Introduction
The temporomandibular joint (TMJ) is a complex
skeletal structure that is essential for jaw movement in mammals
(1). The TMJ is comprised of
multiple tissues, including the mandibular condyle, glenoid fossa,
a fibrocartilaginous articular disc located between these two bones
that divides the joint cavity into two compartments, and a variety
of associated tendons and muscles (2,3).
Furthermore, the tendons of the pterygoid muscle and various
surrounding ligaments are associated with the TMJ (4). Disorders of the TMJ affect numerous
individuals, and may lead to difficulty in chewing function and
chronic myofacial pain (5).
The embryonic development of the TMJ shares a
similar development process across various mammalian species, and
differs significantly from that of other synovial joints (6). In contrast with the formation of long
bone joints by cleavage or segmentation within a single skeletal
condensation, the TMJ develops from two distinct and widely
separated mesenchymal condensations; the glenoid fossa blastema and
the condylar blastema (7). The
glenoid fossa blastema derives from the otic capsule and undergoes
transmembranous ossification (8,9). The
condylar blastema develops rapidly towards a rectangular cell
condensation located lateral to and above Meckel's cartilage, and
is subsequently attached medially by the lateral pterygoid muscle
as a result of rapid cellular proliferation (4). Simultaneously, the condylar blastema
develops out of the secondary condyle cartilage of the mandible and
forms a bone via endochondral ossification, subsequently extending
in an anterior/medial direction and capping the condylar blastema
(10,11).
The intervening mesenchyme between the glenoid fossa
and condylar blastemas condenses, prior to the separation of the
two primordia of the TMJ by an articular disc (12). As the condyle develops continuously
upward approaching the glenoid fossa, the mesenchyme differentiates
into layers of fibrous tissues, ultimately separating the upper and
lower synovial cavities (13). In
addition to cellular proliferation and differentiation, the condyle
anlage is configured into a typical secondary cartilage and is
superficially covered with a thick layer of flat fibrous cells
(14,15). The glenoid fossa exhibits
intramembranous ossification, which corresponds to condyle
differentiation (8). During the
development of the skeletal elements of the TMJ, morphogenesis of
the soft tissues surrounding the joint continues (16). Following the completion of
cavitation, the TMJ exhibits marked ossification and growth of the
condyle and glenoid fossa, functional remodeling of the articular
disc via an avascular event and substantial condensation (17). Furthermore, enclosure of the joint
bone prominences and the articular disc through the joint capsule
occurs, and the development of the muscles and ligaments proceeds
(18,19). Although the structure and function of
the TMJ has been well characterized (12–19), the
molecular and cellular mechanisms underlying its formation and
development remain unclear. Therefore, the aim of the present study
clarify was to investigate the processes underlying the formation
and development of the mouse TMJ using various immunohistochemical
staining methods.
Materials and methods
Animal preparation
A total of 30 pregnant BALB/c mice, aged 2 months
and weighing 33.46±3.57 g, were purchased from the Shanghai Slack
Laboratory Animal Co., Ltd. (Shanghai, China). Mice were housed
under a 12-h light-dark cycle at 22±1°C and with a humidity of
56±5%, with 5 mice per cage, and received a 0.3% sodium diet, ad
libitum. Animal experiments were conducted in accordance with
the Guide for Care and Use of Laboratory Animals of Fujian
University of Traditional Chinese Medicine (Fuzhou, China). The age
of the mouse embryo was defined based on the ejection of the
vaginal plug from the mother. The day of the morning following this
occurrence was defined as embryonic day 0.5 (E0.5). Pregnant,
embryonic and post-natal mice were sacrificed using carbon dioxide
(cage size, 7×11×5 inches; flow rate, 1.3l/min), according to the
Guide for the Care and Use of Animals (8th edition, 2011). Embryos
were subsequently extracted in phosphate-buffered saline (PBS; pH
7.4; GE Healthcare Life Sciences, Logan, UT, USA). Following
collection, embryonic mice heads were stored in ice-cold
phosphate-buffered saline. Heads collected on days E13.5, E14.5 and
E15.5 were fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis,
MO, USA) and PBS overnight at 4°C, while heads collected on days
E16.5, E17.5, E18.5 and post-natal days 0 (P0), P7, P14, P21 and
P180 were fixed and decalcified using a Surgipath Decalcifier I
(Leica Microsystems GmbH, Wetzlar, Germany) for various periods of
time depending on the age of the mouse (2).
Histological analyses
Mouse heads were dehydrated using a graded ethanol
series, cleared with xylene, embedded in paraffin (both
Sigma-Aldrich) and sectioned at 10 µm using a Leica RM2235
microtome (Leica Microsystems GmbH). For histological analysis of
the TMJ, serial sections were subjected to standard
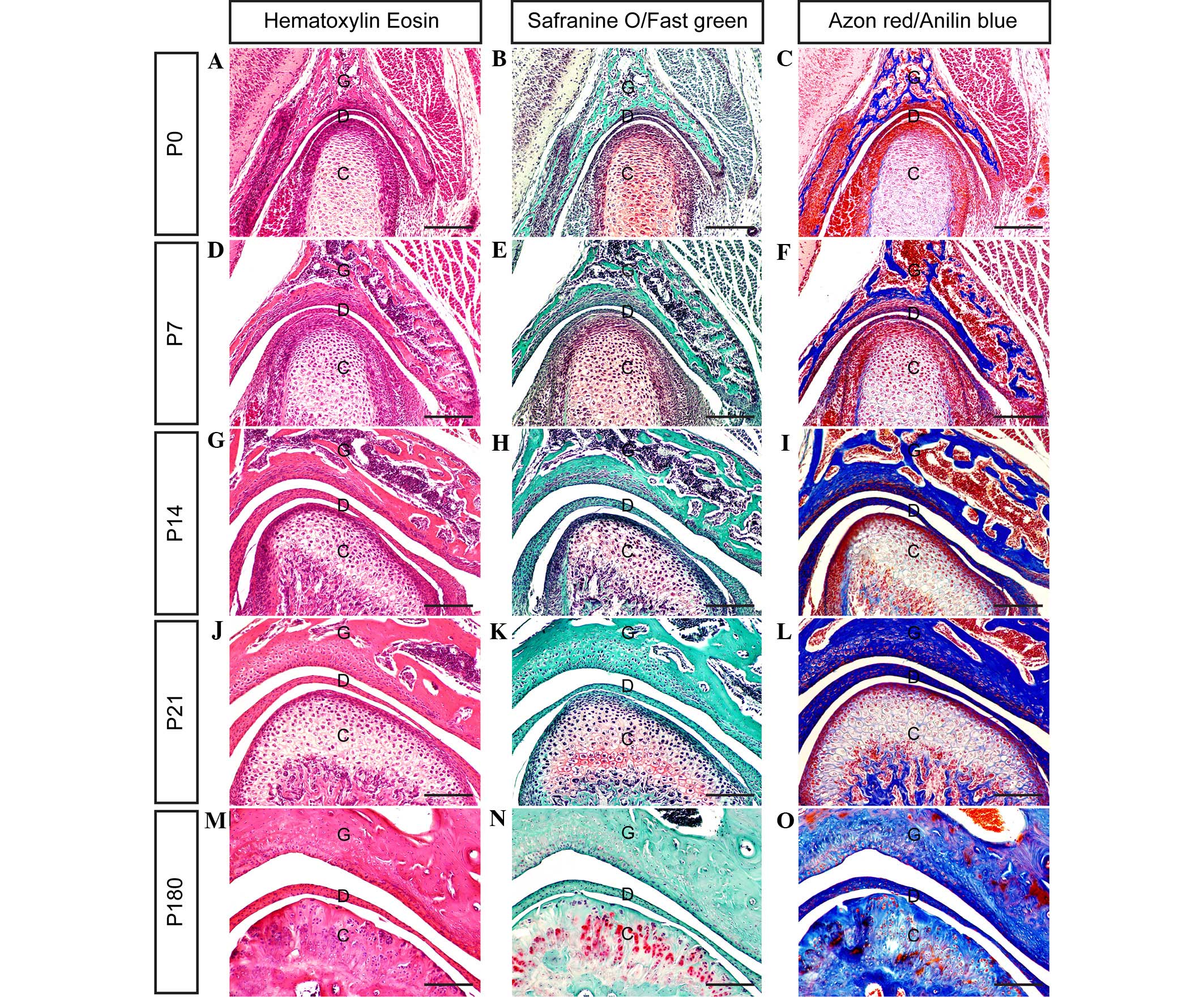
hematoxylin-eosin (HE) (20),
Safranin O-Fast green (21) and Azon
red/Anilin blue staining (2,9) (all Sigma-Aldrich), according to
previously described methods. HE staining is essential for
recognizing various tissue types and morphological alterations, and
it facilitates visualization of a range of nuclear, cytoplasmic and
extracellular matrix (ECM) features. Hematoxylin stains nucleic
acids a deep blue-purple color, whereas eosin is pink and stains
proteins non-specifically. Therefore, in a typical tissue, nuclei
are stained blue and the cytoplasm and ECM components exhibit
varying degrees of pink staining. Safranin O/Fast green staining is
frequently used to stain articular cartilage. Safranin O is a
cationic dye that stains acidic proteoglycans present in cartilage
tissues, which are indicators of cell chondrogenesis, with an
orange-red color. Fast green, which is the contrast stain of
Safranin O, is a sulfate group containing acidic substrate that
binds to protein amino groups, thereby staining non-collagen sites.
Azon red/Anilin blue is a double stain containing Alizarin (bone)
and alcian blue (cartilage) compounds which is used to identify
cartilage and bone.
In situ hybridization and
immunohistochemistry
Mouse heads were embedded in paraffin and sectioned
at 10 µm for in situ and 8 µm for immunohistochemical
analysis. Non-radioactive riboprobes, including SOX-9 [nucleotides
(nt), 116–856; NM_011448), RUNX2 (nt, 3183–3812; NM_001146038),
Osterix (nt, 40–1727; NM_130458) and IHH (nt, 897–1954; NM_010544),
were synthesized using in vitro transcription labeling with
Digoxigenin-11-UTP, according to the manufacturer's instructions
(Roche Diagnostics GmbH, Mannheim, Germany) (9). Briefly, 10 µm sections were pretreated
with 10 µg/ml proteinase K (Sigma-Aldrich), fixed in 4%
paraformaldehyde, hybridized with riboprobes at 50°C for 16 h, and
washed with 2X standard saline citrate (Sigma-Aldrich) containing
50% formamide (Sigma-Aldrich) at 50°C. Maleic acid buffer and
blocking reagent (Roche Diagnostics GmbH) were added for blocking
and antibody washing steps. Signals were developed with BM purple
alkaline phosphatase substrate (Sigma-Aldrich). Immunohistochemical
staining was performed according to the manufacturer's instructions
(2). Paraffin sections were
deparaffinized and rehydrated in a descending series of alcohol
dilutions, heated in 10 mM sodium citrate buffer (pH 6.0;
Sigma-Aldrich) at 100°C for 20 min for antigen retrieval, then
cooled to room temperature. The sections were blocked with goat
serum (1:10; Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad,
CA, USA), and incubated for 15 min at room temperature.
Subsequently, the sections were incubated with polyclonal
antibodies against runt-related transcription factor 2 (RUNX2;
1:1,000; ab76956), sex determining region Y-box 9 (SOX-9; 1:500;
ab26414), collagen I (1:500; ab34710), collagen II (1:200;
ab53047), aggrecan (1:500; ab36861), matrix metalloproteinase-9
(MMP-9; 1:300; ab38898), MMP-13 (1:50; ab75606) and Indian hedgehog
homolog (IHH; 1:200; ab39634) from Abcam (Cambridge, MA, USA)
overnight at 4°C. The slides were then washed three times using
PBS; and incubated with a biotinylated horseradish peroxidase goat
anti-rabbit secondary antibody (1:1,000; A-11034; Invitrogen;
Thermo Fisher Scientific, Inc.) for 20 min at 37°C. The slides were
washed three times following incubation with the secondary antibody
using PBS. Immunolabeling was visualized with 0.05%
diaminobenzidine (Invitrogen; Thermo Fisher Scientific, Inc.) in
PBS for 5 min at room temperature, then slides were rinsed for 10
min under running tap water. The morphology of
immunohistochemically stained TMJ sections was observed using a
BH-2 light microscope (Olympus Corporation, Tokyo, Japan).
In situ zymography and
4′6′-diamidino-2-phenylindole dihydrochloride (DAPI) staining
Heads of the P0 mice were immersed in zinc-based
fixative containing 36.7 mM ZnCl, 27.3 mM
ZnAc2·2H2O and 0.63 mM calcium acetate in 0.1
mM Tris (pH 7.4; Sigma-Aldrich) for 2 h at room temperature,
dehydrated using 15 and 30% sucrose at 4°C overnight, frozen in
optimal cutting temperature compound (Sakura Finetek USA, Inc.,
Torrance, CA, USA), then sectioned at 10 µm using a Leica CM1850
cryostat (Leica Microsystems GmBH). DQ-gelatin (1 mg/ml; E12055;
Molecular Probes; Thermo Fisher Scientific, Inc., Grand Island, NY,
USA) was used as the substrate at 1:10 dilution in the in
situ zymography buffer, according to the manufacturers
instructions (2). Next, 100 µl
mixture was applied to the sections, which were incubated at 37°C
for 2 h in a dark humid chamber. In order to visualize the DNA in
the frozen sections, sections were incubated with 100 ng/ml DAPI
(Sigma-Aldrich) in PBS for 30 min. The gelatinolytic activity and
DAPI-stained TMJ frozen sections were observed as green
fluorescence using a Axioskop 50 fluorescence microscope (Carl
Zeiss AG, Oberkochen, Germany).
Bromodeoxyuridine (BrdU) labeling for
cell proliferation, and terminal deoxynucleotidyl transferase dUTP
nick-end labeling (TUNEL) for cell apoptosis assays
Six pregnant mice were injected with labeling
reagent (1.5 ml/100 g) from a BrdU Labeling Detection Kit II (Roche
Diagnostics GmbH) for 2 h prior to sacrifice using carbon dioxide
euthanasia. Subsequently, three heads of E13.5-E18.5 were fixed in
Carnoy's fixative containing ethanol absolute (Merck & Co.,
Inc., Whitehouse Station, NJ, USA), chloroform (Sigma-Aldrich) and
glacial acetic acid (Merck & Co., Inc.) at a ratio of 6:3:1.
The heads were then ethanol-dehydrated, paraffin-embedded and
sectioned at 5 µm. Sections were subjected to immunodetection for
analysis of cellular proliferation, according to the manufacturers
instructions (9). Heads of
E13.5-E18.5 were subjected for cell proliferation assay, and three
adjacent sections from each head were observed for BrdU labeling.
Three heads of the P0 mice were sectioned at 5 µm, and subjected to
immunodetection for cell apoptosis analysis, according to the
manufacturer's instructions (2).
Apoptosis was measured using an in situ Cell Death Detection
Kit (Roche Diagnostics GmbH), and three adjacent sections from each
head were observed using TUNEL labeling.
Statistical analysis
Statistical analyses were performed using the
Student's t-test with SPSS software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). Data were expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
Formation of the TMJ during the
embryonic development in mice
In mice, the mesenchymal condensation of the condyle
develops at day E13.5 (Fig. 1A–C);
however, the glenoid fossa primordium does not form by this time
point. The location of the glenoid fossa is indicated by the
surrounding lateral pterygoid muscle, trigeminal ganglion and
Meckel's cartilage. In serial coronal sections, the posterior
portion of the condylar primordium appears to be isolated from the
mandible, while its anterior portion is closely connected to the
primordium of the mandible.
By day E14.5 (Fig.
1D–F), the condylar primordium is rearranged and shaped into a
reverse positioned cone, with a tip pointed toward the mandibular
bone. Medially, the lateral pterygoid muscle runs into the
cone-shaped condyle. Above the condyle, the zygomatic process and
squamosal plate combine to form a concave-shaped glenoid fossa
primordium.
By day E15.5 (Fig.
1G–I), the cartilage that has developed in the center of the
condyle is surrounded by a thick layer of perichondrium. At the
apex of condyle development, numerous layers of cells from the
surface of the condyle are compacted into a strip, representing the
articular disc anlage. Furthermore, the glenoid fossa becomes
enlarged, and begins to ossify in the center of the fossa. However,
the space between the developing condyle and glenoid fossa remains
wide.
At day E16.5 (Fig.
1J–L) a cleft, generated by the upward movement of loose
mesenchymal cells in the articular space, forms between the glenoid
fossa and articular disc, indicating the initiation of cavitation.
At this point there is no indication of the formation of lower
cavitation; however, the strip of disc anlage is separated
completely from the condyle surface. Furthermore, the cells of the
medial area of the disc anlage combine with the lateral pterygoid
muscle cells. The rapidly developing condyle differentiates to form
various layers, including fibrous, polymorphic, flattened and
hypertrophic cell layers. The rapid growth of the condyle and
glenoid fossa leads to the narrowing of the articular space.
At day E17.5 (Fig.
1M–O), the formation of a definite articular disc with numerous
tightened layers of fiber results in the formation of a narrow
cavity between the condyle and articular disc. The lateral fiber
tangle of the articular disc, with the tendon fibers derived from
the masseter muscle, and its medial fibers combine with the tendon
fibers extended from the lateral pterygoid muscle.
At day E18.5 (Fig.
1P–R), all of the major anatomical features of the TMJ are
present. A definite and compact articular disc is clearly present,
separating the upper and lower synovial cavities. As shown in
Fig. 2, the present results clarify
the stages of TMJ development from immaturity to maturity in
post-natal mice.
Regulation of TMJ development by
ECM
The ECM appears to serve crucial functions in TMJ
development, in addition to those served by transcription and
growth factors. TMJ structure-function associations are described
in terms of its most abundant ECM components, including collagen,
glycosaminoglycans and proteoglycans (22,23).
These ECM components are critical for resistance against
compressive forces and for maintaining the tensile properties of
the tissue, and are increased or decreased in a number of cartilage
pathologies and across the different stages of a single pathology
(24).
Collagen belongs to a family of structural proteins
with distinct chemical compositions, morphologies and functions
(25). To date, >27 types of
collagen have been classified (25).
Certain types of collagen, including collagen I, II and IV, are
capable of aggregating molecules to form the articular disc,
condyle and glenoid fossa of the TMJ (26,27).
Aggrecan, the best understood of the lecticans, is fundamental for
cartilage function and skeletal development (28,29). The
biological function of aggrecan is the ability to bind hyaluronan
in order to provide the basis for the viscoelastic properties
necessary for load distribution over the articular surface
(28). In addition, aggrecans are
crucially involved in skeletal development, as a key molecular
component of the cartilage templates in the process of endochondral
ossification (29). In order to
investigate the alternation of ECM during TMJ development in the
present study, protein expression levels of collagen I, collagen II
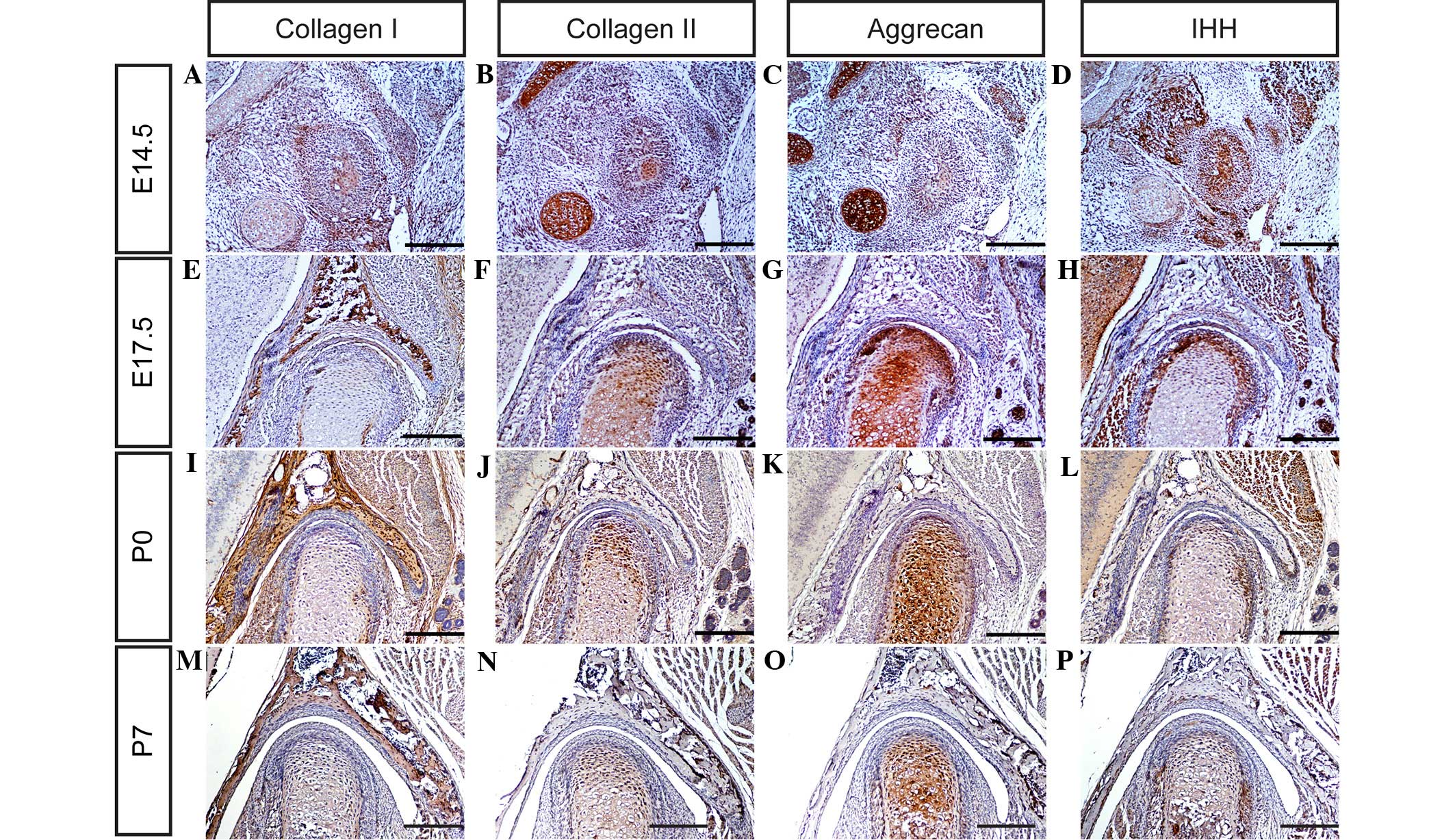
and aggrecan in the TMJ were determined using immunohistochemistry.
It was observed that collagen I, collagen II and aggrecan are
expressed weakly in the TMJ at day E14.5 (Fig. 3A–C), and markedly by days E17.5, P0
and P7 (Fig. 3E–G, I-K and M-O,
respectively). These observations suggest that all the major ECM
components of the TMJ are well-formed by these time points, and may
therefore serve critical functions in TMJ development in mice.
Regulation of TMJ development by
transcription factors
During TMJ development, a large number of
transcription factors and growth factors have been implicated in
the development of primary cartilage and endochondral ossification,
including SOX-9, RUNX2, IHH and Osterix (30–32). The
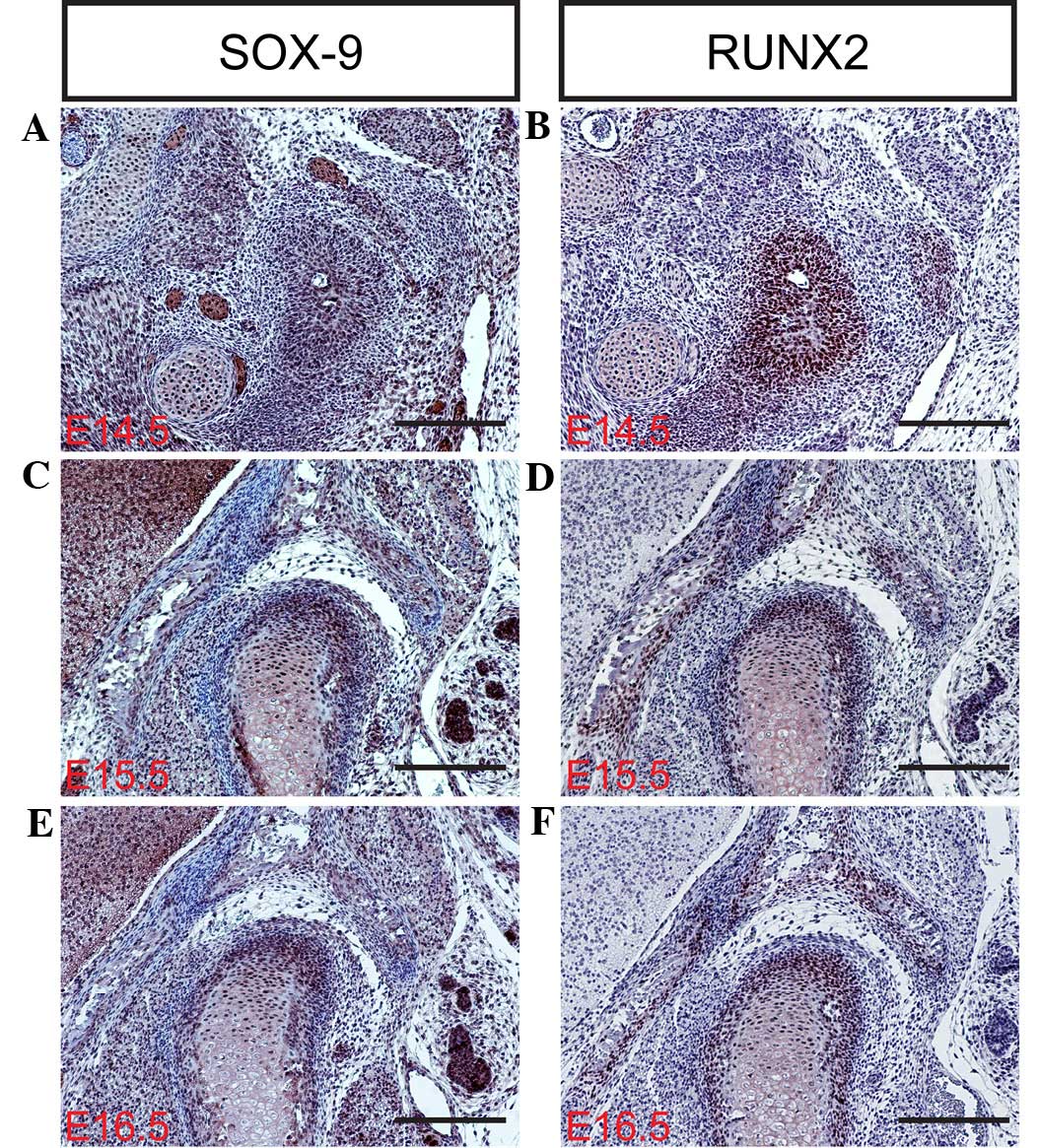
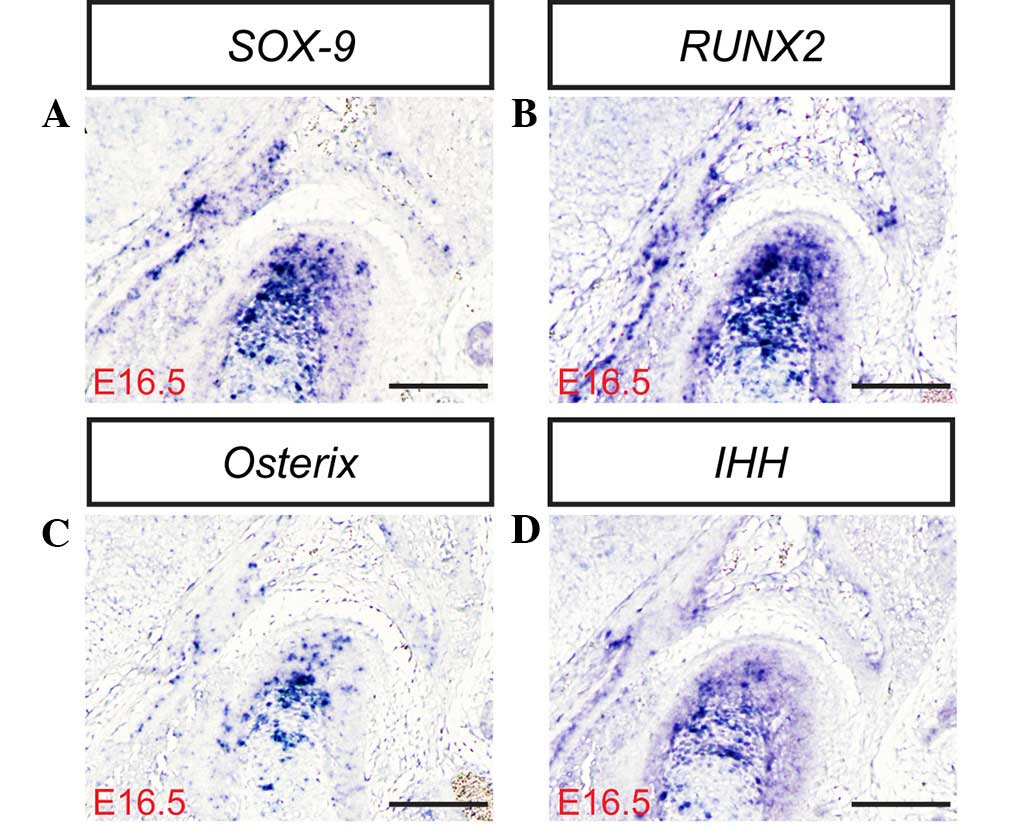
transcription factor SOX-9 is expressed in mesenchymal
condensations and proliferating chondrocytes of the condyle.
Targeted inactivation of SOX-9 in neural crest cells results in the
complete ablation of the condyle due to the failed formation of the
condylar blastema (30–33). In contrast with SOX-9, the RUNX2
transcription factor appears to serve a distinct function in the
development of the condylar and primary cartilage. RUNX2 is
initially expressed in the mesenchymal condensation of the condyle,
and subsequently in the newly formed cartilage and bone collar,
which is similar to its expression during long bone formation
(9). Osterix, a novel
zinc-finger-containing transcription factor, is another essential
factor for osteoblast differentiation and functions downstream of
RUNX2 in the lineage of osteoblast differentiation. RUNX2 and
Osterix are essential for primary cartilage or bone formation.
RUNX2 is primarily involved in the differentiation of mesenchymal
cells into preosteoblasts (34),
whereas Osterix is associated with the differentiation of
preosteoblasts into mature osteoblasts (30). IHH, a member of the Hedgehog family,
serves a pivotal role in long bone development and digit joint
formation (24). IHH regulates
chondrocyte proliferation and the rate of chondrocyte hypertrophy
in cooperation with parathyroid hormone-related protein (PTHrP) in
the periarticular region (1,35). In mice, IHH expression is initially
detected in the condylar condensation, and becomes marked in the
condylar cartilage, in addition to PTHrP expression, at day E15.5
(36,37), suggesting that the IHH-PTHrP
regulatory loop operates in the developing condyle. In the present
study, the mRNA and protein expression levels of SOX-9, RUNX2, IHH
and Osterix (Fig. 3Q) were
determined in the mouse TMJ using in situ hybridization
and/or immunohistochemistry (Figs. 3D,
H, L and P; 4A–F; and 5A–D). The results of the present study
indicate that SOX-9, RUNX2, IHH and Osterix are expressed in the
TMJ at different stages of development. These results are
consistent with those of previous studies (2,6,7,9), which
indicate that transcription factors are important regulators of the
formation of the TMJ during the various stages of mouse embryonic
development.
Regulation of TMJ development by cell
proliferation and apoptosis
Cells proliferate throughout ontogenesis to
facilitate tissue remodeling and renewal, and the repair of damaged
areas during wound healing (6). Cell
proliferation involves replication by normal complete division
cycles, which are sequential and associated with the cell cycle
(7,9). Cell proliferation may be evaluated
using a labeling index (such as the percentage of cells in S phase)
following the administration of DNA precursor labels such as
tritiated thymidine or BrdU, or by immunostaining the endogenous
cell replication marker proliferating cell nuclear antigen
(38,39). In order to investigate differences in
cellular proliferation during TMJ development, a BrdU assay was
used to evaluate cellular proliferation in the condyle and glenoid
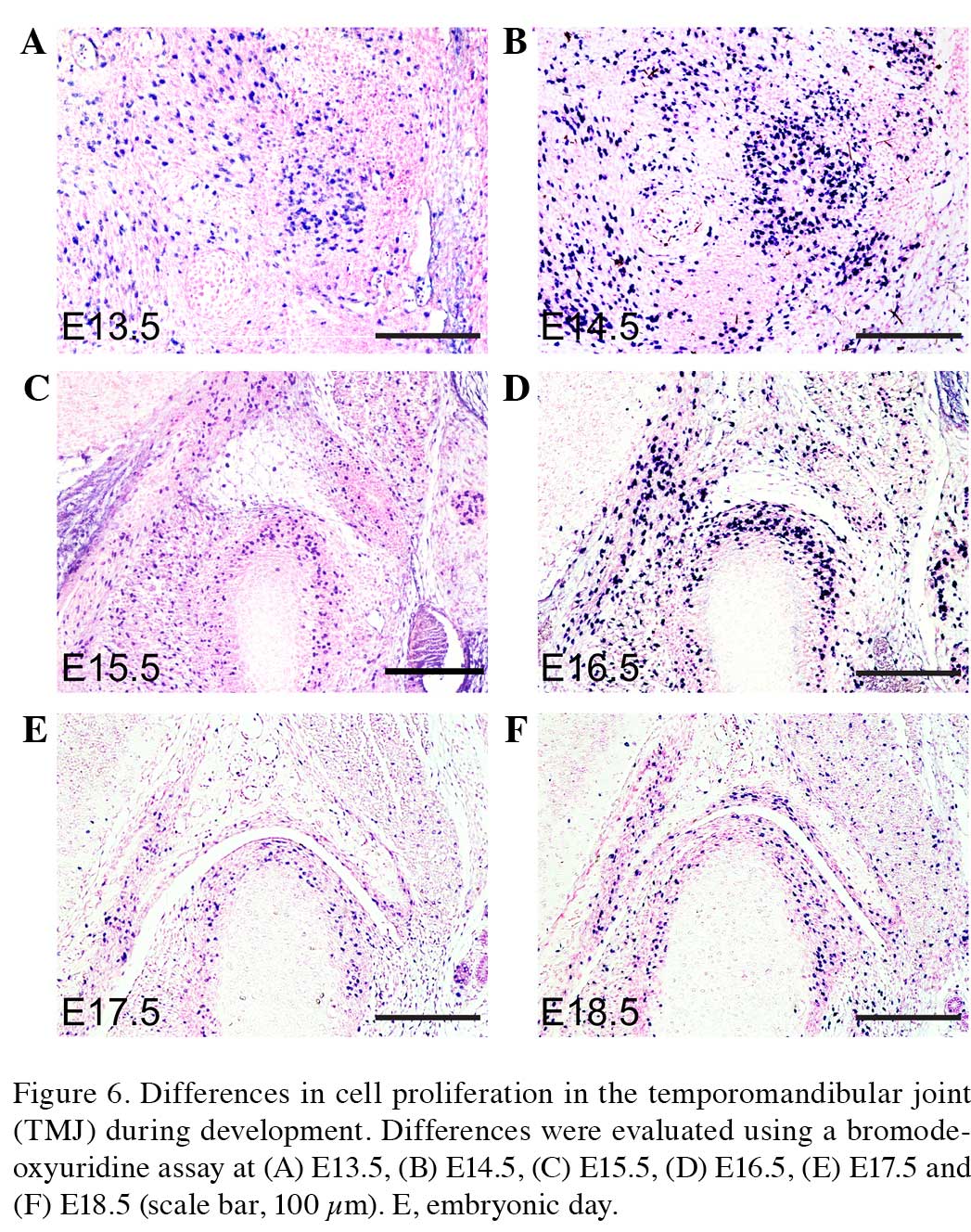
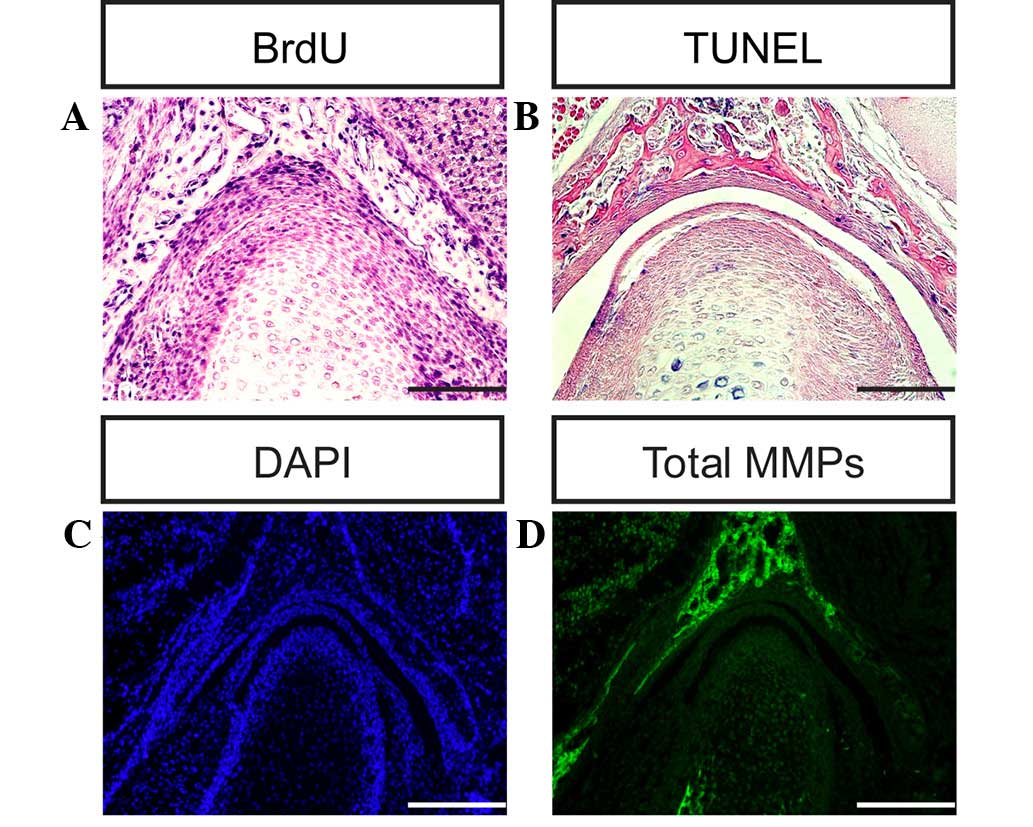
fossa of the TMJ in the present study (Figs. 6A–F and 7A). The cells of the condyle and glenoid
fossa undergo rapid cellular proliferation during embryonic
development. The ratio of cell proliferation exhibits a gradually
declining trend between days E13.5 and P0. Apoptosis, or programmed
cell death, is a form of traumatic cell death that results from
acute cellular injury (40).
Apoptosis typically confers a number of advantages during an
organism's lifecycle compared with unprogrammed cell death,
including cell shrinkage, blebbing, chromatin condensation, nuclear
fragmentation (41) and chromosomal
DNA fragmentation. Apoptosis is responsible for the growth
cartilage and endochondral ossification of condyle and glenoid
fossa (Fig. 7B and C) (6,7).
Regulation of TMJ development by MMP
activity
A well-coordinated remodeling of the ECM is a
pre-requisite for TMJ development and homeostasis (42). The turnover of collagens and
proteoglycans proceeds via various pathways, and may be dependent
on endocytic processes, or involve extracellular reactions
exclusively (43). ECM degradation
requires the action of secreted or membrane MMPs, which are
zinc-dependent endopeptidases capable of degrading ECM components,
including collagens and proteoglycans (44). MMP-9 degrades denatured collagen II,
which is initially cleaved by activated MMP-1, and additionally
degrades noncartilage collagens such as collagen IV and collagen V
(45). Notably, MMP-9 is able to
degrade the core protein aggrecan (46,47).
MMP-13 exhibits a substrate preference for degrading fibrillar
collagens, including collagen II; however, MMP-13 is also able to
degrade aggrecan, a hydrodynamic molecule in the cartilage
(48,49). The results of the present study
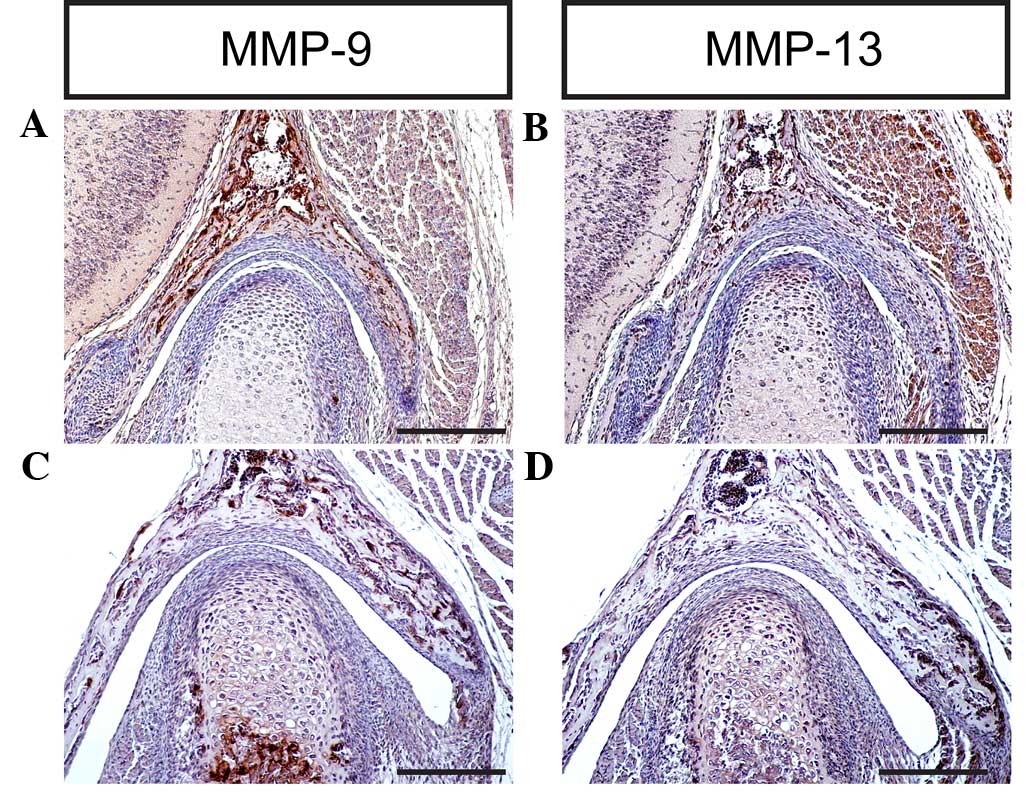
indicate that MMP-9 and MMP-13 are expressed in the TMJ at
different stages, which is consistent with previous studies
(2,6,7,9), implying that MMPs are key regulators of
cartilage growth and endochondral ossification during TMJ formation
and development in mice (Figs. 7D
and 8A–D).
In the present study, the developmental processes of
the mouse TMJ were systemically investigated using HE, Safranin
O-Fast green and Azon red/Anilin blue staining methods, and a large
number of transcription factors and growth factors have been
implicated in the regulation of TMJ development, such as Sox9,
Runx2, Ihh, TGF-β2 and EGF (1–3,50). Using in situ hybridization,
immunohistochemistry and in situ zymography methods, the
expression levels of SOX-9, RUNX2, IHH, collagen I, collagen II,
aggrecan, MMP-9 and MMP-13 were evaluated during the process of TMJ
formation and development. In addition, the cellular proliferation
and apoptosis activity during TMJ formation and development in mice
were analyzed using BrdU and TUNEL assays, respectively.
Generally, the development of the TMJ in mammals has
not been extensively studied. A number of factors in TMJ
development remain unclear, including the profile of the signaling
molecules that mediate the interactions of TMJ tissue during the
developmental process, and the effects of the glenoid fossa on
articular disc formation. Future studies are required, which may
employ transgenic mice models in addition to experimental
embryology, molecular biology and cell biology approaches, to
elucidate the cellular and molecular mechanisms of TMJ
development.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81202645 and
81230087), the Program for New Century Excellent Talents in Fujian
Province University (no. JA14150), and Fujian University of
Traditional Chinese Medicine Tube Project (no. X2015021).
References
|
1
|
Purcell P, Joo BW, Hu JK, Tran PV,
Calicchio ML, O'Connell DJ, Maas RL and Tabin CJ: Temporomandibular
joint formation requires two distinct hedgehog-dependent steps.
Proc Natl Acad Sci USA. 106:18297–18302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Liu H, Gu S, Liu C, Sun C, Zheng Y
and Chen Y: Replacing Shox2 with human SHOX leads to congenital
disc degeneration of the temporomandibular joint in mice. Cell
Tissue Res. 355:345–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owtad P, Park JH, Shen G, Potres Z and
Darendeliler MA: The biology of TMJ growth modification, A review.
J Dent Res. 92:315–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bravetti P, Membre H, El Haddioui A,
Gérard H, Fyard JP, Mahler P and Gaudy JF: Histological study of
the human temporo-mandibular joint and its surrounding muscles.
Surg Radiol Anat. 26:371–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soydan SS, Deniz K, Uckan S, Unal AD and
Tutuncu NB: Is the incidence of temporomandibular disorder
increased in polycystic ovary syndrome? Br J Oral Maxillofac Surg.
52:822–826. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Liang W, Ye H, Weng X, Liu F, Lin P
and Liu X: Overexpression of Indian hedgehog partially rescues
short stature homeobox 2-overexpression-associated congenital
dysplasia of the temporomandibular joint in mice. Mol Med Rep.
12:4157–4164. 2015.PubMed/NCBI
|
|
7
|
Li X, Liang W, Ye H, Weng X, Liu F and Liu
X: Overexpression of Shox2 leads to congenital dysplasia of the
temporomandibular joint in mice. Int J Mol Sci. 15:13135–13150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Liu C, Rohr J, Liu H, He F, Yu J,
Sun C, Li L, Gu S and Chen Y: Tissue interaction is required for
glenoid fossa development during temporomandibular joint formation.
Dev Dyn. 240:2466–2473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu S, Wei N, Yu L, Fei J and Chen Y:
Shox2-deficiency leads to dysplasia and ankylosis of the
temporomandibular joint in mice. Mech Dev. 125:729–742. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Velasco Mérida JR, Rodríguez Vázquez JF,
De la Cuadra Blanco C, Campos López R, Sánchez M and Mérida Velasco
JA: Development of the mandibular condylar cartilage in human
specimens of 10-15 weeks' gestation. J Anat. 214:56–64. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yokohama-Tamaki T, Maeda T, Tanaka TS and
Shibata S: Functional analysis of CTRP3/cartducin in Meckel's
cartilage and developing condylar cartilage in the fetal mouse
mandible. J Anat. 218:517–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Gong Z, Li J, Meng Q, Fang W and
Long X: The pilot study of fibrin with temporomandibular joint
derived synovial stem cells in repairing TMJ disc perforation.
Biomed Res Int. 2014:4540212014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu S, Wu W, Liu C, Yang L, Sun C, Ye W, Li
X, Chen J, Long F and Chen Y: BMPRIA mediated signaling is
essential for temporomandibular joint development in mice. PLoS
One. 9:e1010002014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vinkka-Puhakka H and Thesleff I:
Initiation of secondary cartilage in the mandible of the Syrian
hamster in the absence of muscle function. Arch Oral Biol.
38:49–54. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kenzaki K, Tsuchikawa K and Kuwahara T: An
immunohistochemical study on the localization of type II collagen
in the developing mouse mandibular condyle. Okajimas Folia Anat
Jpn. 88:49–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loughner B, Miller J, Broumand V and
Cooper B: The development of strains, forces and nociceptor
activity in retrodiscal tissues of the temporomandibular joint of
male and female goats.
|
|
17
|
Owtad P, Potres Z, Shen G, Petocz P and
Darendeliler MA: A histochemical study on condylar cartilage and
glenoid fossa during mandibular advancement. Angle Orthod.
81:270–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Kaneko S and Soma K: Glenoid fossa
responses to mandibular lateral shift in growing rats. Angle
Orthod. 77:660–667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaki Y, Tsuchikawa K, Nagasawa T and
Hiroyasu K: Embryological study of the development of the rat
temporomandibular joint, Highlighting the development of the
glenoid fossa. Odontology. 93:30–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Zhang M, Chen YJ, Zhou Q, Wang YJ
and Liu J: Psychological stress alters microstructure of the
mandibular condyle in rats. Physiol Behav 110-111. 129–139. 2013.
View Article : Google Scholar
|
|
21
|
Ricks ML, Farrell JT, Falk DJ, Holt DW,
Rees M, Carr J, Williams T, Nichols BA, Bridgewater LC, Reynolds
PR, et al: Osteoarthritis in temporomandibular joint of Col2a1
mutant mice. Arch Oral Biol. 58:1092–1099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Willard VP, Arzi B and Athanasiou KA: The
attachments of the temporomandibular joint disc, A biochemical and
histological investigation. Arch Oral Biol. 57:599–606. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu Z, Feng J, Shibata T, Hu J and Zhang Z:
Type II collagen and aggrecan mRNA expression by in situ
hybridization in rabbit temporomandibular joint posterior
attachment following disc displacement. Arch Oral Biol. 48:55–62.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garnero P, Rousseau JC and Delmas PD:
Molecular basis and clinical use of biochemical markers of bone,
cartilage, and synovium in joint diseases. Arthritis Rheum.
43:953–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuber M, Zia F, Zia KM, Tabasum S, Salman
M and Sultan N: Collagen based polyurethanes-A review of recent
advances and perspective. Int J Biol Macromol. 80:366–374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Natiella JR, Burch L, Fries KM, Upton LG
and Edsberg LE: Analysis of the collagen I and fibronectin of
temporomandibular joint synovial fluid and discs. J Oral Maxillofac
Surg. 67:105–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Q, Opstelten D, Samman N and Tideman
H: Experimentally induced unilateral tooth loss, Expression of type
II collagen in temporomandibular joint cartilage. J Oral Maxillofac
Surg. 61:1054–1060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aspberg A: The different roles of aggrecan
interaction domains. J Histochem Cytochem. 60:987–996. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu Z, Jin X, Feng J, Shibata T, Hu J, Zhan
J and Hu Y: Type II collagen and aggrecan mRNA expressions in
rabbit condyle following disc displacement. J Oral Rehabil.
32:254–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mori-Akiyama Y, Akiyama H, Rowitch DH and
de Crombrugghe B: Sox9 is required for determination of the
chondrogenic cell lineage in the cranial neural crest. Proc Natl
Acad Sci USA. 100:9360–9365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibata S, Suda N, Suzuki S, Fukuoka H and
Yamashita Y: An in situ hybridization study of Runx2, Osterix, and
Sox9 at the onset of condylar cartilage formation in fetal mouse
mandible. J Anat. 208:169–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ochiai T, Shibukawa Y, Nagayama M, Mundy
C, Yasuda T, Okabe T, Shimono K, Kanyama M, Hasegawa H, Maeda Y, et
al: Indian hedgehog roles in post-natal TMJ development and
organization. J Dent Res. 89:349–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu MJ, Gu ZY and Sun W: Effects of
hydrostatic pressure on cytoskeleton and BMP-2, TGF-beta, SOX-9
production in rat temporomandibular synovial fibroblasts.
Osteoarthritis Cartilage. 16:41–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jing J, Hinton RJ, Jing Y, Liu Y, Zhou X
and Feng JQ: Osterix couples chondrogenesis and osteogenesis in
post-natal condylar growth. J Dent Res. 93:1014–1021. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishizuka Y, Shibukawa Y, Nagayama M,
Decker R, Kinumatsu T, Saito A, Pacifici M and Koyama E: TMJ
degeneration in SAMP8 mice is accompanied by deranged Ihh
signaling. J Dent Res. 93:281–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shibukawa Y, Young B, Wu C, Yamada S, Long
F, Pacifici M and Koyama E: Temporomandibular joint formation and
condyle growth require Indian hedgehog signaling. Dev Dyn.
236:426–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suda N, Shibata S, Yamazaki K, Kuroda T,
Senior PV, Beck F and Hammond VE: Parathyroid hormone-related
protein regulates proliferation of condylar hypertrophic
chondrocytes. J Bone Miner Res. 14:1838–1847. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hume WJ and Thompson J: Double labelling
of cells with tritiated thymidine and bromodeoxyuridine reveals a
circadian rhythm-dependent variation in duration of DNA synthesis
and S phase flux rates in rodent oral epithelium. Cell Tissue
Kinet. 23:313–323. 1990.PubMed/NCBI
|
|
39
|
Herring SW, Decker JD, Liu ZJ and Ma T:
Temporomandibular joint in miniature pigs, Anatomy, cell
replication, and relation to loading. Anat Rec. 266:152–166. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuda S, Mishima K, Yoshimura Y, Hatta T
and Otani H: Apoptosis in the development of the temporomandibular
joint. Anat Embryol (Berl). 196:383–391. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sato I, Uneno R, Miwa Y and Sunohara M:
Distribution of tenascin-C and tenascin-X, apoptotic and
proliferating cells in postnatal soft-diet rat temporomandibular
joint (TMJ). Ann Anat. 188:127–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wattanachai T, Yonemitsu I, Kaneko S and
Soma K: Functional lateral shift of the mandible effects on the
expression of ECM in rat temporomandibular cartilage. Angle Orthod.
79:652–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao Y, Liu S, Huang J, Guo W, Chen J,
Zhang L, Zhao B, Peng J, Wang A, Wang Y, Xu W, Lu S, Yuan M and Guo
Q: The ECM-cell interaction of cartilage extracellular matrix on
chondrocytes. Biomed Res Int. 2014:6484592014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Okada Y: Matrix-degrading
metalloproteinases and their roles in joint destruction. Mod
Rheumatol. 10:121–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Almeida LE, Caporal K, Ambros V, Azevedo
M, Noronha L, Leonardi R and Trevilatto PC: Immunohistochemical
expression of matrix metalloprotease-2 and matrix metalloprotease-9
in the disks of patients with temporomandibular joint dysfunction.
J Oral Pathol Med. 44:75–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malemud CJ: Matrix metalloproteinases: R
ole in skeletal development and growth plate disorders. Front
Biosci. 11:1702–1715. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases, Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stickens D, Behonick DJ, Ortega N, Heyer
B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P and
Werb Z: Altered endochondral bone development in matrix
metalloproteinase 13-deficient mice. Development. 131:5883–5895.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Inada M, Wang Y, Byrne MH, Rahman MU,
Miyaura C, López-Otín C and Krane SM: Critical roles for
collagenase-3 (Mmp13) in development of growth plate cartilage and
in endochondral ossification. Proc Natl Acad Sci USA.
101:17192–17197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oka K, Oka S, Sasaki T, Ito Y, Bringas P
Jr, Nonaka K and Chai Y: The role of TGF-beta signaling in
regulating chondrogenesis and osteogenesis during mandibular
development. Dev Biol. 303:391–404. 2007. View Article : Google Scholar : PubMed/NCBI
|