Introduction
The schwannoma, also known as the Schwann cytoma, is
the most common type of intraspinal tumor (1), accounting for approximately one-quarter
of all primary intraspinal tumors. Due to the limited space in the
spinal canal, the gradual growth of the schwannoma compresses the
spinal cord and nerve roots, thus causing severe spinal and nerve
root dysfunction. Early surgical treatment can result in a
promising outcome (2–7).
To the best of our knowledge, few studies have
systematically investigated cases of cervicothoracolumbar spinal
schwannoma (CSS) (8); however, we
propose that different surgical approaches should be applied
according to different tumor growth sites, in order to improve the
surgical safety. Pollo et al (8) and Jankowski et al (9) reported the use of an anterior-posterior
combined surgical approach in the treatment of lumbar schwannoma.
For thoracolumbar schwannomas, a suitable approach may be to
perform a first-phase posterior tumor resection, i.e. posterior
vertebral decompression plus intraspinal tumor resection, followed
by extraspinal tumor resection. Canbay et al (10) suggested that spinal tumors measuring
>2.5 cm should be classified as giant tumors. Since
intraoperative dissection of these tumors could damage the brachial
plexus nerve and the adjacent vessels, thus generating
complications, a suitable approach in such cases may be to first
perform posterior vertebral decompression plus intraspinal tumor
resection, followed by anterior tumor dissection and resection.
The aim of the present study was to investigate the
surgical methods and efficacies for CSS. A total of 52 patients
with CSS were surgically treated in the First Affiliated Hospital
of Xinjiang Medical University (Urumqi, China) between 2011 and
2013, and their cases were retrospectively analyzed.
Materials and methods
General information
Fifty-two patients with intraspinal schwannoma were
treated in the First Affiliated Hospital of Xinjiang Medical
University between September 2011 and September 2013. The patients
included 25 males and 27 females, who were aged 26–84 years (mean
age, 47.5 years). Of the lesions, 2 were at the cervical spine, 23
were at the thoracic spine and 27 were at the lumbosacral spine,
and radicular pain was the main clinical symptom. Twenty-six
patients experienced radicular pain as the first symptom, which
worsened when the patient slept at night or adopted a prostrate
position and was alleviated when the patient moved or sat. A total
of 27 patients exhibited numbness, weakness and hyperesthesia in
the tumor-corresponding plane, and 2 patients exhibited painless
scoliosis. This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Xinjiang Medical University. Written informed consent was
obtained from all participants.
Auxiliary examination
All patients underwent normal spinal radiography, as
well as spinal computed tomography (CT) and magnetic resonance
imaging (MRI) examination of the corresponding sites. The general
manifestations of X-ray and CT were an expanded intervertebral
foramen and a widened spinal canal. MRI was considerably more
effective at reflecting the status of the schwannoma:
Extramedullary schwannomas, for example, were usually located at
the dorsolateral side of the spinal cord, appearing class-round on
the spinal surface. The Tl images showed iso- or slightly lower
signals, while the T2 images showed iso- or slightly higher
signals, with clear boundaries. The enhanced scanning showed
homogeneous enhancement, and the cystic degeneration of the tumor
was rare.
Surgical methods
In all patients, the surgery was performed under
endotracheal intubation and intravenous general anesthesia with the
patients in a prone position. The surgical methods for the thoracic
and lumbar schwannomas were divided into types I and II according
to the preoperative classification (11–14), and
the patients were correspondingly assigned to groups 1 and 2,
respectively.
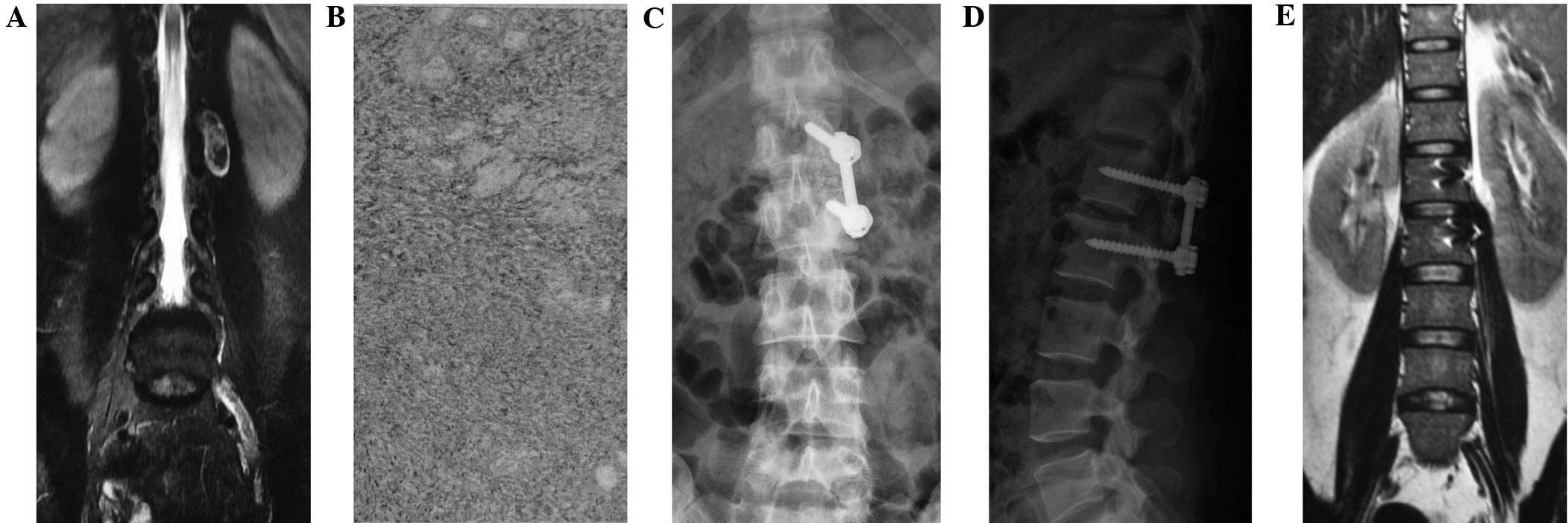
The type I method comprised a posterior midline
approach semi-laminectomy with tumor resection and internal
fixation with pedicle screws (Fig.
1), according to the preoperative diagnosis and analysis. The
C-arm was used to position the lesion segment, and then used as the
center to separate the subperiosteal paraspinal muscles, in order
to expose the segmental spinous process affected by the tumor and
the semi-lamina. The semi-laminectomy was performed towards the
lesion side to resect the ipsilateral medial side of the facet
process, in order to fully reveal the intervertebral foramen around
the tumor. The longitudinal dura was subsequently incised along the
para-tumor midline under the microscope and suspended bilaterally
to expose the subdural space. When fully exposed, the boundaries of
the tumor, nerves or spinal cord were clear, facilitating their
identification. Normally, the arachnoid layer was closely attached
to the tumor; this arachnoid layer exhibited a porous structure,
independently surrounding the dorsal and ventral nerve roots.
Subsequent to cutting this arachnoid layer, the tumor could be
separated along the gaps between the tumor and pericoccygeal nerve,
and the intraspinal part of tumor could then be resected. This was
then tracked outwards along the affected vertebral foramen, and the
section of the tumor between the vertebral foramen and the spinal
canal could be gradually stripped and removed. If the exposure was
limited, the operating table could be rotated towards the lesion
side to facilitate the microscopic illumination and tumor exposure
until the whole tumor was resected. Following complete resection,
the dura was tightly sutured. The muscle or fascia tissues were
used to cover the foramen and dural defects to prevent the leakage
of cerebrospinal fluid. When lumbar tumors were found to be wrapped
in coccygeal nerve or conus terminalis, the nerve roots were
carefully separated to provide sufficient exposure to prevent
recurrence.
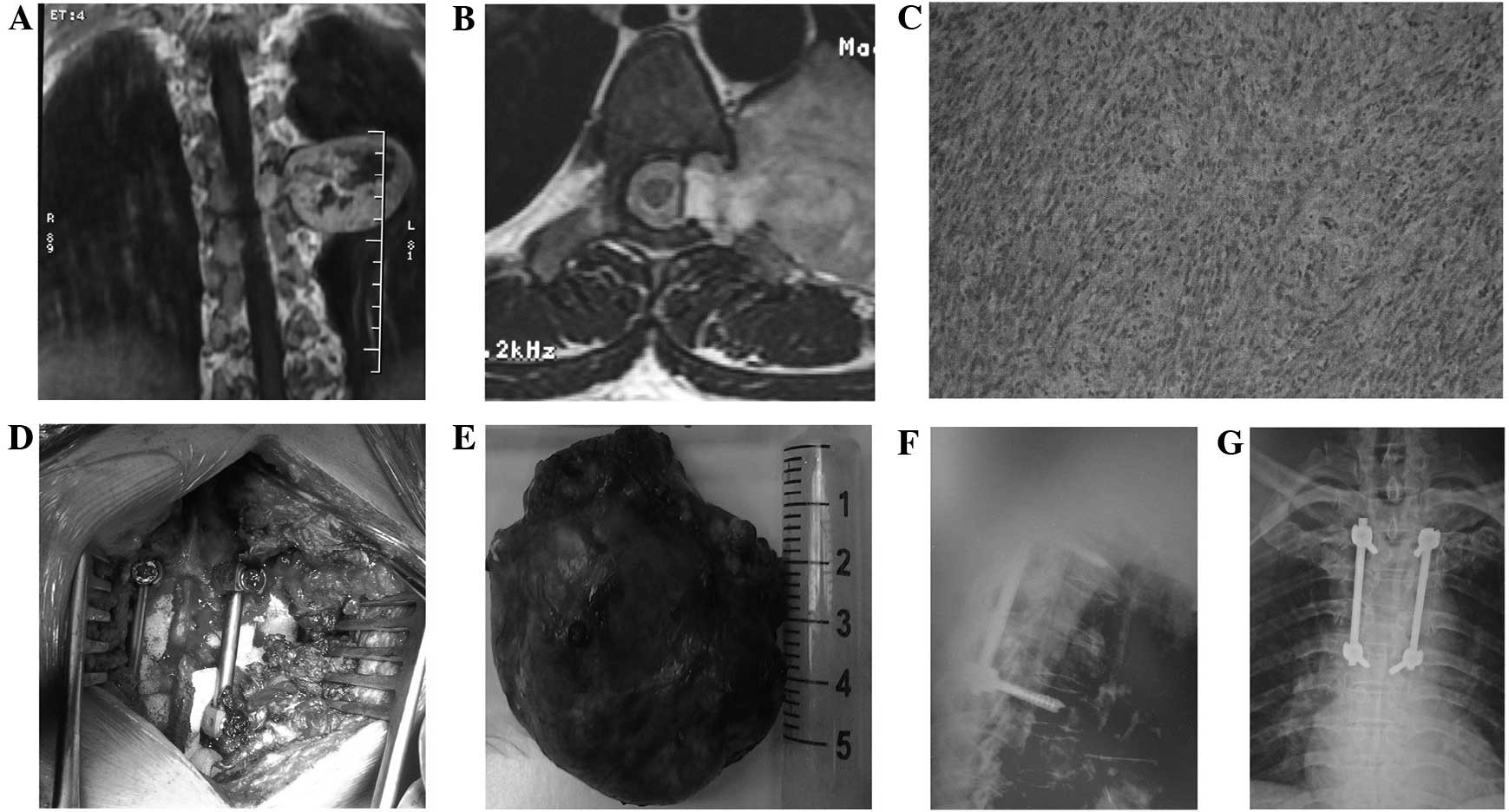
The type II method comprised a posterior midline
approach laminectomy with tumor resection and internal fixation
with pedicle screws (Fig. 2).
Monitored by a C-arm machine, the corresponding segments were
subjected to pedicle screw implantation, laminectomy or
lesion-ipsilateral laminectomy, lesion-ipsilateral facet resection
and transverse process resection to reveal the whole tumor.
Resection of the intraspinal section of the tumor was performed in
an identical manner to that described in the type I method. The
surgery was performed in the following sequence: Resection of the
intraenvelope part, and separation and resection of the tumor
envelope, in order to avoid damaging the coccygeal nerve or
surrounding vital structures. If the dura was opened, it was
sutured tightly. Following the resection of the tumor, the
posterolateral spinal graft fusion and internal fixation of the
pedicle screw were performed.
Postoperative treatment
Following surgery, antibiotics were routinely
administered intravenously, prior to being withdrawn 48 h later.
The patients with dural incision were additionally intravenously
administered 80 mg methylprednisolone and asked to adopt a supine
position postoperatively, while patients with spinal meninges
incision were requested to lie in the Trendelenburg position.
Patients with internal fixation were able to perform ambulation 2
days later, while the patients without internal fixation could
start functional exercise in bed 1 week later according to the
situation. X-rays were carried out after 1 month to decide the
ambulatory status according to the bone fusion conditions (15). Patients were asked to wear a
conventional hard brace for 3 months of activities and to attend
X-ray and MRI appointments every 3 months. Follow-ups were
performed for between 6 months and 3 years.
Evaluation of efficacies
The clinical status of each patient was evaluated
pre- and postoperatively using the visual analog scale (VAS; 0 mm,
no pain; 100 mm, worst imaginable pain), the Oswestry Disability
Index (ODI) (16) and the Japanese
Orthopedic Association (JOA) scores (17). Functional improvement was expressed
by the recovery rate of the JOA scores. Pre- and postoperative
neurological recovery was graded according to Frankel
classification (18).
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. The results are
presented as the mean ± standard deviation or range. The Student's
t-test was used to perform the statistical comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Surgical methods
Thoracic and lumbar schwannomas were surgically
resected using the aforementioned two methods (types I and II), and
the patients were divided into two groups according to the
different methods. In group 1 (posterior midline approach
semi-laminectomy with tumor resection and internal fixation with
pedicle screws), which included 24 cases, the intraoperative blood
loss was 300±20 ml, the duration of surgery was 2.4±1.3 h and the
length of hospitalization was 8.4±5.8 days. In group 2 (posterior
midline approach laminectomy with tumor resection and internal
fixation with pedicle screws), which included 26 cases, the
intraoperative blood loss was 561±210 ml, the duration of surgery
was 2.8±1.8 h and the length of hospitalization was 10.4±5.8 days
(P<0.05), intraoperative blood loss was 561±210 ml (P<0.05),
the duration of surgery was 2.8±1.8 h (P<0.05) and the length of
hospitalization was 10.4±5.8 days (P<0.05). Significant
differences were detected between the two groups in length of
hospitalization, intraoperative blood loss and the duration of
surgery. The postoperative follow-up time was between 6 months and
3 years. During follow-up, no patients reported an exacerbation of
the condition, and significant improvements in the radicular pain
and spinal function were noted. The numbness and hyperesthesia were
relieved to different extents, and the postoperative MRI revealed
no recurrence during the 6-month to 3-year follow-up.
Pre- and postoperative clinical
status
Prior to surgery, the mean VAS score was 31.5 in
group 1 and 43.1 in group 2. Three months after surgery, the score
decreased to 16.3 (P<0.001) in group 1 and 17.0 (P<0.001) in
group 2, and at the final follow-up, the score had improved further
to 12.1 (P<0.001) in group 1 and 12.4 (P<0.001) in group 2
(Table I). For each postoperative
time-point, the difference between the two groups was
insignificant. The mean preoperative ODI score in group 1 was 49.2,
compared with 22.4 (P<0.001) at the 3-month follow-up and 15.2
(P<0.001) at the final follow-up. The mean preoperative ODI
score in group 2 was 62.8, compared with 23.8 (P<0.001) at 3
months postoperatively and 16.9 (P<0.001) at the final follow-up
(Table I). At each follow-up
time-point, no significant difference was found between the two
groups. The JOA scores for groups 1 and 2 were as follows: 14.4 and
9.4 prior to surgery, 19.6 and 18.2 at 3 months after surgery and
22.7 and 21.6 at the final follow-up, respectively (Table I). No significant difference was
found between the two groups postoperatively.
 | Table I.VAS, ODI and JOA scores of the two
groups. |
Table I.
VAS, ODI and JOA scores of the two
groups.
| Score | Group 1 | Group 2 | P-value |
|---|
| VAS |
|
|
|
|
Pre-operative | 31.5±14.1 | 43.1±13.9 | <0.05 |
| 3 months
postoperative | 16.3±9.2 | 17.0±10.3 | NS |
| Final
follow-up | 12.1±7.4 | 12.4±7.6 | NS |
| ODI |
|
|
|
|
Pre-operative | 49.2±15.2 | 62.8±11.8 | <0.05 |
| 3 months
postoperative | 22.4±9.3 | 23.8±9.1 | NS |
| Final
follow-up | 15.2±9.3 | 16.9±9.5 | NS |
| JOA |
|
|
|
|
Pre-operative | 14.4±2.0 | 9.4±3.0 | <0.05 |
| 3 months
postoperative | 19.6±3.3 | 18.2±3.1 | NS |
| Final
follow-up | 22.7±3.2 | 21.6±2.4 | NS |
At the final follow-up, 45 patients (86.5%)
considered their outcome to be excellent or good, 6 patients fair
and 1 patient unchanged. The difference between the two groups was
not statistically significant at the final follow-up. Frankel
classification of neural function prior to surgery was as follows:
Frankel B, 9 patients; Frankel C, 16 patients; Frankel D, 15
patients; and Frankel E, 12 patients. Neural function after
follow-up was as follows: Frankel C, 1 patient; Frankel D, 6
patients; and Frankel E, 45 patients. The results for the
postoperative Frankel classification are shown in Table II.
 | Table II.Improvements in the postoperative
Frankel classification. |
Table II.
Improvements in the postoperative
Frankel classification.
|
|
| Postoperative Frankel
classification |
|---|
|
|
|
|
|---|
| Preoperative Frankel
classification | Cases (n) | A (n) | B (n) | C (n) | D (n) | E (n) |
|---|
| A | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 9 | 0 | 0 | 1 | 3 | 5 |
| C | 16 | 0 | 0 | 0 | 3 | 13 |
| D | 15 | 0 | 0 | 0 | 0 | 15 |
| E | 12 | 0 | 0 | 0 | 0 | 12 |
Discussion
Due to the biological characteristics of
schwannomas, such as expansive growth, the surrounding important
blood vessels, organs and spinal motor nerve roots can be
dislodged; thus, surgical methods for the resection of the tumor
capsule and the exposure and resection of the tumor should be
selected to avoid damage to the surrounding organs and nerves
(19–21). The posterior resection of the
thoracic and lumbar schwannomas in the present study was found to
be relatively safe, and the intraoperative internal fixation
maximally retained the spinal biological stability; consequently,
the patients rehabilitated more rapidly, and the duration of
surgery and the length of hospitalization were shortened.
Postoperative cerebrospinal fluid leakage occurred in 13 patients
in this study, but this was cured through lumbar subarachnoid
catheterization.
The thoracic and lumbar schwannomas in this study
were surgically resected using two methods: The type I method was
used in 24 cases and the type II method was used in 26 cases.
Although patients in group 1 had a shorter duration of surgery and
less intraoperative blood loss, the cases in group 2 involved a
larger intraspinal tumor mass; therefore, the tumor tissues in the
group 2 cases had to be fully exposed in order avoid damage to the
spinal cord and nerve roots and prevent tumor residues. The
surgical approach should be preoperatively selected according to
the positions of the tumor and spinal nerve roots, so that surgical
field exposure can be ensured. During thoracic schwannoma
resection, the transverse process and ribs can be resected if
necessary, and the maintenance of long-term stability should be
ensured through precise intraoperative internal fixation and bone
grafting. In total, 50 patients with thoracic schwannoma in this
study underwent internal fixation, and the follow-up found no
occurrence of spinal instability.
By analyzing the cases in the present study we found
that methods I and II could both be used to completely resect CSSs.
It was often difficult to reach larger schwannomas beside the
cervical vertebra through the posterior approach, due to the
abundance of the anterior cervical neurovascular structures, such
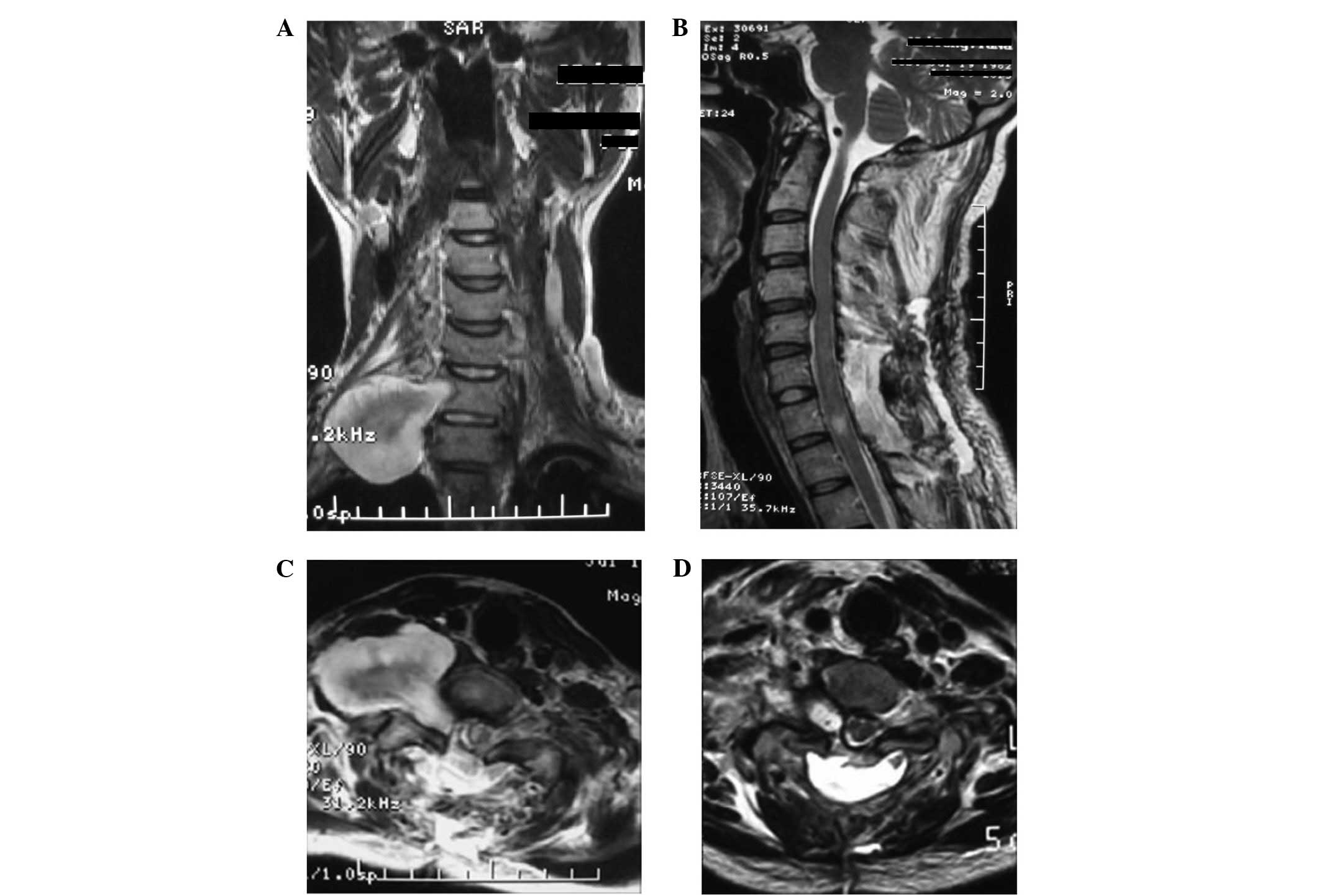
as the brachial plexus nerves. In this study, 2 cases of
dumbbell-shaped giant cervical schwannoma was resected using an
anterior-posterior combined approach. First, C6 and 7 posterior
laminar decompression was performed, followed by partial resection
of the schwannoma and the affected right C7 nerve root. The
dumbbell-shaped giant schwannoma body was then resected through the
anterior approach, due to the adherence of the tumor to the upper
and lower trunks of the brachial plexus nerve. The right brachial
plexus nerve function gradually returned to preoperative levels 2
days later. Since only the cervical 6 and 7 laminae were resected,
the cervical stability was affected minimally; therefore, internal
fixation was not performed (Fig.
3).
Most schwannomas are located on the dorsal or
lateral side of the spinal cord, and can be easily viewed when the
dura is opened through the posterior approach. The tumors can thus
be clearly intraoperatively separated, disconnected and isolated,
and the capsule wall surface can be subjected to electric
coagulation to shrink the tumor volume. The proximal and distal
nerve roots of the tumor should be cut off to enable the removal of
the whole tumor. For giant tumors, the intracapsular resection
should be performed first to realize the intracapsular
decompression, and the nerve roots of tumor origin should be cut
off. The majority of dumbbell-shaped thoracolumbar tumors can be
exposed by expanding the intervertebral foramen, and the whole
facet resection of one side can increase the paraspinal exposure,
thus enabling access to any extension of the tumor through the
intervertebral foramen. Using this technique, all dissections can
be performed on the tumor surface, the nerves can be identified
from the proximal end and the nerve damage can be further reduced.
Preserving the nerve roots at the expense of failing to perform a
thorough tumor resection can lead to a higher local recurrence rate
and a correspondingly increased necessity for further surgery
(22).
Findings regarding the consequence of retaining or
resecting the nerve roots affected by the tumor are not currently
consistent (22,23). In the majority of cases in which the
nerve roots were resected, no permanent or serious dysfunction
occurred, although a few cases exhibited slight sensory
disturbances (22). In the present
study, 52 affected nerve roots were resected without any obvious
aggravation of sensory impairment. A possible explanation for the
finding of this study is that the affected nerve roots gradually
became deactivated with the long-term disease duration, leading to
the adjacent spinal nerve roots compensatorily covering the surface
area that was previously dominated by the affected nerve roots. We
therefore propose that resecting the necessary affected nerve roots
is likely to cause only mild sensory loss. In the 52 patients in
the present study, the affected nerve roots were resected according
to the situation; only 3 patients experienced skin numbness in the
corresponding segmental areas following resection, and the skin
numbness was improved significantly 1 year later. No permanent
sensory impairments were caused.
The 52 patients in this study achieved satisfactory
results following surgery; therefore, we believe that a detailed
preoperative evaluation based on the preoperative MRI could be used
to select the appropriate approach for surgery. This selection of
the optimal surgical method could facilitate the complete removal
of the tumor while causing a reduction in trauma.
References
|
1
|
Gottfried ON, Binning MJ and Schmidt MH:
Surgical approaches to spinal schwannomas. Contemp Neurosurg.
27:1–9. 2005. View Article : Google Scholar
|
|
2
|
Lee SE, Chung CK and Kim HJ:
Intramedullary schwannomas: Long-term outcomes of ten operated
cases. J Neurooncol. 113:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colosimo C, Cerase A, Denaro L, Maira G
and Greco R: Magnetic resonance imaging of intramedullary spinal
cold schwannomas: Report of two case and review of the literature.
J Neurosurg. 99(1 Suppl): 114–117. 2003.PubMed/NCBI
|
|
4
|
Nakamura M, Iwanami A, Tsuji O, Hosogane
N, Watanabe K, Tsuji T, Ishii K, Toyama Y, Chiba K and Matsumoto M:
Long-term surgical outcomes of cervical dumbbell neurinomas. J
Orthop Sci. 18:8–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuoka H, Itoh Y, Numazawa S, Tomii M,
Watanabe K, Hirano Y and Nakagawa H: Recapping hemilaminoplasty for
spinal surgical disorders using ultrasonic bone curette. Surg
Neurol Int. 3:702012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu D, Ba Z, Huang Y, Zhao W, Shen B and
Kan H: Totally cystic schwannoma of the lumbar spine. Orthopedics.
36:e679–e682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozawa H, Kokubun S, Aizawa T, Hoshikawa T
and Kawahara C: Spinal dumbbell tumors: An analysis of a series of
118 cases. J Neurosurg Spine. 7:587–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pollo C, Richard A and De Preux J:
Resection of a retroperitoneal schwannoma by a combined approach.
Neurochirurgie. 50:53–56. 2004.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jankowski R, Szmeja J, Nowak S, Sokół B
and Blok T: Giant schwannoma of the lumbar spine. A case report.
Neurol Neurochir Pol. 44:91–95. 2010.PubMed/NCBI
|
|
10
|
Canbay S, Hasturk AE, Basmaci M, Erten F
and Harman F: Management of thoracal and lumbar schwannomas using a
unilateral approach without instability: An analysis of 15 cases.
Asian Spine J. 6:43–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiedemayer H, Sandalcioglu IE, Aalders M,
Wiedemayer H, Floerke M and Stolke D: Reconstruction of the laminar
roof with miniplates for a posterior approach in intraspinal
surgery: Technical consideration and critical evaluation of
follow-up results. Spine (Phila Pa 1976). 29:E333–E342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGirt MJ, Garcés-Ambrossi GL, Parker SL,
Sciubba DM, Bydon A, Wolinksy JP, Gokaslan ZL, Jallo G and Witham
TF: Short-term progressive spinal deformity following laminoplasty
versus laminectomy for resection of intradural spinal tumors:
Analysis of 238 patients. Neurosurgery. 66:1005–1012. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yimaz MR, Bek S, Bekmezci T, Gökduman C
and Solak AS: Malignant triton tumor of the lumbar spine. Spine
(Phila Pa 1976). 29:E399–E401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: World Health Organization Classification of Tumours
of Soft Tissue and Bone (4th). Lyon: IARC Press. 2013.
|
|
15
|
Theodosopoulos T, Stafyla VK, Tsiantoula
P, Yiallourou A, Marinis A, Kondi-Pafitis A, Chatziioannou A,
Boviatsis E and Voros D: Special problems encountering surgical
management of large retroperitoneal schwannomas. World J Surg
Oncol. 6:1072008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fairbank JC, Couper J and Davies JB: The
Oswestry Low Back Pain Questionnaire. Physiotherapy.
1980.66:271–273. PubMed/NCBI
|
|
17
|
Izumida S and Inoue S: 1986.Assessment of
treatment for low back pain. J Jpn Orthop Assoc. 60:391–394
|
|
18
|
Frankel HL, Hancock DO, Hyslop G, Melzak
J, Michaelis LS, Ungar GH, Vernon JD and Walsh JJ: The value of
postural reduction in the initial management of closed injuries of
the spine with paraplegia and tetraplegia. I. Paraplegia.
7:179–192. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravnik J, Potrc S, Kavalar R, Ravnik M,
Zakotnik B and Bunc G: Dumbbell synovial sarcoma of the
thoracolumbar spine: A case report. Spine (Phila Pa 1976).
34:E363–E366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yokoi H, Arakawa A, Inoshita A and Ikeda
K: Novel use of a Weerda laryngoscope for transoral excision of a
cervical ganglioneuroma: A case report. J Med Case Rep. 6:882012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Celii P, Trillò G and Ferrante L: Spinal
extradural schwannoma. J Neurosurg Spine. 2:447–456. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schulthiess R and Gullotta G: Resection of
relevant nerve roots in surgery of spinal neurinomas without
persisting neurological deficit. Acta Neurochir (Wien). 122:91–96.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seppälä MT, Hatia MJ, Sankila RJ,
Jääskeläinen JE and Heiskanen O: Long-term outcome after removal of
spine schwannoma: A clinicopathological study of 187 cases. J
Neurosurg. 83:621–626. 1995. View Article : Google Scholar : PubMed/NCBI
|