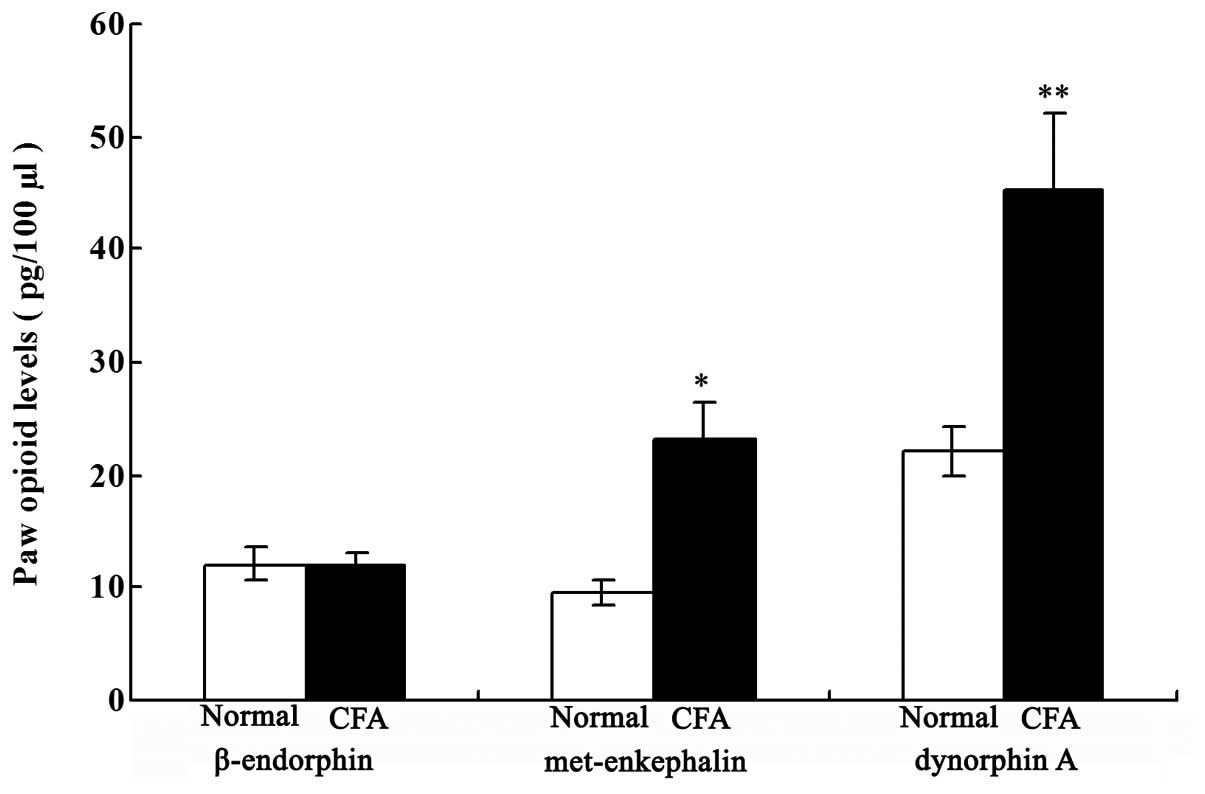
Local opioid levels in the paw
inflammatory tissue
As shown in Fig. 3,
the levels of Met-ENK and DYN A were significantly increased in the
CFA group when compared with those in the normal group on day 18
after the CFA injection (P<0.05 and P<0.01, respectively).
However, no statistically significant difference in the β-END level
was observed between the two groups.
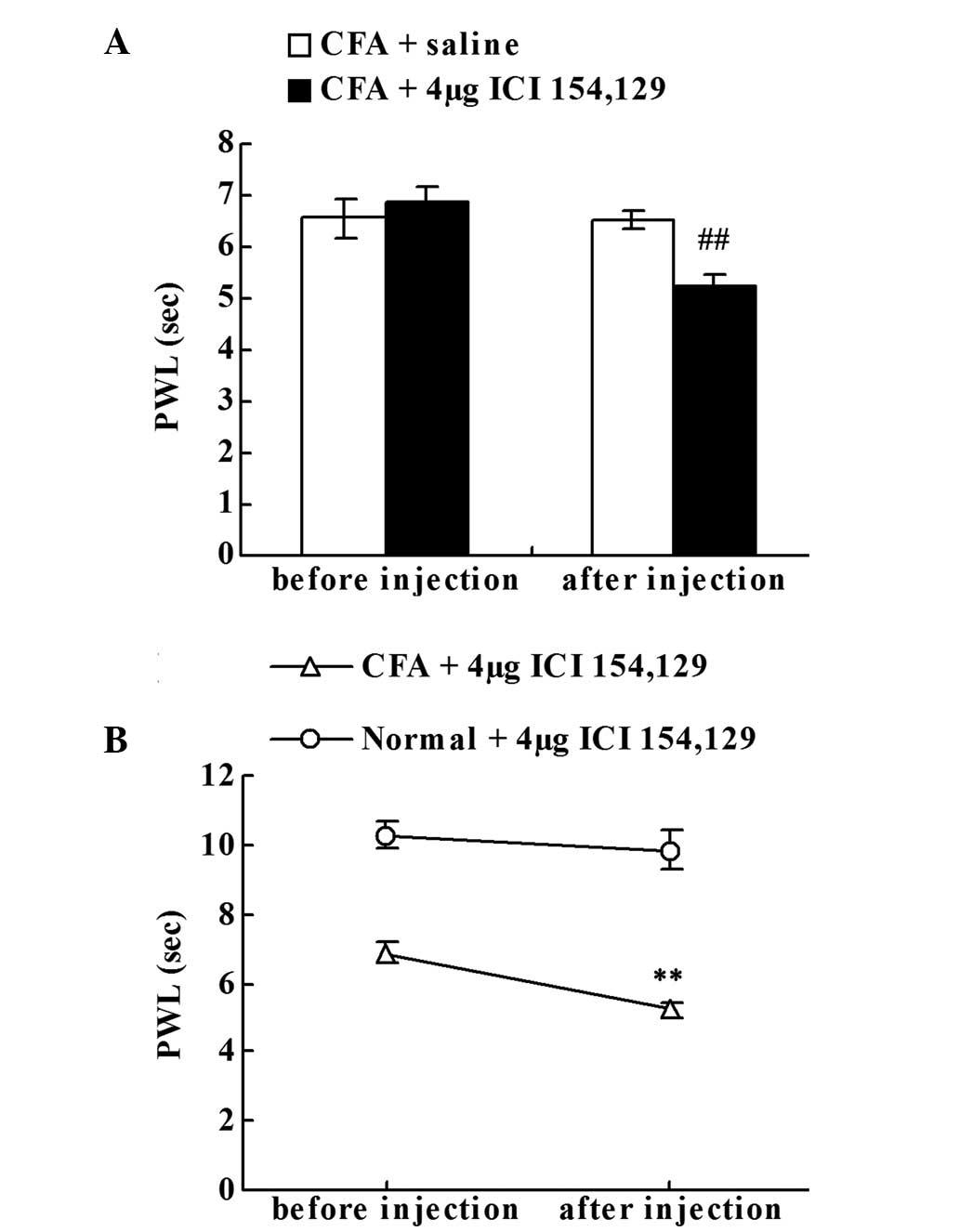
Effect of the intraplantar injection
of the DOR antagonist on chronic inflammatory pain
To investigate whether the upregulation of Met-ENK
suppressed chronic inflammatory pain, the effect of ICI 154,129
(DOR antagonist) local administration was examined. Prior to the
saline or ICI 154,129 injection, no statistically significant
difference in the PWLs of the right hind paws was observed between
the CFA + saline and CFA + DOR antagonist groups on day 18. The
local ICI 154,129 injection significantly decreased the PWLs of the
CFA-injected rats when compared with the CFA + saline group
(P<0.01; Fig. 4A). As shown in
Fig. 4B, compared with the PWL prior
to injection on day 18, local administration of ICI 154,129
significantly decreased the PWLs of the CFA-injected rats
(P<0.01; Fig. 4B), but failed to
significantly reduce the PWLs of the normal rats.
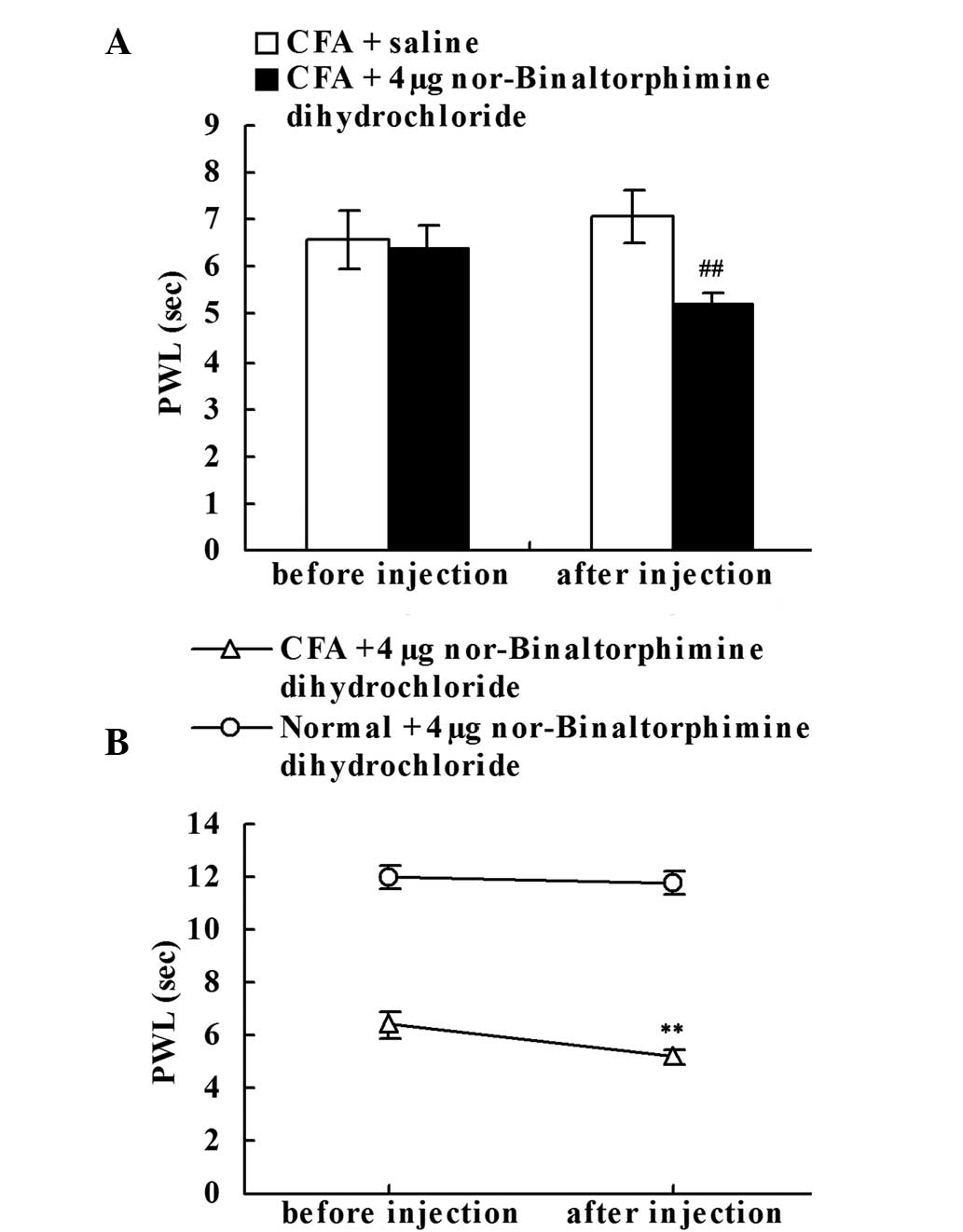
Effect of the intraplantar injection
of the KOR antagonist on chronic inflammatory pain
To determine whether the upregulation of DYN A
played a role in intrinsic analgesia, the effect of local
administration of nor-Binaltorphimine dihydrochloride (KOR
antagonist) was investigated. Prior to the saline or
nor-Binaltorphimine dihydrochloride injection, no statistically
significant difference in the PWLs of the right hind paws was
observed between the CFA + saline and CFA + KOR antagonist groups
on day 18. However, the local nor-Binaltorphimine dihydrochloride
injection significantly decreased the PWLs of the CFA-injected rats
when compared with the CFA + saline group (P<0.01; Fig. 5A). As shown in Fig. 5B, when compared with the PWLs prior
to injection on day 18, local administration of nor-Binaltorphimine
dihydrochloride significantly decreased the PWLs of the
CFA-injected rats (P<0.01; Fig.
5B), but failed to significantly reduce the PWLs of the normal
rats.
Discussion
Peripheral opioids are attracting increasing
attention for the development of peripherally restricted strategies
for pain relief. However, limited data exist with regard to their
profiles in long-lasting inflammatory pain. The present study, for
the first time, demonstrated the involvement of peripheral Met-Enk
and DYN A in intrinsic analgesia in a rat model of CFA-induced
long-lasting inflammatory pain.
Inflammation can trigger the migration of
opioid-containing immunocytes to the inflammatory tissue, causing
the release of opioid peptides (13,14).
Unlike peripheral intrinsic analgesia, which is primarily mediated
by β-END in the early stage of CFA-induced inflammatory pain (on
day 4 after CFA injection) (6,15,16),
β-END was shown to remain at an almost normal level in the later
stages of inflammatory pain (on day 18 after CFA injection).
However, the levels of Met-Enk and DYN A were significantly
increased in the paw inflammatory tissue. The different profiles of
the peripheral opioids in the early and later stages of CFA-induced
inflammatory pain may be the result of the distinct characteristics
of the locally infiltrating immunocytes (9). The most commonly referred to
stimulators of the peripheral opioids released from immunocytes in
inflammatory sites are CRF, IL-1β and other inflammatory cytokines
(6,17–19). A
normal CRF level and a significantly elevated IL-1β level were
observed in the present study, indicating that the increased levels
of peripheral opioids in long-lasting inflammatory pain may be more
closely associated with inflammatory intrinsic cytokines, such as
IL-1β, but not local CRF. CRF levels have been reported to be
elevated and play an important role in immune cell-mediated
intrinsic antinociception under stress conditions, such as a cold
water swim (20).
To testify the hypotheses that upregulation of
Met-Enk and DYN A may mediate intrinsic analgesia to prevent
further injury or more serious pain (4,21), the
effects of the local administration of DOR and KOR antagonists on
inflammatory pain were investigated. Locally delivered DOR and KOR
antagonists resulted in a more substantial pain to the CFA-injected
rats. However, the antagonists did not significantly affect the
pain thresholds of the normal rats, indicating that the
upregulation of Met-Enk and DYN A contribute to intrinsic analgesia
in long-lasting inflammatory pain. These observations are in
accordance with a previous study, which demonstrated that locally
administered naloxone (opioid receptor antagonist) can exacerbate
pain under inflammatory conditions (4). Peripheral intrinsic analgesia mediated
by Met-ENK and DYN A may be regarded as the body's negative
feedback of inflammation, a subsequent protective mechanism to
prevent further pain under long-lasting inflammatory
conditions.
In conclusion, the present study demonstrated that
Met-ENK and DYN A, but not β-END levels, were increased in
long-lasting inflammatory pain, unlike that observed for
early-stage of CFA-induced pain where β-END is the predominant
elevated peripheral opioid. In addition, upregulation of Met-ENK
and DYN A were shown to contribute to peripheral intrinsic
analgesia. The results provide valuable data for developing
peripherally restricted strategies for pain relief under
long-lasting inflammatory conditions.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81072855 and 81303039),
the Zhejiang Provincial Natural Science Foundation of China (nos.
Z2100979 and LY12H27015) and the Key Subject of State
Administration of Traditional Chinese Medicine of China
(Acupuncture and Moxibustion).
References
|
1
|
Tanaka N, Sakahashi H, Sato E, Hirose K
and Ishii S: The efficacy of intra-articular analgesia after total
knee arthroplasty in patients with rheumatoid arthritis and in
patients with osteoarthritis. J Arthroplasty. 16:306–311. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalso E, Smith L, McQuay HJ and Andrew
Moore R: No pain, no gain: clinical excellence and scientific
rigour - lessons learned from IA morphine. Pain. 98:269–275. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunha TM, Roman-Campos D, Lotufo CM, et
al: Morphine peripheral analgesia depends on activation of the
PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci
USA. 107:4442–4447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stein C, Hassan AH, Lehrberger K, Giefing
J and Yassouridis A: Local analgesic effect of endogenous opioid
peptides. Lancet. 342:321–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mousa SA, Bopaiah CP, Stein C and Schäfer
M: Involvement of corticotropin-releasing hormone receptor subtypes
1 and 2 in peripheral opioid-mediated inhibition of inflammatory
pain. Pain. 106:297–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schäfer M, Carter L and Stein C:
Interleukin 1 beta and corticotropin-releasing factor inhibit pain
by releasing opioids from immune cells in inflamed tissue. Proc
Natl Acad Sci USA. 91:4219–4223. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mousa SA, Shakibaei M, Sitte N, Schäfer M
and Stein C: Subcellular pathways of beta-endorphin synthesis,
processing, and release from immunocytes in inflammatory pain.
Endocrinology. 145:1331–1341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stein C, Schäfer M and Hassan AH:
Peripheral opioid receptors. Ann Med. 27:219–221. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rittner HL, Brack A, Machelska H, et al:
Opioid peptide-expressing leukocytes: identification, recruitment,
and simultaneously increasing inhibition of inflammatory pain.
Anesthesiology. 95:500–508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
da Silva MA, Bersani-Amado CA,
Ishii-Iwamoto EL, Bracht L and Caparroz-Assef SM: Protective
effects of indomethacin and cyclophosphamide but not of infliximab
on liver metabolic changes caused by adjuvant-induced arthritis.
Inflammation. 34:519–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals8th.
Washington (DC): National Academies Press (US); 2011
|
|
12
|
Ren WK, Yin J, Zhu XP, et al: Glutamine on
intestinal inflammation: A mechanistic perspective. Eur J Inflamm.
11:315–326. 2013.
|
|
13
|
Rittner HL, Machelska H and Stein C:
Leukocytes in the regulation of pain and analgesia. J Leukoc Biol.
78:1215–1222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mousa SA: Morphological correlates of
immune-mediated peripheral opioid analgesia. Adv Exp Med Biol.
521:77–87. 2003.PubMed/NCBI
|
|
15
|
Mousa SA, Zhang Q, Sitte N, Ji R and Stein
C: beta-Endorphin-containing memory-cells and mu-opioid receptors
undergo transport to peripheral inflamed tissue. J Neuroimmunol.
115:71–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Machelska H, Schopohl JK, Mousa SA, et al:
Different mechanisms of intrinsic pain inhibition in early and late
inflammation. J Neuroimmunol. 141:30–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cabot PJ, Carter L, Schäfer M and Stein C:
Methionine-enkephalin- and Dynorphin A-release from immune cells
and control of inflammatory pain. Pain. 93:207–212. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Binder W, Mousa SA, Sitte N, Kaiser M,
Stein C and Schäfer M: Sympathetic activation triggers endogenous
opioid release and analgesia within peripheral inflamed tissue. Eur
J Neurosci. 20:92–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puehler W, Rittner HL, Mousa SA, et al:
Interleukin-1 beta contributes to the upregulation of kappa opioid
receptor mRNA in dorsal root ganglia in response to peripheral
inflammation. Neuroscience. 141:989–998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schafer M, Mousa SA, Zhang Q, Carter L and
Stein C: Expression of corticotropin-releasing factor in inflamed
tissue is required for intrinsic peripheral opioid analgesia. Proc
Natl Acad Sci USA. 93:6096–6100. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sehgal N, Smith HS and Manchikanti L:
Peripherally acting opioids and clinical implications for pain
control. Pain Physician. 14:249–258. 2011.PubMed/NCBI
|