Introduction
Spinal cord injury (SCI) is a catastrophic injury
that has a high disability rate and effects feeling, movement and
autonomic functions, and has a number of serious secondary
life-threatening complications (1).
As society develops, the incidence of SCI increases (2). Osteoporosis (OP) is a primary
complication of SCI, affecting primarily patients below the level
of injury, and increases the probability of fracturing lower limbs
(3). The pathological mechanism that
causes secondary OP is currently uncertain, and there is,
therefore, a lack of systematic and efficient treatment.
According to traditional Chinese medicine theories,
the main pathogenesis of OP resulting from SCI is marrow deficiency
and kidney asthenia (4,5). Therefore, kidney reinforcing and
marrow-beneficial (KRMB) traditional Chinese medicines may be
prescribed (6). A number of studies
have demonstrated that KRMB can significantly increase bone mineral
density (BMD) in rats without ovaries, improve bone tissue, and
promote the growth and differentiation of osteoblast (OB) cells
(7). In addition, it has been
reported that KRMB intervention in rats (weight, 28.125 g/kg;
gavage 1 h later; 25% concentration) promotes bone marrow stromal
cell proliferation and osteogenic differentiation (8).
The present study aims to investigate the effect of
KRMB on kidney and bone marrow metabolism-related factor expression
following SCI, and to study the pathomechanism of SCI and OP. This
may lay the foundation for the prevention and treatment of OP
resulting from SCI using traditional Chinese medicine.
Materials and methods
Animals
A total of 240 pathogen-free Sprague-Dawley rats
(weight, 200±20 g; 120 male and 120 female; age, 3 months) were
obtained from the Experimental Animal Center of Chinese Medical
Sciences University (Shenyang, China). Animals were maintained in
grouped-housing in a temperature (20–25°C) and humidity (40–55%)
controlled environment, with a 12 light/dark cycle and ad
libitum access to food and water.
Preparation of reagents
KRMB was prepared as a suspension containing 10 g
lyophilized powder of fresh antler (Animal Husbandry of Shunda,
Jilin, China), 5 g oyster powder and 15 g Epimedium
brevicornum decoction (both purchased from Jinzhou pharmacy
market, Jinzhou, China) and refrigerated at 4°C.
Drug administration
Rats were allocated at random into the following
groups (n=4 per group): Normal; sham + KRMB; normal + KRMB; SCI +
KRMB; and SCI model groups. The KRMB dose was 28.125 g/kg body
weight (suspension volume, 1 ml), and the normal group was
administered an equivalent volume of saline for 10 weeks, once a
day, by gavage. Following the experiments, rats were sacrificed by
an anesthetic overdose (10% chloral hydrate; 300 mg/kg; China
Shanghai National Medicine Group Corporation, Shanghai, China).
Surgical procedure
Rats were fasted for 24 h, with free access to
water, prior to the operation. Rats were anesthetized with an
intraperitoneal injection of 10% chloral hydrate (300 mg/kg) and
laid in the prone position. The thoracic T9-11 vertebra was marked
as the center, and an aseptic operation along the spinous process
was performed. A longitudinal incision (~4 cm) was made, blunt
separation stripped the fascia, fat and paravertebral muscle, bite
T7-9 spinous process and a laminectomy was performed on the T8
vertebrae in order to fully expose the back and sides of the dural
sac. The endorachis and spinal cord were entirely transected using
a 10 scalpel (Jinzhou Medical Instruments Factory, Changchun,
China), and rat hind limbs convulsed several times prior to flaccid
paralysis. Next, a 2-mm incision was made through spinal cord
tissues below the T10 spinal segment, and a gelfoam sponge (Jinzhou
Medical Instruments Factory) was placed at the broken ends of
spinal cord. The endorachis was opened by incision and covered with
a fasciai patch, and sutured layer by layer. The sham operation cut
off the spinous process and lamina to expose the spinal cord, but
there was no resection to the spinal cord (9–11). At 1,
2, 4, 6, 8 and 10 weeks after the surgery, 8 rats were randomly
selected from each group and specimens were collected.
Once blood was collected from the rats, eliminated
attachment of the muscle fascia, retained periosteum, taken the
left hind limb, flushed by the physiological saline and preserved
at −80°C. BMD expression in the rat distal femur was detected using
Lunar Prodigy dual-energy X-ray absorptiometry (GE Healthcare Life
Sciences, Chalfont, UK). Post-injury motor behavior is assessed
using the Basso, Beattie and Bresnahan (BBB) locomotor scale method
(12). Rats are placed on an
operating table to observe the hip joint, knee joint, ankle joint,
the movement and coordination of walking, the torso and the tail.
Rats were analyzed for 4 min between 8 and 9 p.m. following
micturition. The average score of the rats' hind legs was then
recorded using a single blind method.
Detection of bone
gamma-carboxyglutamic-acid containing protein (BGP) expression
Rats were anesthetized with an intraperitoneal
injection of 10% chloral hydrate (300 mg/kg), blood samples were
extracted from the abdominal aortic separation and blood serum was
separated by centrifugation for 10 min at 3,000 × g, and preserved
at −80°C. BGP expression was detected using an Osteocalcin (BGP)
enzyme linked immunosorbent assay (ABE20719; R&D Systems China
Co., Ltd., Shanghai, China) according to the manufacturer's
instructions.
Detection of hepcidin mRNA
expression
Hepcidin mRNA expression in the liver was determined
using an RNA polymerase chain reaction (PCR) kit (AMV) (version
3.0; Takara Biotechnology Co., Inc., Dalian, China) and a 600 bp
DNA ladder marker (Beijing TransGen Biotech Co., Ltd., Beijing,
China). Primer Premier version 5.0 software (Premier Biosoft
International, Palo Alto, CA, USA) was used to design PCR primer
sequences for β-actin and hepcidin, based on the rat β-actin and
hepcidin gene sequences registered in GenBank (http://www.ncbi.nlm.nih.gov/genbank/).
Subsequently, ~100 mg fresh rat liver tissue was homogenized in
liquid nitrogen. Total RNA (1 µl) extraction was performed using
TRIzol reagent (Takara Biotechnology Co., Inc.) according to the
manufacturer's instructions. A reverse transcription-PCR (RT-PCR)
kit (Takara Biotechnology Co., Inc.) was used to synthesize the
first strand of cDNA. The reaction conditions were as follows: 42°C
for 30 min, 99°C for 5 min, 5°C for 5 min followed by preservation
at 4°C. The reaction mixture (total volume, 10 µl) contained 2 µl
MgCl2, 1 µl 10X RT Buffer, 3.75 µl RNase Free
dH2O, 1 µl dNTP mixture (10 mM), 0.25 µl RNase
inhibitor, 0.5 µl AMV Reverse Transcriptase, 0.5 µl Oligo dT and 1
µl RNA. The hepcidin PCR protocol began with initial denaturation
for 5 min at 94°C, followed by amplification for 30 sec at 94°C for
30 cycles, and 10 min at 72°C. The β-actin PCR protocol began with
initial denaturation for 40 sec at 72°C, followed by an
amplification program for 30 s at 55°C. PCR products were
electrophoresed using a 3-µl DNA ladder marker (DL600) with
molecular weight standards (100 bp) as the reference.
Electrophoresis was performed at 90 V for 1 h. The primer sequences
were as follows: β-Actin forward, 5′-GGAGATTACTGCCCTGGCTCCTA-3′ and
reverse, 5′-GACTCATCGTACTCCTGCTTGCTG-3′; and hepcidin forward,
5′-GAAGGCAAGATGGCACTAAGCA-3′ and reverse,
5′-TCTCGTCTGTTGCCGGAGATAG-3′. A gel imaging analysis system (Alpha
Innotech ChemiImager 5500; BioSurplus, Inc., San Diego, CA, USA)
was used to analyze the PCR results.
Determination of bone sialoprotein
(BSP)
Frozen tibia tissues (100 mg) were lysed in 1 ml
ice-cold homogenization radioimmunopreciptation assay buffer (Wuhan
Boster Biological Technology, Ltd., Wuhan, Boster) containing a
protease inhibitor. The homogenates were centrifuged at 2,580 × g
for 5 min at 4°C and the supernatant was collected. The protein
content was determined using a bicinchoninic acid assay (Beijing
Tiandz Biological Technology Co., Ltd.), ensuring that each 20 µl
contained 50 µg protein, and samples were stored at −20°C. Each
sample (6.08–6.44 µg) was separated using 10% sodium dodecyl
sulfonate gel electrophoresis (60–120 V; 2 h) and transferred to a
polyvinylidene difluoride membrane, then semi dry transfer
membranes (both purchased from Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China) were blocked with 5% calf
serum albumin (Beyotime Biotechnology Co., Ltd., Shanghai, China)
at room temperature for 1 h. Following this, the membranes were
washed 3 times for 5 min with Tris-buffered saline with 0.05%
Tween-20 (TBST) (Wuhan Boster Biological Technology, Ltd.). Then,
the membranes were incubated overnight at 4°C in blotting buffer
containing a primary rabbit polyclonal antibody (1:300; BA2329;
Wuhan Boster Biological Technology, Ltd.). Membranes were then
washed using Tris-buffered saline (Wuhan Boster Biological
Technology, Ltd.) and incubated for 1 h at room temperature in
blotting buffer containing poly-horseradish peroxidase-conjugated
streptavidin mouse anti-goat IgG antibody (1:3,000; bs-0294Ms;
Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China).
Membranes were then washed again with TBST for 5 min and a western
blot was performed using 5-bromo-4-chloro-3-indolyl phosphate and
p-nitroblue tetrazolium reagent (Beijing Tiandz Biological
Technology Co., Ltd.) and β-actin (42 kD; Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China) was employed as the
internal reference. Membranes were scanned using a Gel Imaging
Analyzer (Six One Instrument Factory, Beijing, China), absorbance
was measured using a D8 quasi dual beam UV-visible
spectrophotometer [Runqee (Shanghai) Instrument Technology Co.,
Ltd., Shanghai, China] and the absorbance of protein bands was
analyzed using gel analysis software (Image J; version 1.47;
National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analysis of the
experimental data. Data are presented as the mean ± standard
deviation. The electrophoresis results were determined using
FluorChem software, version 2.0 (Gene Genus; Syngene, Frederick,
MD, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
BBB score
During the experimental process, a total of 32 rats
succumbed to mortality; 18 rats succumbed to mortality in the SCI
model group, and 14 rats succumbed to mortality in the SCI + KRMB
group, according to the experimental conditions of strict
supplements. As presented in Table
I, the BBB scores in the SCI and SCI + KRMB groups were
significantly reduced in comparison to the normal group (P<0.01)
at 1, 3, 5 and 7 days after the operation. These results suggest
that the SCI model was successfully established.
 | Table I.BBB scores (n=8 per group). |
Table I.
BBB scores (n=8 per group).
|
| BBB score (days) |
|---|
|
|
|
|---|
| Group | 1 | 3 | 5 | 7 |
|---|
| Normal | 20.75±0.460 | 20.50±0.53 | 20.63±0.520 | 20.50±0.530 |
| Sham + KRMB | 20.63±0.520 | 20.75±0.46 | 20.50±0.530 | 20.38±0.520 |
| Normal + KRMB | 20.63±0.520 | 20.75±0.46 | 20.63±0.520 | 20.75±0.460 |
| SCI + KRMB |
0.000±0.000a |
0.375±0.51a |
0.750±0.463a |
1.625±0.744a |
| SCI model |
0.000±0.000a |
0.250±0.46a |
0.625±0.518a |
1.500±0.756a |
BMD detection
As presented in Table
II and Fig. 1, the expression of
BMD was not significantly different among the groups at 1 or 2
weeks following the operation. However, BMD levels in the SCI model
group were significantly lower than those in the normal group
(P<0.01). Furthermore, BMD levels in the SCI group were
significantly lower than that in the SCI + KRMB group at 6
(P<0.05), 8 and 10 weeks (P<0.01).
 | Table II.BMD detection results (n=8 per
group). |
Table II.
BMD detection results (n=8 per
group).
|
| BMD
(g/cm2; weeks) |
|---|
|
|
|
|---|
| Group | 1 | 2 | 4 | 6 | 8 | 10 |
|---|
| Normal | 0.210±0.010 | 0.209±0.010 |
0.211±0.009a |
0.209±0.008a |
0.208±0.009a |
0.209±0.005a |
| Sham + KRMB | 0.210±0.010 | 0.211±0.011 |
0.209±0.009a |
0.211±0.009a |
0.210±0.009a |
0.210±0.013a |
| Normal + KRMB | 0.210±0.009 | 0.209±0.011 |
0.208±0.013a |
0.208±0.012a |
0.210±0.011a |
0.209±0.010a |
| SCI + KRMB | 0.210±0.009 | 0.203±0.011 | 0.196±0.007 |
0.190±0.007b |
0.188±0.007a |
0.190±0.006a |
| SCI model | 0.209±0.012 | 0.202±0.011 | 0.189±0.010 | 0.178±0.009 | 0.172±0.010 | 0.172±0.009 |
Serum BGP expression levels
As presented in Table
III, the serum expression levels of BGP in the SCI model group
were significantly higher than those in the normal, sham + KRMB
(P<0.01) and normal + KRMB (P<0.05) groups at each time
point, and significantly lower than the normal + KRMB group
(P<0.05), and the normal and sham + KRMB group (P<0.01). The
level of serum BGP in the SCI + KRMB group was significantly
increased compared with the normal, sham + KRMB and normal + KRMB
group at each time point (P<0.01).
 | Table III.BGP levels in rat serum. |
Table III.
BGP levels in rat serum.
|
| Serum BGP (pg/ml;
weeks) |
|---|
|
|
|
|---|
| Group | 1 | 2 | 4 | 6 | 8 | 10 |
|---|
| Normal |
96.67±5.90a |
96.74±7.96a |
96.31±6.50a |
94.77±6.35a |
96.13±6.63a |
97.50±6.34a |
| Sham + KRMB |
101.20±5.88a |
99.16±6.98a |
101.85±6.25a |
96.49±5.71a |
97.90±6.49a |
98.88±5.47a |
| Normal + KRMB |
97.84±6.32a |
98.35±9.05a |
100.83±6.01a |
96.87±5.51a |
97.03±4.55a |
98.51±6.36a |
| SCI + KRMB |
131.93±7.80b–d |
138.88±4.43b–d |
142.08±7.16b–d |
162.50±7.31b–d |
160.40±10.13b–d |
156.81±6.90b–d |
| SCI model |
124.79±4.81b–e |
123.23±4.80b–e |
120.65±4.60b–e |
115.76±5.06b–e |
112.97±5.17b–e |
112.45±4.70b–e |
Hepcidin mRNA expression
The primer internal reference gene and target genes
of each group were subjected to RT-PCR amplification, which was
performed using rat liver tissue. RT-PCR analysis revealed two
bands at 200 and 263 bp (Fig. 2). As
presented in Table IV, the image
analysis software indicated that the expression of hepcidin mRNA in
the normal, sham + KRMB and normal + KRMB group was significantly
higher than that in the SCI + KRMB and SCI model groups at each
time point (P<0.01). Hepcidin mRNA expression in the SCI + KRMB
group was significantly higher than that in the SCI model group at
1, (P<0.05), 2, 4, 6, 8 and 10 weeks (P<0.01).
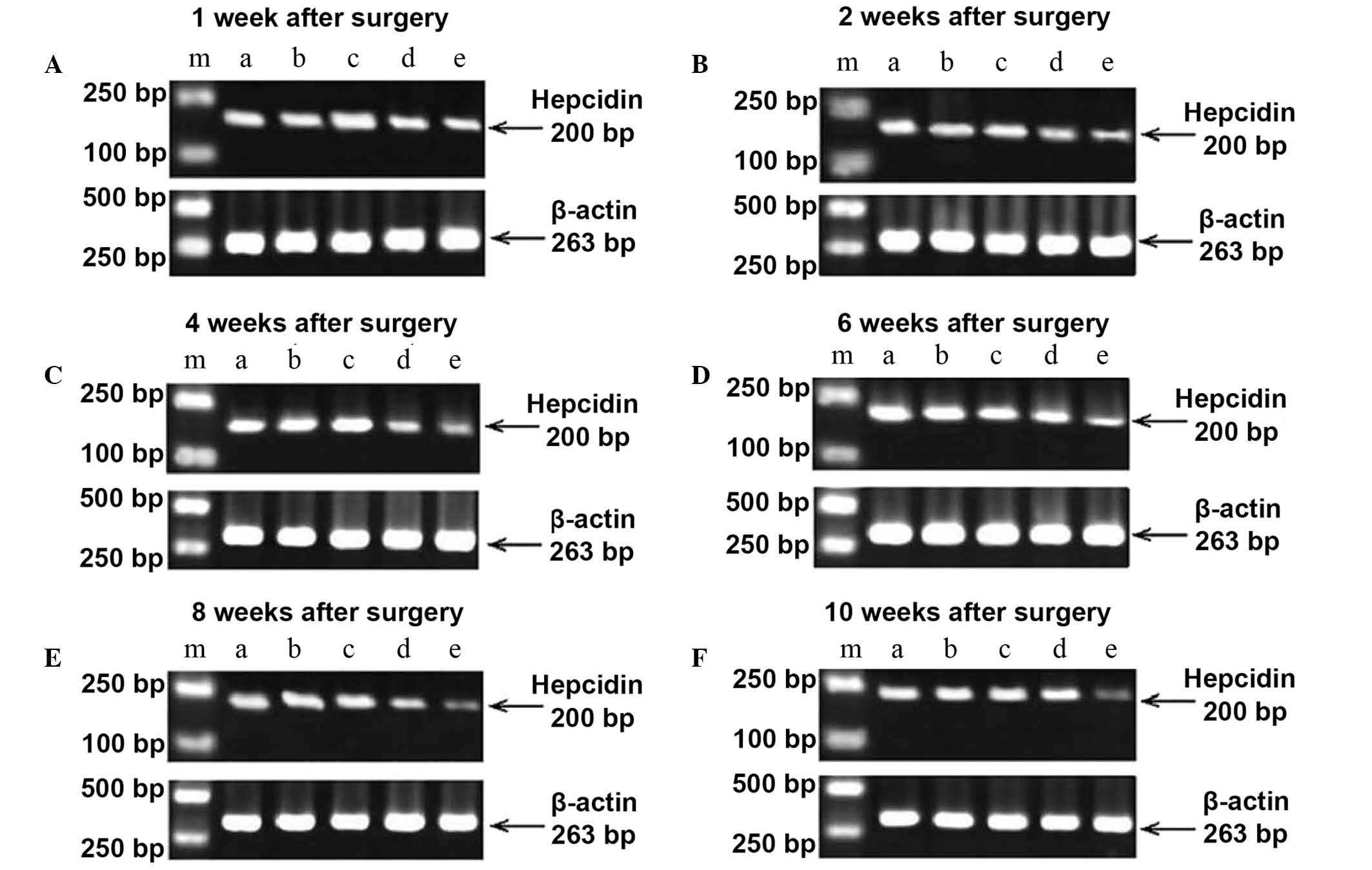 | Figure 2.Gel images from reverse transcription
polymerase chain reaction amplification of hepcidin mRNA expression
at (A) 1, (B) 2, (C) 4, (D) 6, (E) 8 and (F) 10 weeks after
operation. m, DNA marker; a, normal group; b, sham operation + KRMB
group; c, normal + KRMB group; d, SCI + KRMB group; e, SCI model
group. KRMB, kidney reinforcing and marrow-beneficial medicine;
SCI, spinal cord injury. |
 | Table IV.Hepcidin mRNA expression in rat liver
tissue (n=5 per group). |
Table IV.
Hepcidin mRNA expression in rat liver
tissue (n=5 per group).
|
| Optical density
(Hepcidin/β-actin; weeks) |
|---|
|
|
|
|---|
| Group | 1 | 2 | 4 | 6 | 8 | 10 |
|---|
| Normal |
0.621±0.030a,b |
0.620±0.031a,b |
0.620±0.038a,b |
0.622±0.032a,b |
0.623±0.039a,b |
0.618±0.033a,b |
| Sham + KRMB |
0.609±0.018a,b |
0.608±0.020a,b |
0.613±0.027a,b |
0.616±0.029a,b |
0.621±0.021a,b |
0.627±0.019a,b |
| Normal + KRMB |
0.614±0.017a,b |
0.617±0.045a,b |
0.619±0.039a,b |
0.622±0.039a,b |
0.628±0.020a,b |
0.641±0.011a,b |
| SCI + KRMB | 0.370±0.017 | 0.387±0.023 | 0.415±0.021 | 0.428±0.020 | 0.429±0.033 | 0.444±0.033 |
| SCI model |
0.341±0.015c |
0.323±0.018a |
0.313±0.016a |
0.300±0.011a |
0.299±0.010a |
0.293±0.018a |
BSP expression in rat tibial bone
tissue
As presented in Table
V and Fig. 3, there are no
statistically significant differences in BSP expression among the
normal, SCI + KRMB and normal + KRMB groups. However, the BSP
expression levels in the SCI + KRMB and SCI model groups were
significantly higher compared with the normal, sham + KRMB and
normal + KRMB groups at each time point (P<0.01). In addition,
the expression of BSP in the SCI model group was higher than that
in the SCI + KRMB group at 1 (P<0.05) 2, 4, 6, 8 and 10 weeks
(P<0.01).
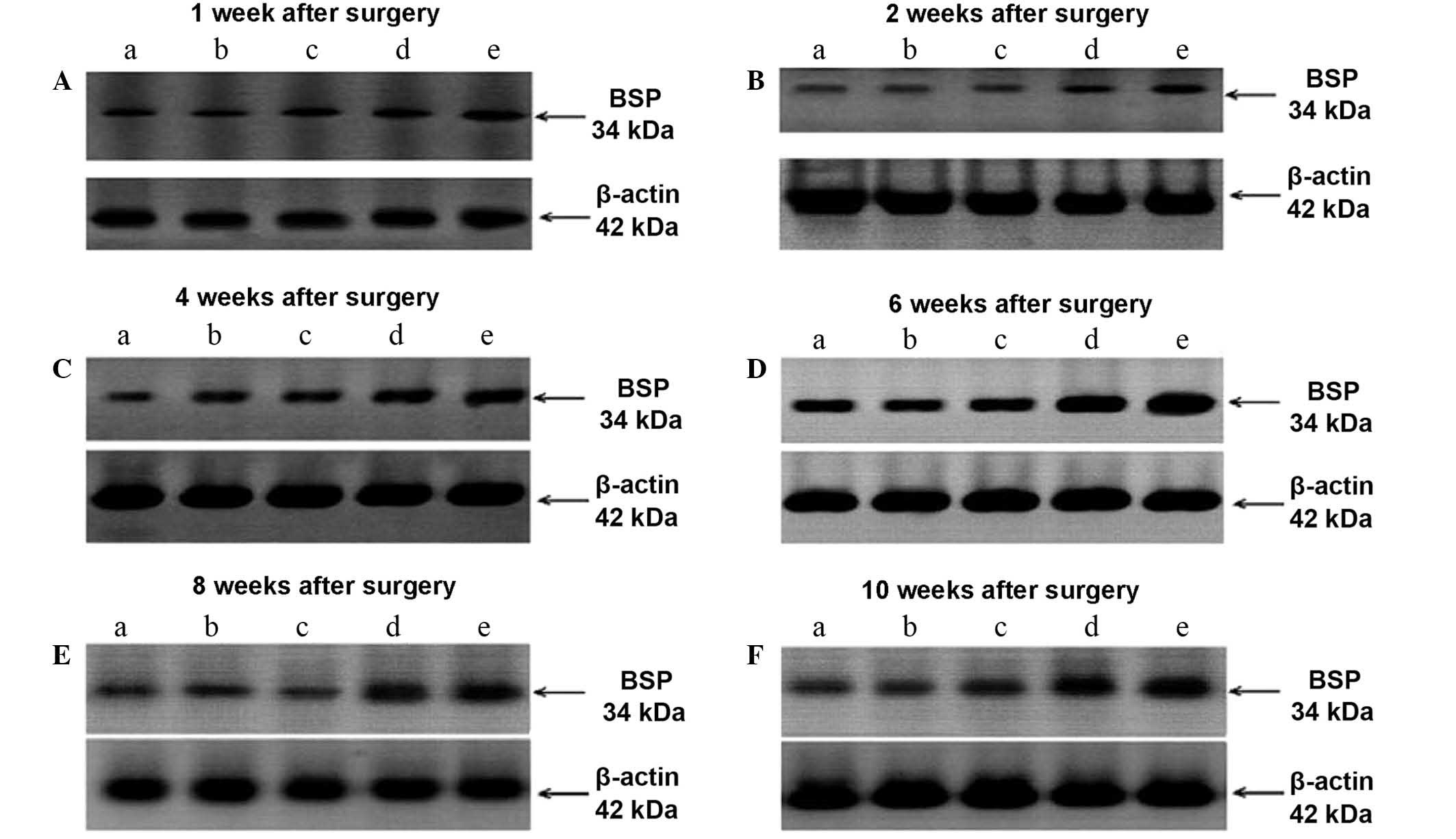 | Figure 3.Western blots of BSP expression at (A)
1, (B) 2, (C) 4, (D) 6, (E) 8 and (F) 10 weeks after operation. A,
normal group; B, sham operation + KRMB group; C, normal + KRMB
group; D, SCI + KRMB group; E, SCI model group. BSP, bone
sialoprotein; KRMB, kidney reinforcing and marrow-beneficial
medicine; SCI, spinal cord injury. |
 | Table V.BSP expression in rat tibial bone
tissue (n=5 per group). |
Table V.
BSP expression in rat tibial bone
tissue (n=5 per group).
|
| Grey level ratio
(BSP/β-actin; weeks) |
|---|
|
|
|
|---|
| Group | 1 | 2 | 4 | 6 | 8 | 10 |
|---|
| Normal |
0.223±0.017a,b |
0.227±0.010a,b |
0.229±0.009a,b |
0.224±0.012a,b |
0.231±0.011a,b |
0.230±0.009a,b |
| Sham + KRMB |
0.229±0.008a,b |
0.226±0.009a,b |
0.238±0.011a,b |
0.228±0.010a,b |
0.225±0.008a,b |
0.227±0.013a,b |
| Normal + KRMB |
0.234±0.013a,b |
0.232±0.010a,b |
0.228±0.013a,b |
0.225±0.009a,b |
0.223±0.007a,b |
0.223±0.004a,b |
| SCI + KRMB | 0.320±0.014 | 0.313±0.008 | 0.306±0.014 | 0.281±0.012 | 0.281±0.016 | 0.282±0.009 |
| SCI model |
0.339±0.009c |
0.343±0.013a |
0.372±0.019a |
0.399±0.018a |
0.397±0.015a |
0.388±0.025a |
Discussion
According to the theory of traditional Chinese
medicine, bone ingrowth relies on providing bone marrow with
sufficient nutrition (5,6). The main pathogenesis of OP resulting
from SCI is marrow deficiency and kidney asthenia, and the
mechanism underlying KRMB Chinese medicine in treating OP is a
current area of interest (6).
At present, a number of methods exist to establish
the SCI model, including the spinal cord transection model that is
commonly used in studies as a result of its simple operational
procedure and light secondary reaction (13). The present study cut the T10 dura and
spinal cord in rats, removed 2 mm spinal cord tissue from below the
T10 spinal segment and filled the gap with gelfoam sponge. The
advantage of using this surgical procedure is that it operates at
the correct anatomical position and is consistent with the degree
of injury. The operation results in motor and sensory function loss
below the cross section, causing dysfunction that is attributed to
the primary injury of the spinal cord and diminishing the risk of
human error (14).
Characteristics of hind limb motor function were
assessed in accordance with the BBB scale (15–17). At
1, 3, 5 and 7 days following the operation, the BBB scores of the
SCI model and SCI + KRMB groups were significantly decreased
compared with the normal group (P<0.01), suggesting that the SCI
model was duplicated successfully.
BMD is a reliable criterion for evaluating the
effect of drugs in treating OP, and is an important index to
quantify bone mineralization in bone metabolism; therefore, it is
regarded as the gold standard criteria for diagnosing OP (18). BMD typically decreases 1 week
following SCI, a reduction in bone mass appears at 2 weeks and
marked OP appears at 4 weeks, reaching its peak and flattening at 6
weeks (19). In the present study,
the BMD levels in the SCI model group were significantly lower
compared with the SCI + KRMB group at 6 (P<0.05), 8 and 10 weeks
(P<0.01). These results indicate that KRMB increases BMD in rats
with OP following SCI.
A preliminary study of bone metabolism following SCI
demonstrated that osteoclastic resorption performance, with or
without slight enhancement of bone formation, is the primary cause
of the high-turn-over OP (20), and
that an increase in the expression of BGP indicates bone formation
(21). In the current study, the
expression levels of serum BGP in the SCI model group were
significantly higher than those in the normal group (P<0.01),
and that, consistent with the literature (22), this was associated with mild bone
formation enhancement following SCI. The measurement of serum BGP
is used in evaluating the effect and efficacy of treatments for OP
(23,24). Furthermore, the expression levels of
serum BGP in the SCI + KRMB group were significantly higher
compared with the normal group at each time point (P<0.01).
Therefore, KRMB may be able to upregulate the expression of serum
BGP.
The association between iron metabolism and OP is
being increasingly recognized, and both clinical and experimental
studies suggest that an iron overload may be a risk factor for OP
(24,25). In addition, a previous study has
demonstrated that hepcidin expression in OP model groups is
significantly different compared with control groups (26). Hepcidin can significantly decrease
the apoptosis rate of human fetal OB 1.19 cells and enhance their
calcification (27). These studies
suggest that hepcidin may have a correlation with OB cell
metabolism. In the present study, hepcidin expression in the SCI
model group was reduced compared with the SCI + KRMB group at 1
(P<0.05), 2, 4, 6, 8 and 10 weeks (P<0.01). This result
suggests that KRMB Chinese medicine is able to increase the
expression of hepcidin mRNA in rat livers, which may be involved in
the development of OP following SCI.
BSP is the predominant non-collagen material in bone
extracellular matrix that participates in cell adhesion, transfer
and signal identification associated with the formation of bone
tissue and alteration (28). The
expression of BSP serves a crucial function in the process of bone
absorption (29,30). BSP expression in the SCI model group
was significantly higher than that in the normal group at each time
point (P<0.01), and was increased compared with the SCI + KRMB
group at 1 (P<0.05), 2, 4, 6, 8 and 10 weeks (P<0.01). This
suggests that the expression of BSP in the early stage of SCI in
rats may be involved in activating the process of bone resorption.
Therefore, it may be suggested that KRMB Chinese medicine delays
the progression of OP following SCI by reducing the expression of
BSP in tibia bone tissue in rats, resulting in the reduction of
bone resorption.
References
|
1.
|
Nielson JL, Guandique CF, Liu AW, Burke
DA, Lash AT, Moseanko R, Hawbecker S, Strand SC, Zdunowski S,
Irvine A, et al: Development of a database for translational spinal
cord injury research. J Neurotrauma. 31:1789–1799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hamid S and Hayek R: Role of electrical
stimulation for rehabilitation and regeneration after spinal cord
injury: An overview. Eur Spine J. 17:1256–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Troy KL and Morse LR: Measurement of bone:
Diagnosis of SCI-induced osteoporosis and fracture risk prediction.
Top Spinal Cord Inj Rehabil. 21:267–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Li J, Meng Q, Shan Q, Mao Z, Wang B, Zhang
F, Zhao T and Yu P: Extract of Cornus officinalis SIEB
ameliorates osteoporosis in spinal cord injured rats. Zhong Guo Gu
Zhi Shu Song Za Zhi She. 60:627–633. 2015.(In Chinese).
|
|
5.
|
Niu Y and Zheng HX: Effect of herbs with
function of reinforcing kidney and replenish marrow on mRNA and
protein expression of calcium binding protein - D9K in kidney
tissue of rats with glucocorticoid-induced osteoporosis. Ji Lin
Zhong Yi Yao. 32:68–69. 2012.(In Chinese).
|
|
6.
|
Wang J, Zheng HX, Liu Y, Zhang JP and Liu
RH: Effect of reinforcing kidney to replenish marrow herbs compound
on mRNA and protein expression of osterix in kidney tissues of rats
with glucocorticoid-induced osteoporosis. Guang Ming Zhong Yi Za
Zhi. 27:673–677. 2012.(In Chinese).
|
|
7.
|
Wang J, Zheng HX, Zong ZH, Yang F, Zhu H
and Zhang ZG: Effect of herbs with function of reinforcing kidney
and replenish marrow on mRNA and protein expression of Runx2 in
bone tissue of rats with glucocorticoid-induced osteoporosis. Zhong
Guo Gu Zhi Shu Song Za Zhi. 17:120–125. 2011.(In Chinese).
|
|
8.
|
Zhou DA, Deng YN and Liu L: Effect of
kidney-reinforcing and marrow-beneficial traditional Chinese
medicine-intervened serum on the proliferation and osteogenic
differentiation of bone marrow stromal cells. Exp Ther Med.
9:191–196. 2015.PubMed/NCBI
|
|
9.
|
Xie L, Shen YX and Fan ZH: Establishment
of rat models of complete spinal cord transection and several
relative problems. Ji Zhu Wai Ke Za Zhi. 8:377–380. 2010.(In
Chinese).
|
|
10.
|
Meng BL, Ba YC, Song SN, Chen SS and Wang
TH: Establishment of spinal cord transection injury models in rats.
Zhong Guo Zu Zhi Gong Cheng Yan Jiu Yu Lin Chuang Kang Fu.
15:1215–1218. 2011.(In Chinese).
|
|
11.
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open filed
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen M, Kong XY, Ma Q, Zheng X, Zhao S and
Zheng XY: Establishment of completely transected spinal cord model
and influence of minocycline pretreatment on GFAP expression in
rats. Jie Po Xue Za Zhi. 37:352–355. 2014.(In Chinese).
|
|
13.
|
Nakae A, Nakai K, Yano K, Hosokawa K,
Shibata M and Mashimo T: The animal model of spinal cord injury as
an experimental pain model. J Biomed Biotechnol. 2011:9390232011.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lim JH, Piedrahita JA, Jackson L,
Ghashghaei T and Olby NJ: Development of a model of sacrocaudal
spinal cord injury in cloned Yucatan minipigs for cellular
transplantation research. Cell Reprogram. 12:689–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yu D, Li M, Ni B, Kong J and Zhang Z:
Induction of neuronal mitophagy in acute spinal cord injury in
rats. Neurotox Res. 24:512–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lukovic D, Moreno Manzano V, Stojkovic M,
Bhattacharya SS and Erceg S: Concise review: Human pluripotent stem
cells in the treatment of spinal cord injury. Stem Cells.
30:1787–1792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ward PJ and Hubscher CH: Persistent
polyuria in a rat spinal contusion model. J Neurotrauma.
29:2490–2498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rufus P, Mohamed N and Shuid AN:
Beneficial effects of traditional Chinese medicine on the treatment
of osteoporosis on ovariectomised rat models. Curr Drug Targets.
14:1689–1693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ye CQ, Ji SR, Zhang QM, Du LJ and Ren XF:
The influence of spinal cord injury on bone metabolism and bone
mass density in rats. Zhong Guo Kang Fu Yi Xue Za Zhi. 20:258–260.
2005.(In Chinese).
|
|
20.
|
Battaglino RA, Lazzari AA, Garshick E and
Morse LR: Spinal cord injury-induced osteoporosis: Pathogenesis and
emerging therapies. Curr Osteoporos Rep. 10:278–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lin SJ, Ho HC, Lee YF, Liu NC, Liu S, Li
G, Shyr CR and Chang C: Reduced osteoblast activity in the mice
lacking TR4 nuclear receptor leads to osteoporosis. Reprod Biol
Endocrinol. 10:432012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Liang HD, Yu F, Tong ZH, Zhang HQ and
Liang W: Cistanches herba aqueous extract affecting serum
BGP and TRAP and bone marrow Smad1 mRNA, Smad5 mRNA, TGF-β1 mRNA
and TIEG1 mRNA expression levels in osteoporosis disease. Mol Biol
Rep. 40:757–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Atalay S, Elci A, Kayadibi H, Onder CB and
Aka N: Diagnostic utility of osteocalcin, undercarboxylated
osteocalcin, and alkaline phosphatase for osteoporosis in
premenopausal and postmenopausal women. Ann Lab Med. 32:23–30.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rossi F, Perrotta S, Bellini G, Luongo L,
Tortora C, Siniscalco D, Francese M, Torella M, Nobili B, Di Marzo
V and Maione S: Iron overload causes osteoporosis in thalassemia
major patients through interaction with transient receptor
potential vanilloid type 1 (TRPV1) channels. Haematologia.
99:1876–1884. 2014. View Article : Google Scholar
|
|
25.
|
Tsay J, Yang Z, Ross FP,
Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB,
Grady RW, Giardina PJ, et al: Bone loss caused by iron overload in
a murine model: Importance of oxidative stress. Blood.
116:2582–2589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Jensen ED, Gopalakrishnan R and Westendorf
JJ: Regulation of gene expression in osteoblasts. Biofactors.
36:25–32. 2010.PubMed/NCBI
|
|
27.
|
Marie PJ and Kassem M: Osteoblasts in
osteoporosis: Past, emerging, and future anabolic targets. Eur J
Endocrinol. 165:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kruger TE, Miller AH, Godwin AK and Wang
J: Bone sialoprotein and osteopontin in bone metastasis of
osteotropic cancers. Crit Rev Oncol Hematol. 89:330–341. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yang Y, Mkhonto D, Cui Q and Sahai N:
Theoretical study of bone sialoprotein in bone biomineralization.
Cells Tissues Organs. 194:182–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Uccello M, Malaguarnera G, Vacante M and
Motta A: Serum bone sialoprotein levels and bone metastases. J
Cancer Res Ther. 7:115–119. 2011. View Article : Google Scholar : PubMed/NCBI
|

















