Introduction
Hepatic artery pseudoaneurysm (HAP) after liver
transplantation is a rare and serious vascular complication.
Pseudoaneurysm rupture and hemorrhage may endanger the life of the
patients (1,2). A timely and accurate diagnosis prior to
aneurysm rupture has been shown to be favorable for HAP prognosis.
Common ultrasound is the preferred examination method of imaging
following liver transplantation. Contrast-enhanced ultrasonography
(CEUS) is a new technology with the ability of carrying out
microvascular imaging (3,4). This method is real-time and convenient
and can be considered as a type of point-of-care test (POCT).
Advantages of CEUS make it a more efficient method compared with
computed tomography (CT), magnetic resonance imaging (MRI) and
digital subtraction angiography (DSA).
In the present study, the diagnostic value of common
ultrasound was compared to the CEUS in HAP subsequent to liver
transplantation. The results showed that, CEUS is a convenient and
effective diagnostic method for HAP following liver
transplantation.
Patients and methods
Clinical data
From January 2005 to November 2015, information on
2,085 cases of orthotopic liver transplantation was collected from
the Organ Transplantation Research Institute, General Hospital of
Chinese People's Armed Police Forces (Beijing, China). The cases
included 1,617 men and 468 women. The present study was approved by
the Ethics Committee of the General Hospital of Chinese People's
Armed Police Forces. Written informed consent for the CEUS
examination was obtained.
Instrument and contrast agent
Acuson Sequoia 512 (Siemens Medical Solutions,
Mountain View, CA, USA) with contrast pulse sequencing imaging
software and Mylab Twice (Esaote S.p.A., Genoa, Italy) with CnTI
imaging software were used. SonoVue (Bracco Imaging S.p.A., Milan,
Italy) was used as the contrast agent. Peripheral venous infusion
was applied after conventional configuration. Physiological saline
(5 ml) was flushed quickly, and a single dose of 0.5–2.4 ml/time
was applied.
Inspection method
Conventional ultrasound examination after surgery
was conducted on all liver transplant recipients to observe the
echo of liver parenchyma and the vascular hemodynamic of all
anastomotic vessels. The recipients were checked once per day
within a week and after one week they were checked once every 3–7
days until the discharge. Examination frequency for CEUS was 1–2
weeks after the operation, and once when abnormal arterial blood
flow parameters were detected. Enhanced CT was performed prior to
discharge from hospital, or when abnormal arterial blood flow
parameters were detected during ultrasound and contrast-enhanced
ultrasound. Observation on hepatic artery included the echo of the
hepatic artery, the artery line, the peak velocity, the resistance
index and the acceleration time. Abnormal blood flow parameters of
hepatic artery were: peak velocity <25 cm/sec, RI <0.5,
and/or SAT >80 msec.
The diagnostic standard for HAP by common ultrasound
involved follicular structure with disrupted blood flow and
arterial blood flow signal existed along and around the hepatic
artery. The diagnostic standard for HAP by CEUS was carried out as:
After peripheral intravenous injection with contrast agent and
hepatic artery infusion with contrast agent, the contrast agent
perfusion in the follicular contrast enhancement zone beside the
hepatic artery synchronized with the perfusion in hepatic artery,
and it was visible that the contrast agent was sprayed from the
artery to a vesicle-like structure along with the arterial
pulse.
Diagnostic gold standard
The diagnostic gold standard of HAP after liver
transplantation was CT angiography (CTA) or operation. Six cases of
HAP were diagnosed by CTA, and 2 cases were confirmed by emergency
operation.
Statistical analysis
PASW Statistics 18.0 SPSS 18.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis.
Results
Imaging diagnosis
Of the 2,085 patients, 8 cases of HAP following
liver transplantation were diagnosed (0.38%). There were 7 men and
1 woman. The condition occurred 12–44 days after the operation
(average time, 28.3 days). Clinical manifestation and imaging
diagnosis of the 8 cases of HAP is shown in Table I. Three HAP cases were diagnosed
using common ultrasound while 5 cases were missed. Sensitivity,
specificity and diagnostic accuracy for this method was 37.5, 100
and 99.76%, respectively. In total, 6 cases of HAP were diagnosed
using CEUS while only 2 cases were missed. Sensitivity, specificity
and diagnostic accuracy for CEUS method was 75, 100 and 99.9%,
respectively.
 | Table I.Imaging diagnosis and clinical
treatment for 8 cases with HAP. |
Table I.
Imaging diagnosis and clinical
treatment for 8 cases with HAP.
| Cases | Occurrence time | Common
ultrasound | Contrast-enhanced
ultrasonography | Clinical
manifestation | Treatment | Prognosis |
|---|
| 1 | 29 | Definite
diagnosis | Definite
diagnosis | Intra-abdominal
hemorrhage | Emergency arterial
embolization | Lateral shoot
formation |
| 2 | 15 | Definite
diagnosis | Definite
diagnosis | Intra-abdominal
hemorrhage | Tumor resection and
artery reconstruction | Well |
| 3 | 44 | Definite
diagnosis | Definite
diagnosis | Hemobilia | Tumor resection and
hepatic artery ligation | Lateral shoot
formation and biliary complication |
| 4 | 38 | Missed diagnosis | Definite
diagnosis | None | No treatment | Well |
| 5 | 45 | Missed diagnosis | Definite
diagnosis | None | No treatment | Well |
| 6 | 25 | Missed diagnosis | Definite
diagnosis | None | Tumor resection and
artery bypass reconstruction | Well |
| 7 | 12 | Missed diagnosis | Missed diagnosis | Intra-abdominal
hemorrhage | Tumor resection and
hepatic artery ligation | Hepatic
retransplantation |
| 8 | 18 | Missed diagnosis | Missed diagnosis | Intra-abdominal
hemorrhage | Abdominal
laparotomy | Death |
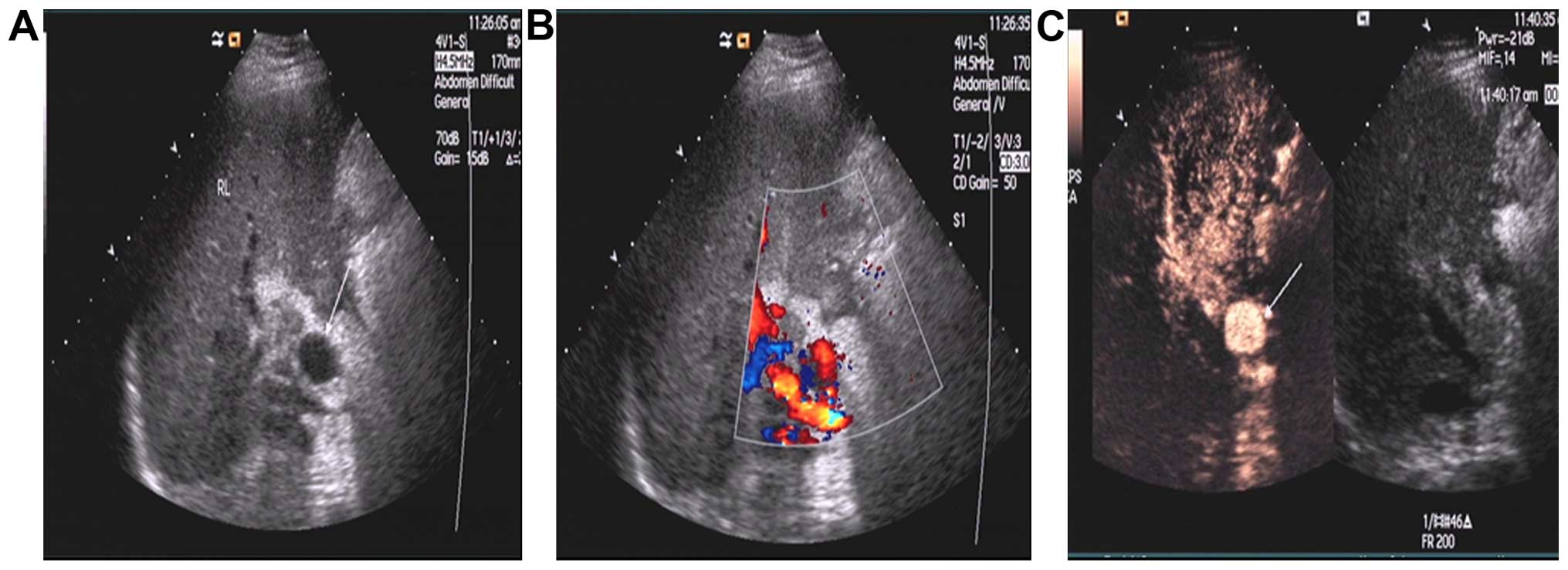
Hepatic artery pseudoaneurysm in
hepatic artery anastomosis
Of the 8 cases of HAP, 3 cases were definitely
diagnosed by the two methods, follicular structure was visible in
hilar through grayscale ultrasound, arterial blood flow signal was
detected during color Doppler ultrasound, and CEUS showed
synchronous enhancement of contrast agent in a vesicle-like
structure and the hepatic artery (Fig.
1). Three cases that were not diagnosed correctly by common
ultrasound, were diagnosed by CEUS. In 2 of 3 cases, follicular
structure was invisible in hilar through grayscale ultrasound,
color Doppler ultrasound showed RI <0.5 and SAT >80 msec in
hepatic artery, and CEUS showed that synchronous enhancement of
contrast agent in the vesicle-like structure and the hepatic artery
(Fig. 2).
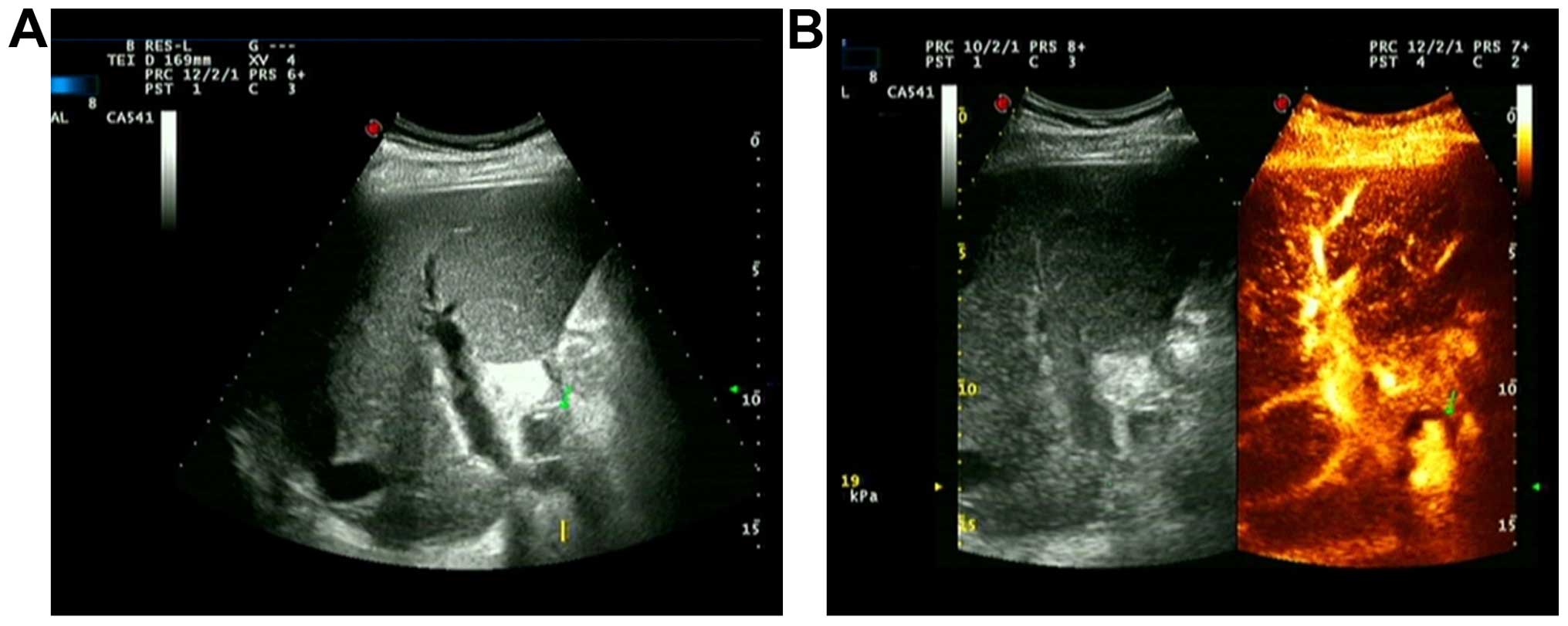
In another case, the postoperative clinical
manifestation was multiple biliary tract hemorrhage. During the
first examination on the 38th day after operation, we observed no
abnormal echo in hilar during grayscale ultrasound and no
significantly abnormal hepatic artery blood flow parameters. During
the second examination on the 44th day after surgery, low echo area
was detected in hilar by common ultrasound and no blood flow signal
was detected during the examination with color Doppler ultrasound.
With CEUS, in low echo area, the contrast agent sprayed into tumors
along with arterial pulse, and gradually filled them (Fig. 3). There were 2 HAP cases that were
not diagnosed by common ultrasound or CEUS. There was a single case
that was diagnosed by CTA. The position of HAP was far from the
hilar. The other case was confirmed by emergency surgery due to
abdominal bleeding.
Discussion
Liver transplantation is the only effective
treatment for patients suffering from terminal stages of liver
disease. Postoperative arterial complications are the most
important factors affecting the success of the transplant (5,6). The
most common hepatic artery complications are hepatic artery
stenosis, embolization, hepatic artery dissection and HAP (7–10). HAP
is the rarest and most dangerous complication with an incidence
rate of 0.5–2.6% (11,12). HAP may lead to mortality due to
rupture and hemorrhage. The mortality rate, after liver
transplantation, due to HAP is extremely high (2.7%). Timely
detection, diagnosis and treatment can effectively reduce the
mortality rate (1,13).
The preferred method of blood flow monitoring after
liver transplantation is the common ultrasound (14), CTA, MRI and DSA. Other large
equipment cannot be used for POCT, and are often applied as
supplementary examination and qualitative diagnosis. CT, MRI and
DSA are not routine examinations, and are usually applied prior to
hospital discharge or in the event of a possible vascular
complication following ultrasonic examination. In this study, we
used CEUS as a postoperative routine imaging examination method.
CEUS, has been widely used for the differential diagnosis of
abdominal organs with space-occupying lesion (15,16), and
it has also been applied for the diagnosis of vascular
complications following liver transplantation (17–19).
Ultrasound contrast agent, which also served as a blood pool
tracer, can be used in real-time ultrasound contrast imaging to
identify large blood vessels and the micro-vascular system
(20).
HAP mostly occurs in the anastomotic site, and is
often linked to infection, bile leakage or multiple TACE treatment
prior to transplantation. Certain technical difficulties are
involved in the detection of arterial anastomosis with common
ultrasound. HAP, which is located near the hilar, possessing
disrupted artery blood fow, was lined with the artery, and common
ultrasound was able to make a diagnosis (21), as in the 3 cases of HAP diagnosed
using common ultrasound in the study. Detection of anastomosis is
difficult when located far from the hilar. This is due to the
interference caused by gas, intestine and omentum. In this study, 3
cases of HAP that were far from the hilar, demonstrated abnormal
hemodynamic parameters in the common ultrasound examination. CEUS
successfully diagnosed 2 of the cases, while the other one was
diagnosed by CTA. In the HAP cases, during examination with CEUS,
we observed a circular contrast agent perfusion area around the
artery after the perfusion of contrast agent.
In one case of HAP, the diagnosed primary diseases
were liver cirrhosis and hepatocellular carcinoma. TACE was
performed 6 times prior to operation and postoperative clinical
manifestations were two occasions of biliary tract hemorrhage. In
the first biliary tract hemorrhage, common ultrasound showed no
abnormalities while in the second one, grayscale ultrasound
identified a hilar hypoechoic mass. Color Doppler ultrasound did
not identify any sign of blood flow in lumps. CEUS showed that, in
the arterial phase, contrast agent was sprayed into the tumor with
arterial pulse in the low echoic block. Emergency laparotomy was
carried out and tumor resection and arterial ligation were
conducted under the guidance of ultrasound. During the operation,
we detected a large tumor with high probability to rupture and
cause hemorrhage.
If the surgery had been delayed the patient would
have faced a life-threatening condition. The case was analyzed to
determine the reason for the failure of color Doppler ultrasound to
detect any blood flow signal in the tumor leading to missed
diagnosis. We hypothesized that the failure was due to the presence
of a smaller fistula orifice between the HAP and hepatic artery
that retarded the blood flow.
Ultrasound contrast agents belong to the blood pool
tracers and mimic the blood flow. Contrast agent perfusion also
occurs, and thus ultrasound has a function of diagnosis. It was
reported that multiple TACE prior to liver transplantation in liver
cancer cases may increase the risk of HAP following liver
transplantation (22). Therefore,
for liver transplantation recipients with multiple TACE treatment,
any of the following symptoms suggest a high risk for occurrence of
HAP: i) Biliary tract bleeding; ii) bile leakage; and iii)
abdominal bleeding. In these cases, the best course of action would
be performing ultrasound angiography, CTA or DSA as early as
possible to avoid missed diagnosis.
CTA, MRI and DSA were not used as routine
examination methods of vascular complications after liver
transplantation. Therefore when postoperative common ultrasound
detects any of these conditions, CEUS or other vascular imaging
should be conducted to verify: i) Arterial hemodynamic
abnormalities; ii) follicular block; and iii) low echoic lump in
hilar. The treatment of HAP should be proactive, whenever possible,
and arterial revascularization treatment should be immediately
carried out to avoid tumor rupture. In the present study, only 1 of
8 cases of HAP succumbed, and this may be attributed to the timely
diagnosis of CEUS and timely surgical intervention.
In summary, CEUS was identified as a convenient and
effective diagnostic method for HAP cases following liver
transplantation. This is a superior method of diagnosis compared
with CTA, as it can be realized in real-time and is considered more
convenient. Poor grayscale image display affected the display
effect of CEUS. For HAP located far from the hilar, there was a
high probability of missed diagnosis. CEUS made up for the
deficiencies associated with enhanced CT, DSA and other large
equipment, and was crucial in the diagnosis of vascular
complications subsequent to liver transplantation.
References
|
1
|
Volpin E, Pessaux P, Sauvanet A, Sibert A,
Kianmanesh R, Durand F, Belghiti J and Sommacale D: Preservation of
the arterial vascularisation after hepatic artery pseudoaneurysm
following orthotopic liver transplantation: Long-term results. Ann
Transplant. 19:346–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nghiem HV: Imaging of hepatic
transplantation. Radiol Clin North Am. 36:429–443. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo Y, Fan YT, Lu Q, Li B, Wen TF and
Zhang ZW: CEUS: A new imaging approach for postoperative vascular
complications after right-lobe LDLT. World J Gastroenterol.
15:3670–3675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rennert J, Dornia C, Georgieva M, Roehrl
S, Fellner C, Schleder S, Stroszczynski C and Jung EM:
Identification of early complications following liver
transplantation using contrast enhanced ultrasound (CEUS). First
results. J Gastrointestin Liver Dis. 21:407–412. 2012.PubMed/NCBI
|
|
5
|
Wertheim JA, Petrowsky H, Saab S,
Kupiec-Weglinski JW and Busuttil RW: Major challenges limiting
liver transplantation in the United States. Am J Transplant.
11:1773–1784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pakosz-Golanowsha M, Lubikowski J, Post M,
Jarosz K, Zasada-Cedro K, Milkiewicz P and Wójcicki M: The arterial
anastomosis in liver transplantation: Complications, treatment and
outcome. Hepatogastroenterology. 57:1477–1482. 2010.PubMed/NCBI
|
|
7
|
Singhal A, Stokes K, Sebastian A, Wright
HI and Kohli V: Endovascular treatment of hepatic artery thrombosis
following liver transplantation. Transpl Int. 23:245–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Zhao JC, Yan LN, Ma YK, Huang B,
Yuan D, Li B, Wen TF, Wang WT, Xu MQ, et al: Risk factors
associated with early and late HAT after adult liver
transplantation. World J Gastroenterol. 20:10545–10552. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frongillo F, Lirosi MC, Nure E, Inchingolo
R, Bianco G, Silvestrini N, Avolio AW, De Gaetano AM, Cina A, Di
Stasi C, et al: Diagnosis and management of hepatic artery
complications after liver transplantation. Transplant Proc.
47:2150–2155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamby BA, Ramirez DE, Loss GE, Bazan HA,
Smith TA, Bluth E and Sternbergh WC III: Endovascular treatment of
hepatic artery stenosis after liver transplantation. J Vasc Surg.
57:1067–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fistouris J, Herlenius G, Bäckman L,
Olausson M, Rizell M, Mjörnstedt L and Friman S: Pseudoaneurysm of
the hepatic artery following liver transplantation. Transplant
Proc. 38:2679–2682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saad WE, Dasgupta N, Lippert AJ, Turba UC,
Davies MG, Kumer S, Gardenier JC, Sabri SS, Park AW, Waldman DL, et
al: Extrahepatic pseudoaneurysms and ruptures of the hepatic artery
in liver transplant recipients: Endovascular management and a new
iatrogenic etiology. Cardiovasc Intervent Radiol. 36:118–127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thorat A, Lee CF, Wu TH, Pan KT, Chu SY,
Chou HS, Chan KM, Wu TJ and Lee WC: Endovascular treatment for
pseudoaneurysms arising from the hepatic artery after liver
transplantation. Asian J Surg. Aug 30–2014.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Girometti R, Como G, Bazzocchi M and
Zuiani C: Post-operative imaging in liver transplantation:
State-of-the-art and future perspectives. World J Gastroenterol.
20:6180–6200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tada T, Kumada T, Toyoda H, Ito T, Sone Y,
Kaneoka Y, Maeda A, Okuda S, Otobe K and Takahashi K: Utility of
contrast enhanced ultrasonography with perflubutane for determining
histologic grade in hepatocellular carcinoma. Ultrasound Med Biol.
41:3070–3078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
D'Onofrio M, Crosara S, De Robertis R,
Canestrini S and Mucelli RP: Contrast-enhanced ultrasound of focal
liver lesions. AJR Am J Roentgenol. 205:W56–W66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fontanilla T, Noblejas A, Cortes C, Minaya
J, Mendez S, Van den Brule E, Hernando CG, Alfageme M, Baños I and
Aguirre E: Contrast-enhanced ultrasound of liver lesions related to
arterial thrombosis in adult liver transplantation. J Clin
Ultrasound. 41:493–500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng RQ, Mao R, Ren J, Xu EJ, Liao M,
Wang P, Lu MQ, Yang Y, Cai CJ and Chen GH: Contrast-enhanced
ultrasound for the evaluation of hepatic artery stenosis after
liver transplantation: Potential role in changing the clinical
algorithm. Liver Transpl. 16:729–735. 2010.PubMed/NCBI
|
|
19
|
Lee SJ, Kim KW, Kim SY, Park YS, Lee J,
Kim HJ, Lee JS, Song GW, Hwang S and Lee SG: Contrast-enhanced
sonography for screening of vascular complication in recipients
following living donor liver transplantation. J Clin Ultrasound.
41:305–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cosgrove D and Harvey C: Clinical uses of
microbubbles in diagnosis and treatment. Med Biol Eng Comput.
47:813–826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanyal R, Zarzour JG, Ganeshan DM,
Bhargava P, Lall CG and Little MD: Postoperative Doppler evaluation
of liver transplants. Indian J Radiol Imaging. 24:360–366. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goel A, Mehta N, Guy J, Fidelman N, Yao F,
Roberts J and Terrault N: Hepatic artery and biliary complications
in liver transplant recipients undergoing pretransplant
transarterial chemoembolization. Liver Transpl. 20:1221–1228. 2014.
View Article : Google Scholar : PubMed/NCBI
|