Introduction
Polyacrylamide hydrogel (PAHG) is a medical soft
tissue filling agent employed for the treatment of a variety of
soft tissue defects, since its introduction in the Ukraine in 1997
(?). PAHG has been used successfully in the plastic and
reconstructive surgery of soft tissue defects. Augmentation
mammoplasty with PAHG injection has also been used with success.
However, there are postoperative complications and the increasing
number of cases result in morbidity (?). The occurrence rate of
such complications was reported to be ≤6–7% (?). Induration
accounted for 58.89% of complications, with 10.11% being hematoma,
7.78% aseptic inflammation, 6% infection, 4.565% myositis, 3.44%
asymmetric, 3.44% of PAHG leakage, 1.22% of displacement and 3% of
other types of complications (1).
From January 2008 to October 2012, 28 patients with
breast augmentation were successfully treated with PAHG injections.
The aim of the present study was to examine the treatment of
complications of breast augmentation with PAHG injections.
Materials and methods
Patients
From January 2008 to October 2012, 28 patients
underwent breast augmentation with PAHG injections. The patients
were 20–40 years of age, with an average age of 30.2 years. The
post-injection time ranged from 2 months to 4 years, and the
injection volume was approximately 150–200 ml. However, the
specific injection volume of the PAHG by individual case was not
determined. The common complications were nodular breast lumps,
chest pain, distorted breast and displacement of the PAHG. The
majority of patients experienced several complications.
Methods
Incision on the subareolar or periareolar or
subglandular plica was performed. After the skin and subcutaneous
tissue incision, the superficial gland envelope was dissected until
the lower pole of the gland. Separation in the deep part was then
performed and the gland post gap was opened. When the PAHG was at
appropriate levels, a granular, soft, jam-like substance was
effused. In the case of several cavity gaps, an incision was
performed in the membranes to form a connected cavity gap. The
suction duct apparatus was subsequently inserted into the gap and
filled with normal saline. During the lavage, the breast was
massage to ensure PAHG effusion. When the gland or muscle was
involved, an incision was made to the gland or myolemma and the
PAHG was removed by curettage. In the case of the envelope being
extremely thick or the sticky deformation of the tissue overly
distinct, part of the muscle or gland was severed and sent for
pathological examination. Normal saline was used to lavage the
sections until the flushing fluid became translucent again. The
gland or muscle tissue was probed mannually to ensure there were no
nodules, and the breast was softer than prior to the procedure.
The present study was approved by the ethics
committee of the First People's Hospital of Xuzhou (Jiangsu,
China). Patient consent was obtained from the patient or the
patient's family.
Results
General
After a 3 month- to 2-year follow-up, preoperative
symptoms, such as sclerosis of the skin, breast nodule and pain had
almost disappeared following surgery. For the patients who
underwent breast augmentation prosthesis, the breasts were soft and
bilaterally symmetric, with a good appearance. There were no
symptoms of infection, pain or lumps in any of the patients. The
patients expressed satisfaction with the postoperative effect.
Typical cases
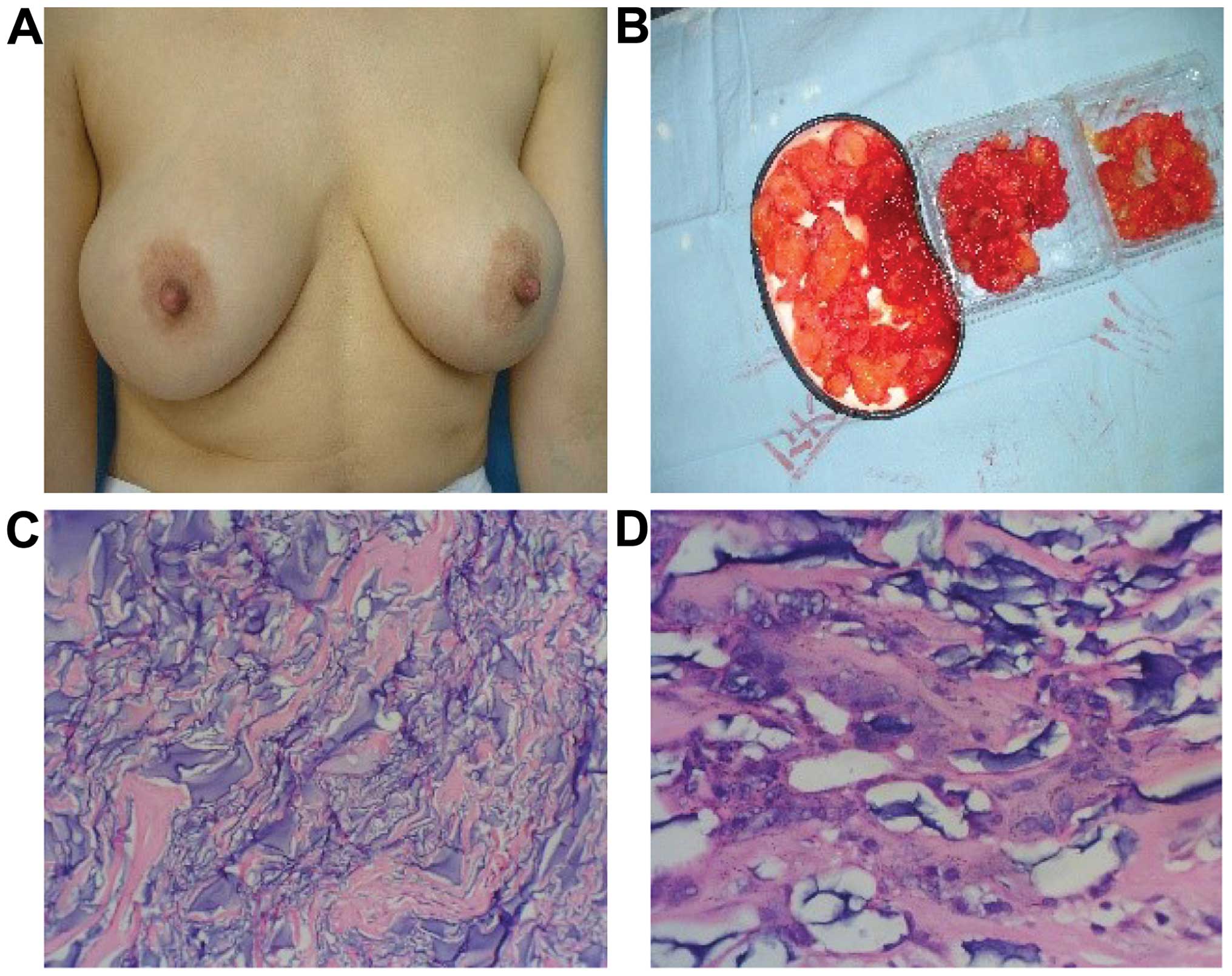
Case 1 was a female patient, aged 36 years and
married. She underwent PAHG injection because of light breast
ptosis in another hospital. Although two months post-surgery the
patient experienced chest pain, especially when she breathed, she
did not pay particular attention to the symptom. After 1 year, the
mammary gland was hardened, distorted and tender. She tried to
communicate with the hospital but failed. After 2 years, the
patient presented at the First People's Hospital of Xuzhou
(Jiangsu, China), where she was diagnosed as having complications
from the augmentation mammoplasty with the PAHG. Surgery was
carried out. Incision over the areolar was performed, and we found
that there were many tender nodules of various shapes and sizes in
the glandular tissue, retromammary cellular space, post-pectoralis
major space, and pectoralis major and minor muscles. The glands and
muscles involved were edematous and distorted. We removed the
granular hydrogel, and the distorted tissue. The pathological
examination showed the fibrous tissues were proliferating, and
masses of inflammatory mononuclear cells were being infiltrated by
the tissue. The paient was advised to undergo prosthetic plombage
surgery. Through a telephone follow-up, we learned that the
symptoms disappeared, and the patient was satisfied with the
results (Fig. 1).
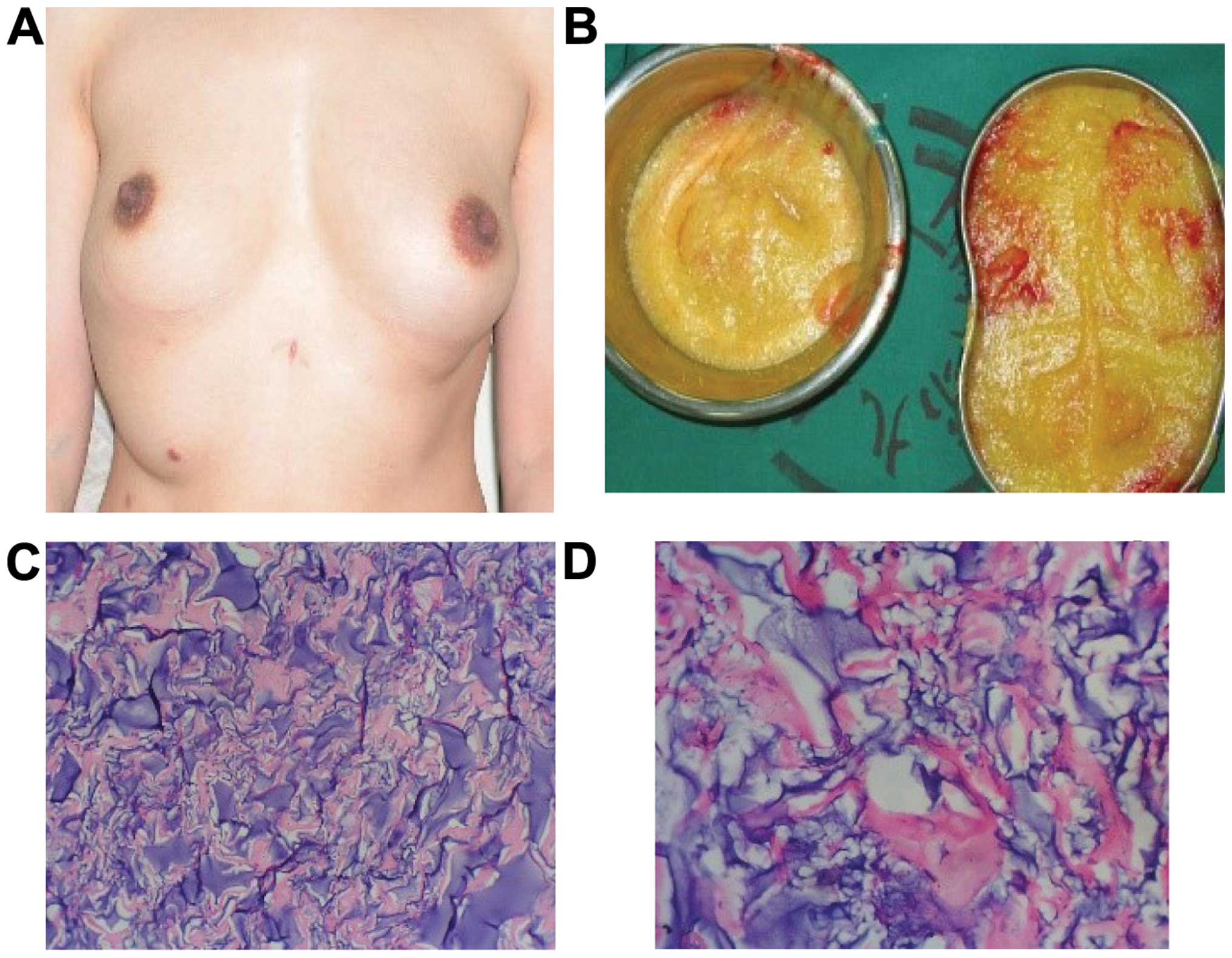
Case 2 was a female patient, aged 32 years and
married. She underwent PAHG injections in another hospital with
relatively positive results and symmetrical breasts. Six months
prior to presenting at the hospital, the patient underwent
laparoscopy for cholecystitis. During surgery, it was found that
there were lumps in her breasts, and the breasts were distorted.
The medical history revealed chest pain subsequent to the surgery.
Owing to the complications of augmentation mammoplasty by PAHG
injection, the hydrogel was removed. Incision of plica under breast
was performed, and the enclosed massed presented in the
subcutaneous tissue and mammary glands in a number of various
shapes and sizes. There were also incomplete and irregular
envelopes in the multilocular cystic condition. An incision was
made in the myolemma of the pectoralis major, and the PAHG that was
distributed in the muscle bundles was drained and then flushed with
normal saline. Part of the removed envelopes, which were sent for
pathological examination, comprised collagenous fiber and
fibroblast. The foreign body surface was infiltrated with a great
number of mononuclear cells. At 2 weeks following surgery, the pain
symptoms disappeared, the breasts were asymmetrical, and the
breasts collapsed. The patient did not undergo any further breast
augmentation (Fig. 2).
Discussion
Hydrophilic polyacrylamide hydrogel, or HPAMG, is
broadly used as an injectant in breast augmentation due to its
relatively good physiological compatibility and steady
physicochemical property (2).
However, the complications of surgery leads to the patient
experiencing pain.
The filling material of the injection in breast
augmentations should be placed in the retromammery cellular space
(6). However, since the injection
performed is obscured, the position into which the material is
injected is often inaccurate. In addition, a large volume of the
material, reaching 150–200 ml, is required (7,8). Under
the influence of gravity, body positions, muscular movement and
postoperative massage, the injectant may infiltrate into the
surrounding tissue and redistribute itself within the body. The
injectants may distribute in the form of crumbs in the outer and
downside of the submammary gland space, inside of the mammary gland
or subcutaneous tissue, or even the outer side of the breasts
(7,8).
The most common complication, which is the formation
of breast induration following breast augmentation by PAHG, has
been identified in almost all the clinical cases (9). The breast induration varies in size and
is distributed in subcutaneous tissue, glands or muscles. Although
the hydrogel is regarded as a biomaterial that cannot cause cancer
or malformation, the formation of lumps has obvious interference
over the differential diagnosis of an early mammary tumor (10). Chest pain is also a common
complication and can primarily be identified in the pectorals where
the hydrogel is located. Previous findings suggest that hydrogel
does not move subsequent to the injection (11). However, there is a part of the
hydrogel that is transferred to the surrounding tissue over time,
with some hydrogel being transferred beyond the scope of breasts
and reaching the armpits or napes.
When selecting a method to remove the hydrogel,
liposuction using the tumescent technique can be used for patients
whose injection in the early period was only limited to
retromammary cellular space (12).
However, for patients with decentralized crumbs, or those whose
injection is located in the pectoralis major, liposuction is not as
easy. Injectants become a granulate and multicystic enclosed mass
after a long period of time has elapsed (12). Additionally, the diolame is separated
in the net structure (12). These
issues make liposuction difficult to perform. In addition, this
method can also damage the surrounding tissue, displace the
hydrogel during the piercing process and develop several cavity
spaces. Thus, incision on the subareolar or periareolar or
subglandular plica is performed. The envelope of glands is stripped
from the subcutaneous tissue and the shell is sectioned, resulting
in the injectants emerging. At the same time, this method can
remove the distorted muscle (13,14).
Owing to the infiltration of hydrogel, we cannot
ensure that the hydrogel is completely removed under direct vision.
However, it plays a decisive role in symptom relief, relieves
psychological burden and there is no interference of early
screening on the mammary tumor (10). Only by complete removal of the
subcutaneous glands and muscle can the hydrogel be thoroughly
removed. Patients remain unsatisfied with the changes in the shape
of the breasts. Breast augmentation prosthesis is therefore not
performed unless the patients consent to the procedure. Six months
post-surgery, a B ultrasonic super-review may be conducted. If no
obvious remaining hydrogel is identified, the patient may consider
accepting the silicone gel breast implant to improve the breast
shape (15,16).
In conclusion, the introduction of PAHGs in breast
augmentation is a recent development and there are insufficient
large samples of animal experiments and clinical trials to
thoroughly determine its effects. Although PAHGs possess better
histocompatibility, there are complications that should be
considered (9). Thus, this method
may not the optimal choice for breast augmentation.
References
|
1
|
Kasi AD, Pergialiotis V, Perrea DN, Khunda
A and Doumouchtsis SK: Polyacrylamide hydrogel (Bulkamid(R)) for
stress urinary incontinence in women: A systematic review of the
literature. Int Urogynecol J. 27:367–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng XL, Yi CX, Peng C, Zhang YM, Yang M,
Wang YR, Guo NQ and Zhou WD: Analysis of the complications induced
by polyacrylamide hydrogel injection. Plast Reconstr Surg.
114:261–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Do ER and Shim JS: Long-term complications
from breast augmentation by injected polyacrylamide hydrogel. Arch
Plast Surg. 39:267–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Z, Liu DL, Lu KH, et al: The research
of complications in syringe breast augmentation using
polyacrylamide hydrogel. Chin J Pratic Aesthet Plast Surg.
16:353–356. 2005.
|
|
5
|
Wang HY, Jiang YX and Qiao Q:
Ultrasonographic value for thecomplications of breast augmentation
with injectable polyacrylamide hydrogel technique. Zhonghua Zheng
Xing Wai Ke Za Zhi. 23:97–100. 2007.(In Chinese). PubMed/NCBI
|
|
6
|
Biggs TM and Yarish RS: Augmentation
mammaplasty: Retropectoral versus retromammary implantation. Clin
Plast Surg. 15:549–555. 1988.PubMed/NCBI
|
|
7
|
Godwin Y, Duncan RT, Feig C, Reintals M
and Hill S: Soft, Brown Rupture: Clinical Signs and Symptoms
Associated with Ruptured PIP Breast Implants. Plast Reconstr Surg
Glob Open. 2:e2492014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim CH: Asian Outcomes of Primary Breast
Augmentation in 162 Consecutive Cases by a Single Surgeon. Plast
Reconstr Surg Glob Open. 3:e5372015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng NX, Wang YL, Wang JH, Zhang XM and
Zhong H: Complications of breast augmentation with injected
hydrophilic polyacrylamide gel. Aesthetic Plast Surg. 26:375–382.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu HL and Cheung WY: Complications of
polyacrylamide hydrogel (PAAG) injection in facial augmentation. J
Plast Reconstr Aesthet Surg. 63:e9–e12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chao MJ and Yin WM: Application of
hydrophilic polyacrylamide gel in augmentation mammoplasty. J Pract
Aesth Plast Surg. 11:16–18. 2000.(In Chinese).
|
|
12
|
Leung KM, Yeoh GP and Chan KW: Breast
pathology in complications associated with polyacrylamide hydrogel
(PAAG) mammoplasty. Hong Kong Med J. 13:137–140. 2007.PubMed/NCBI
|
|
13
|
Wang Z, Li S, Wang L, Zhang S, Jiang Y,
Chen J and Luo D: Polyacrylamide hydrogel injection for breast
augmentation: Another injectable failure. Med Sci Monit.
18:CR399–CR408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmed EM: Hydrogel: Preparation,
characterization, and applications: A review. J Adv Res. 6:105–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Losken A: Early Results Using Sterilized
Acellular Human Dermis (Neoform) in Post-Mastectomy Tissue Expander
Breast Reconstruction. Plast Reconstr Surg. 123:1654–1658. 2009.
View Article : Google Scholar
|
|
16
|
Manafi A, Emami AH, Pooli AH, Habibi M and
Saidian L: Unacceptable results with an accepted soft tissue
filler: Polyacrylamide hydrogel. Aesthetic Plast Surg. 34:413–422.
2010. View Article : Google Scholar : PubMed/NCBI
|