Introduction
Iodinated contrast media used in diagnostic and
therapeutic procedures often causes nephrotoxicity (1). Contrast-induced nephropathy (CIN) is
one of the main adverse effects of iodinated contrast media in
patients undergoing coronary angiography (CAG) and/or percutaneous
coronary intervention (PCI) (2). CIN
refers to an impairment of renal function (usually defined as the
elevation of serum creatinine levels by 0.5 mg/dl or 25%) that
occurs within 3 days following intravascular administration of
contrast media, with the exclusion of other causes (3,4). CIN is
independently associated with a longer stay in hospital, and
results in increased long-term mortality and increased costs of
medical care that partly result from the prolonged hospital stay
(5,6).
The incidence of CIN can be as high as 50% for high
risk patients (3,4,7). Risk
factors associated with CIN include the type and dosage of contrast
media, concomitant nephrotoxic medication, inflammation, diabetes
mellitus, renal insufficiency, congestive heart failure, increasing
age and female gender (5–10). The presence of multiple risk factors
concomitantly can increase the risk of CIN disproportionately
(7). A key step in minimizing the
risk of developing CIN is to identify patients at risk and initiate
appropriate prophylactic measures (6).
When multiple risk factors coexist, patients with
low baseline hematocrit are reported to have a higher incidence of
CIN (11), suggesting that anemia
may be a risk factor for CIN. Low levels of hemoglobin may cause
hypoxia owing to the decreased oxygen transport capacity (12) and their increased affinity for oxygen
when exposed to contrast media (13), which aggravates hypoxic injury and
further results in renal dysfunction (14,15).
However, few studies have assessed the effects of low hemoglobin
levels on CIN in patients without baseline renal insufficiency. In
the present study, an investigation into whether low levels of
hemoglobin are associated with an increased incidence of CIN in
patients undergoing CAG and/or PCI was performed.
Materials and methods
Study population and exclusion
criteria
The present retrospective study included 841
patients (565 males and 276 females; aged 64.92±10.80 years) who
had been diagnosed with coronary heart disease and had undergone
CAG/PCI at the Second Affiliated Hospital of Zhejiang University
School of Medicine (Hangzhou, China), the Affiliated Wenling
Hospital of Wenzhou Medical University (Wenling, China) or Tongde
Hospital of Zhejiang Province (Hangzhou, China) between January
2013 and December 2013. Patients with baseline renal insufficiency,
tumor-associated anemia, severe infection, cardiogenic shock and
any diseases resulting in abnormal levels of hemoglobin (such as
chronic pulmonary heart disease and congestive heart failure) were
excluded from the present study. Patients who had been exposed to
nephrotoxic medication (aminoglycosides and non-steroidal
anti-inflammatory drugs, with the exception of aspirin),
N-acetylcysteine and interventional anemia management, with the
exception of patients who had a diet of enriched foods prior to
developing CIN, were also excluded. For patients who underwent
multiple CAG/PCI procedures during the study period, only the first
procedure was included for analysis.
In the study population, patients with comorbid
coronary heart diseases, hypertension and diabetes mellitus,
medication or treatment was provided according to corresponding
guidelines, including the following: i) The European Society of
Cardiology Guidelines for the management of acute myocardial
infarction in patients presenting with ST-segment elevation or
without persistent ST-segment elevation; ii) the guidelines of
Chinese Hypertension League for the management of hypertension; and
iii) Chinese Diabetes Society Guidelines for the management of
diabetes (16–19). The demographic, clinical and
laboratory data, including details of CAG/PCI, were collected from
study patients. Written informed consent was obtained from all
patients.
Clinical definitions
Patients were divided into the following two groups
according to their hemoglobin levels: i) Patients with low
hemoglobin levels (hemoglobin-L group; n=156); ii) and patients
with normal hemoglobin levels (hemoglobin-N group; n=685). Baseline
renal insufficiency was defined as an increase in serum creatinine
concentration >133 µmol/l (20).
CIN was defined as an acute deterioration in renal function with an
increase in serum creatinine of 0.5 mg/dl (50 µmol/l), or 25% from
the baseline within 96 h following the intravascular administration
of contrast agents, in the absence of alternative risk factors.
Anemia was defined as a hemoglobin concentration <120 g/l for
males and <110 g/l for females, in accordance with the Chinese
criteria (21).
Treatment and measurements
The patients were exposed to four contrast media, as
follows: Iohexol [100 ml (30 g/l) or 50 ml (15 g/l); Shanghai
General Pharmaceutical, Co., Ltd., Shanghai, China]; iodixanol [100
ml (32 g/l) or 50 ml (16 g/l); Shanghai General Pharmaceutical,
Co., Ltd.]; iopamidol [100 ml (37 g/l) or 50 ml (18.5 g/l); Sine
Pharmaceutical, Co., Ltd., Shanghai, China]; and iopromide [100 ml
(30 g/l) or 50 ml (15 g/l); Bayer Vital GmbH, Leverkusen, Germany].
The patients received saline hydration within 3–12 h prior to and
6–24 h following CAG/PCI procedures, as described previously
(22). Patient demographic, clinical
and laboratory data, including details of the CAG/PCI, were
collected. For patients with diabetes mellitus, the administration
of metformin was withheld at least 24 h prior to the procedure, and
replaced with insulin (NovoRapid 30, Novolin 30R or Novolin 70/30;
Novo Nordisk A/S, Bagsvaerd, Denmark) or oral anti-diabetic drugs,
including repaglinide tablets (Hansoh Pharmaceutical Co., Ltd.,
Jiangsu, China), Nateglinide tablets (Beijing Novartis Pharma Ltd.,
Beijing, China) or Acarbose tablets (Bayer HealthCare Company Ltd.,
Beijing, China). Blood samples (5 ml) were measured for serum
creatinine, glucose, uric acid and lipids using an automatic
biochemical analyzer (Roche Module P800; Roche Diagnostics, Basel,
Switzerland) and hemoglobin was detected using a hematology
analyzer (XE-2100; Sysmex Corporation, Kobe, Japan).
The baseline characteristics of patients in the
hemoglobin-L and hemoglobin-N groups were compared. The incidence
of CIN and changes in serum creatinine prior to and following
CAG/PCI were analyzed. Serum creatinine measurements taken prior to
CAG/PCI were recorded as the baseline creatinine level, and the
post-procedure serum creatinine was recorded as the maximum
creatinine level that was measured within 96 hours of CAG/PCI.
Statistical analysis
Statistical analysis was performed using SPSS
version 20.0 (IBM SPSS, Armonk, NY, USA). Continuous data were
expressed as the mean ± standard deviation when normally
distributed. Categorical data were expressed as the absolute value
and percentage. Comparisons of continuous variables among the four
contrast media were performed using one-way analysis of variance
with multiple Scheffe-type comparisons after reciprocal
transformation of the variables to correct for heterogeneity of
variance. In order to evaluate inter- and intra-group differences,
continuous variables were compared using the paired t-test and
independent-samples t-test, and categorical variables were compared
using a χ2 test or Fisher's exact test where
appropriate. The Bonferroni correction was used for multiple
comparisons. Univariate logistic regression was performed to search
for the potential factor of CIN as a dependent variable. Variables
that were statistically significant on univariate analysis and
other potential variables were identified as predictors of CIN in
the final multivariate model using the forward selection method. A
two-sided 95% confidence interval (CI) was constructed around the
point estimate of the relative risk. Receiver operating
characteristic (ROC) curve analysis was performed to predict CIN.
All tests were two-sided, and P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
As presented in Table
I, patients in the hemoglobin-L group were significantly older
compared with patients in the hemoglobin-N group (70.79±9.00 vs.
63.56±10.72 years; P<0.01). The plasma levels of total
cholesterol, triglycerides, red blood cells, hemoglobin and
hematocrit in the hemoglobin-L group were significantly lower
compared with those in the hemoglobin-N group (P<0.05). In the
hemoglobin-L group, a greater number of patients used calcium
channel blockers and a lower number of patients used proton pump
inhibitors compared with the hemoglobin-N group (P<0.05). There
was no significant difference between the groups regarding the
incidence of CIN resulting from the contrast media used (P=0.39;
Table II). Patients in both groups
had been administered similar volumes of iohexol, iodixanol or
iopamidol (P>0.05), but only a small volume of iopromide
(P<0.01).
 | Table I.Baseline characteristics of patients
with normal (Hemoglobin-N) or low (Hemoglobin-L) levels of
hemoglobin. |
Table I.
Baseline characteristics of patients
with normal (Hemoglobin-N) or low (Hemoglobin-L) levels of
hemoglobin.
| Parameter | Hemoglobin-N group
(n=685) | Hemoglobin-L group
(n=156) | P-value |
|---|
| Female, n (%) | 218 (31.8%) | 58 (37.2%) |
0.20 |
| Age (years) | 63.56±10.72 | 70.79±9.00 | <0.01 |
| Body weight
(kg) | 63.17±11.44 | 61.95±12.05 |
0.24 |
| Hypertension, n
(%) | 421 (61.5%) | 100 (64.1%) |
0.54 |
| Diabetes mellitus,
n (%) | 135 (19.7%) | 39 (25.0%) |
0.14 |
| Current smoking, n
(%) | 172 (25.1%) | 30 (19.2%) |
0.12 |
| Medication
used |
|
|
|
|
Aspirin, n (%) | 672 (98.1%) | 153 (98.1%) | 1.00 |
|
Clopidogrel, n (%) | 667 (97.4%) | 150 (96.2%) |
0.42 |
|
ACEI/ARB, n (%) | 519 (75.8%) | 127 (81.4%) |
0.13 |
|
β-blocker, n (%) | 468 (68.3%) | 109 (69.9%) |
0.70 |
| Statin,
n (%) | 653 (95.3%) | 151 (96.8%) |
0.42 |
|
Anticoagulants, n (%) | 300 (43.8%) | 75 (48.1%) |
0.33 |
| CCB, n
(%) | 192 (28.0%) | 23 (14.7%) | <0.01 |
| PPI, n
(%) | 339 (49.5%) | 102 (65.4%) | <0.01 |
|
Nitrate, n (%) | 305 (44.5%) | 74 (46.8%) |
0.60 |
|
Diuretics, n (%) | 139 (20.3%) | 42 (26.9%) |
0.069 |
| LVEF (%) | 60.98±9.63 | 61.40±11.17 |
0.67 |
| Total cholesterol
(mmol/l) | 4.41±1.14 | 4.15±1.11 | <0.01 |
| Triglyceride
(mmol/l) | 1.75±1.22 | 1.30±0.74 | <0.01 |
| LDL-C (mmol/l) | 2.64±0.97 | 2.51±0.95 |
0.13 |
| Fasting blood
glucose (mmol/l) | 5.88±1.96 | 5.85±1.87 |
0.90 |
| Uric acid
(µmol/l) | 355.85±98.12 | 356.20±120.69 |
0.97 |
| Red blood cell
(1012/l) | 4.39±0.43 | 3.61±0.44 | <0.01 |
| Hemoglobin
(g/l) | 134.04±12.70 | 105.11±11.39 | <0.01 |
| Hematocrit (%) |
0.40±0.039 |
0.33±0.057 | <0.01 |
 | Table II.Contrast media volume used in
patients undergoing CAG and/or PCI. |
Table II.
Contrast media volume used in
patients undergoing CAG and/or PCI.
| Parameter | Iohexol
(n=177) | Iodixanol
(n=133) | Iopamidol
(n=260) | Iopromide
(n=271) | P-value |
|---|
| Mean volume
(ml) | 137.49±86.40 | 127.33±63.02 | 125.63±64.30 |
98.49±51.17a | <0.01 |
| CIN, n (%) | 10 (5.6%) | 13 (9.8%) | 19 (7.3%) | 15 (5.5%) | 0.39 |
Changes in levels of serum creatinine,
incidence of CIN and hospital stay
The mean serum creatinine concentration levels
increased significantly following the intravascular administration
of contrast media in both patient groups (P<0.05; Table III). However, the change in serum
creatinine concentration levels prior to and following the use of
contrast media in the hemoglobin-L group was significantly higher
compared with the hemoglobin-N group (P<0.01).
 | Table III.Changes in serum creatinine and
incidence of contrast-induced nephropathy in patients with normal
(Hemoglobin-N) and low (Hemoglobin-L) levels of hemoglobin. |
Table III.
Changes in serum creatinine and
incidence of contrast-induced nephropathy in patients with normal
(Hemoglobin-N) and low (Hemoglobin-L) levels of hemoglobin.
| Parameter | Hemoglobin-N
(n=685) | Hemoglobin-L
(n=156) | P-value |
|---|
| Serum creatinine
before use of contrast media (µmol/l) | 78.00±18.41 | 80.31±23.35 | 0.31 |
| Serum creatinine
after use of contrast media (µmol/l) | 79.40±18.67 | 87.65±33.49 | <0.01 |
| Absolute change in
serum creatinine (µmol/l) | 1.40±12.00 | 7.35±22.60 | <0.01 |
| CIN | 34 (5.0) | 23 (14.7) | <0.01 |
| Females
with CIN | 9 (26.5) | 10 (43.5) | 0.18 |
| Males
with CIN | 25 (73.5) | 13 (56.5) | |
| Total duration of
hospital stay (days) | 7.52±4.03 | 8.87±4.87 | <0.01 |
| Duration of
hospital stay after use of contrast media (days) | 4.59±3.55 | 5.04±3.54 | 0.15 |
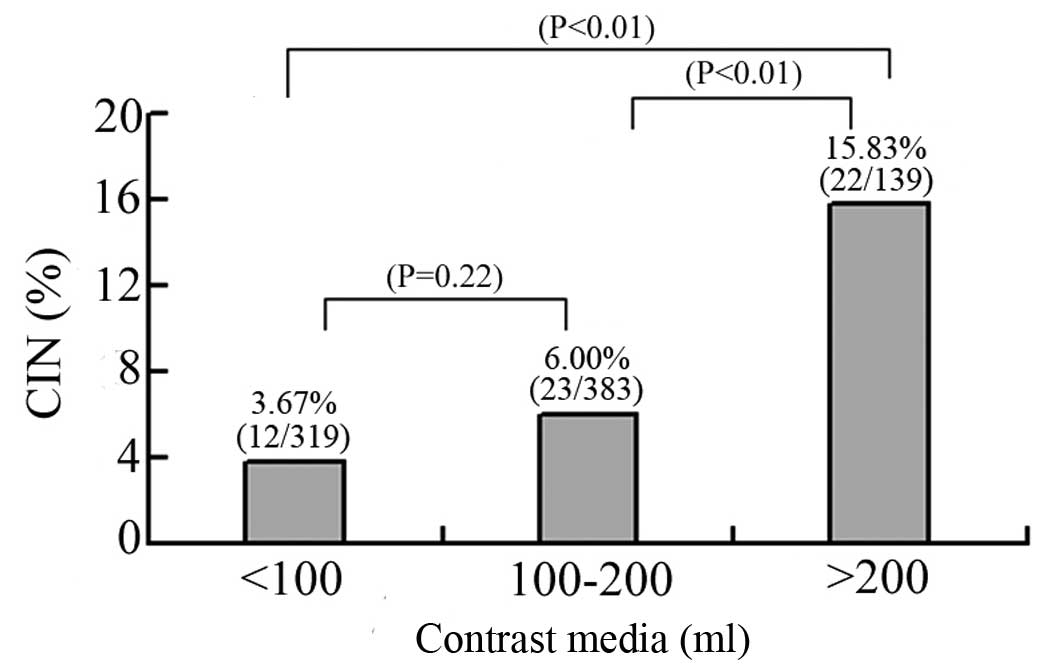
CIN occurred in 6.78% (57 of 841 patients) of the
total patients in the study. Notably, there was no significant
difference in the occurrence of CIN in genders between the two
groups (females, 26.5 vs. 43.5%; males, 73.5 vs. 56.5%; P=0.18;
Table III). Among the 57 patients
who developed CIN, 12 (12/319, 3.76%) had received <100 ml
contrast media, 23 (23/383, 6.00%) received 100–200 ml contrast
media, and 22 (22/139, 15.83%) received ≥200 ml contrast media
(Fig. 1).
The mean total duration of hospital stay of patients
in the hemoglobin-L group was 8.87±4.87 days, which was
significantly longer than that of patients in the hemoglobin-N
group (7.52±4.03 days; P<0.01). The mean duration of hospital
stay following administration of the contrast media was not
significantly different between the two groups (4.59±3.55 vs.
5.04±3.54 days; P=0.15).
Risk factors associated with CIN
In a univariate model, contrast media volume ≥200
ml, diuretic use, low levels of hemoglobin, diabetes mellitus,
hyperuricemia, use of anticoagulants and age ≥70 years were
associated with the development of CIN (P<0.05). Multivariate
analysis was performed to evaluate these baseline univariate
predictors. Contrast media volume ≥200 ml (RR, 4.64; 95% CI,
2.15–9.99; P<0.01), diuretic usage (RR, 3.68; 95% CI, 2.07–6.53;
P<0.01), low hemoglobin levels (RR, 3.07; 95% CI, 1.69–5.56;
P<0.01) and diabetes mellitus (RR, 2.46; 95% CI, 1.35–4.46;
P<0.01) were found to be associated with an increased risk of
CIN in the hemoglobin-L group (Table
IV). For the patients with normal hemoglobin levels, contrast
media volume ≥200 ml (RR, 4.56; 95% CI, 1.73–12.03; P<0.01) and
diuretic usage (RR, 3.31; 95% CI, 1.62–6.76; P<0.01) were shown
to be associated with an increased risk of CIN (Table V).
 | Table IV.Risk factors associated with
contrast-induced nephropathy in the hemoglobin-L group were
determined by univariate and multivariate logistic regression
analyses. |
Table IV.
Risk factors associated with
contrast-induced nephropathy in the hemoglobin-L group were
determined by univariate and multivariate logistic regression
analyses.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Contrast media
volume ≥200 ml | 4.81 |
2.31–10.03 | <0.01 | 4.64 | 2.15–9.99 | <0.01 |
| Use of
diuretics | 3.92 | 2.27–6.78 | <0.01 | 3.68 | 2.07–6.53 | <0.01 |
| Low levels of
hemoglobin | 3.31 | 1.89–5.80 | <0.01 | 3.07 | 1.69–5.56 | <0.01 |
| Diabetes
mellitus | 2.61 | 1.49–4.58 | <0.01 | 2.46 | 1.35–4.46 | <0.01 |
|
Hypercholesterolemia | 2.14 | 1.23–3.70 | <0.01 |
|
|
|
| Use of
anticoagulants | 2.44 | 1.39–4.28 | <0.05 |
|
|
|
| Age ≥70 years | 1.83 | 1.07–3.14 | <0.05 |
|
|
|
| Use of PPI | 1.60 | 0.92–2.80 |
0.096 |
|
|
|
| Contrast media
volume 100–200 ml | 1.63 | 0.80–3.34 |
0.18 |
|
|
|
| LDL-C | 1.32 | 0.70–2.52 |
0.39 |
|
|
|
| Use of
nitrates | 1.20 | 0.70–2.05 |
0.51 |
|
|
|
| Use of statins | 1.29 | 0.30–5.48 |
0.74 |
|
|
|
| Gender
(female) |
1.025 | 0.58–1.81 |
0.93 |
|
|
|
| Use of ACEI | 1.02 | 0.54–1.94 |
0.94 |
|
|
|
 | Table V.Risk factors associated with
contrast-induced nephropathy in the hemoglobin-N group were
determined by univariate and multivariate logistic regression
analyses. |
Table V.
Risk factors associated with
contrast-induced nephropathy in the hemoglobin-N group were
determined by univariate and multivariate logistic regression
analyses.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Contrast media
volume ≥200 ml | 4.53 | 1.73–11.82 | <0.01 | 4.56 | 1.73–12.03 | <0.01 |
| Use of
diuretics | 3.29 | 1.63–6.65 | <0.01 | 3.31 | 1.62–6.76 | <0.01 |
| Diabetes
mellitus | 2.34 | 1.13–4.86 |
0.02 |
|
|
|
| Use of
anticoagulants | 1.30 | 0.65–2.59 |
0.46 |
|
|
|
| Age | 1.30 | 0.63–2.69 |
0.47 |
|
|
|
| Use of PPI | 1.69 | 0.83–3.44 |
0.15 |
|
|
|
| Contrast media
volume 100–200 ml | 1.97 | 0.79–4.91 |
0.15 |
|
|
|
| LDL-C | 1.33 | 0.59–3.02 |
0.49 |
|
|
|
| Use of
nitrates | 1.43 | 0.72–2.85 |
0.31 |
|
|
|
| Use of ACEI | 1.25 | 0.53–2.92 |
0.61 |
|
|
|
| Smoking | 1.08 | 0.49–2.36 |
0.85 |
|
|
|
| Total
cholesterol | 1.05 | 0.46–2.36 |
0.91 |
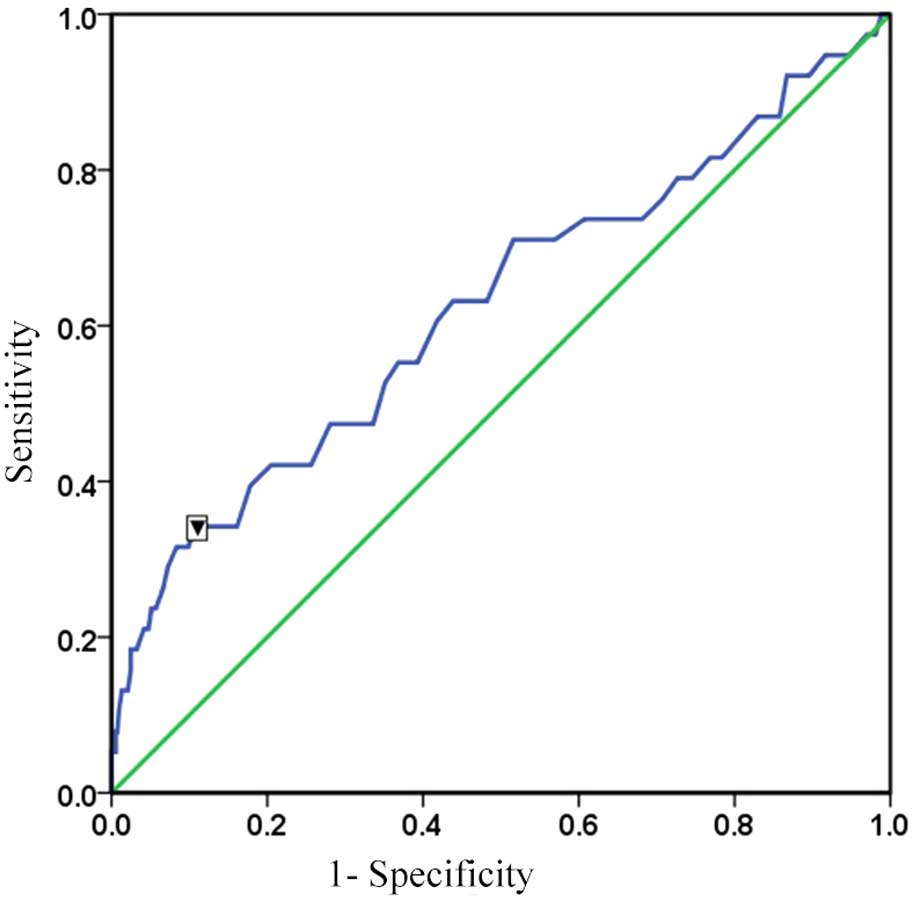
ROC
ROC curve analysis was conducted to determine the
cut-off point at which there is a high probability of developing
CIN. Male and female patients were analyzed separately. It was
observed that the optimal cut-off point of serum hemoglobin
concentration for predicting CIN was 111.5 g/l in females, with a
sensitivity of 63.2% and specificity of 76.3%, and an area under
the curve (AUC) of 0.737 (P<0.01; Fig. 2). A serum hemoglobin concentration of
115.5 g/l was determined as the optimal cut-off point for
predicting CIN in males, with a sensitivity of 34.2% and
specificity of 89.2%, and an AUC of 0.625 (P<0.01; Fig. 3). Maintaining a concentration of
hemoglobin >130 g/l decreased the incidence of CIN by 26.3% and
25.8% for males and females respectively.
Discussion
The present study demonstrated that patients with
low levels of hemoglobin have a nearly three-fold higher incidence
of developing CIN compared with patients with normal levels of
hemoglobin, suggesting that low hemoglobin is a strong risk factor
for developing CIN. Furthermore, patients with low hemoglobin
levels had longer total hospital stay. The cut-off hemoglobin
values for predicting CIN were 115.5 g/l for male and 111.5 g/l for
female patients.
In the current study, although the patients with low
levels of hemoglobin were older, multivariate analysis did not
identify age ≥70 years as a risk factor. It has been reported that
patients ≥70 years have an increased risk of CIN (23,24);
however, the reasoning behind this is multifactorial and partially
due to the reduced renal function typically observed in elderly
patients (7). Thus, it is reasonable
to account for advanced age when planning the administration of
contrast media.
Four different contrast media were investigated in
the present study, including the iso-osmolality medium iodixanol
and the low-osmolality media iohexol, iopamidol and iopromide.
Iso-osmolality media are believed to cause a low incidence of CIN
in comparison with low-osmolality media; however, this remains
controversial (23). Solomon
(25) reported that the risk of CIN
was significantly lower with iodixanol and iopamidol compared with
iohexol, but no significant difference was observed between
iodixanol and iopamidol. In an earlier meta-analysis, it was
observed that there was no difference in the incidence of CIN in
patients without pre-existing renal insufficiency, or injury based
on the use of contrast media with different osmolalities (26), and these results were consistent with
the findings of the present study.
Besides renal injury and changes in serum
creatinine, other risk factors of CIN have been reported, including
high-dose contrast media, diuretic usage and diabetes (26–29). A
previous study reported that the incidence of CIN increases with
higher doses of contrast media (27), and in the present study, patients
receiving ≥200 ml contrast media were more likely to suffer from
CIN. However, patients had a similar risk of developing CIN when
receiving <100 or 100–200 ml contrast media, suggesting that a
potential safe range of contrast media is <200 ml. Thus, it is
possible to decrease the incidence of CIN in patients undergoing
CAG/PCI by reducing the dose of contrast media to <200 ml.
The association of diuretics with the development of
CIN may be a result of their nephrotoxic potential, particularly in
patients with pre-existing renal diseases (28). Patients treated with diuretics have
been reported to have a greater incidence of CIN (30), and it is recommended that diuretics
should be discontinued for at least 24 h prior to the
administration of contrast media, particularly when the glomerular
filtration rate is <60 ml/min per 1.73 m2 (28). The present study determined that the
use of diuretics resulted in a high risk of developing of CIN (RR,
3.68; 95% CI, 2.07–6.53; P<0.01). However, diuretics have
previously been reported to have a potentially protective effect
against kidney injury (31,32). Due to these conflicts of evidence, a
meta-analysis was performed to examine the clinical efficacy of
furosemide administration in preventing CIN (33). Unexpectedly, furosemide, as a
diuretic, has no additional influence beyond saline hydration on
the incidence of CIN (33). However,
the impact of furosemide dose on clinical outcome was not taken
into account. In the present study, the mean daily dose of
furosemide in patients with CIN was 30.37±10.18 mg, which is a
higher dose than that used in similar studies (31). Thus, it remains to be demonstrated
whether a high dose of diuretics is related to a higher risk of
developing CIN.
Diabetic nephropathy has been identified as a strong
and independent risk factor for CIN (34), which is consistent with the findings
of the present study. In addition, individuals with diabetes, even
in the absence of nephropathy, are more likely to develop CIN
compared with non-diabetic individuals (4). Therefore, diabetes mellitus as a risk
factor for developing CIN should not be underestimated, and
patients with diabetes mellitus, regardless of whether they have
renal dysfunction, should be evaluated closely prior to the
administration of contrast media.
The impact that low hemoglobin levels have on the
development of CIN has not been clearly identified. Previous
studies have concluded that low levels of hemoglobin are not a risk
factor for CIN (8,35,36),
while one study observed that a reduction in hematocrit increased
the incidence of CIN in patients with or without chronic kidney
disease (RR, 1.23; 95% CI, 1.14–1.31; P<0.01) (11). It is likely that a number of these
studies did not rigorously exclude the confounders related to low
hemoglobin or serum creatinine, such as chronic kidney disease. Low
levels of hemoglobin may occur as a result of various conditions,
such as immunological disease, malignancy, chemical exposure or
chronic kidney disease, which results in difficulty in assessing
the independent effects of hemoglobin on CIN (29,37–40). In
the present study, however, low levels of hemoglobin were
demonstrated to be an independent risk factor for CIN, increasing
the risk by 3.07 (95% CI, 1.69–5.56; P<0.01), although it was
not the highest risk factor for CIN and was second to contrast
media volume ≥200 ml and the use of diuretics. An ROC analysis for
male and female patients separately was performed in the present
study, and it was determined that the different cut-off values of
serum hemoglobin for developing CIN in males and females was
different, emphasizing that gender should be taken into account
when assessing the risk of developing CIN.
It is important to note that low hemoglobin levels
are often associated with poor prognosis and mortality in patients
(41). It has been reported that
there is a dose-dependent increase in mortality when hemoglobin
levels decrease below the optimal hemoglobin range (42). The risk of mortality increases 2.5
times for every gram reduction in hemoglobin <80 mg/l (43). Furthermore, Shah et al
(42) reported that the risk
associated with low levels of hemoglobin is greater in patients
with myocardial infarction than for those with stable angina.
Therefore, a previous study treated anemic patients with myocardial
injury with blood transfusions and demonstrated favorable outcomes
(44). In addition, patients with
coronary artery disease are given treatment to maintain their
hemoglobin concentrations at a minimum of 100 g/l (45). In each case, prophylactic blood
transfusions may decrease the risk of developing CIN and the risk
of mortality, in particular in anemic patients at risk of
myocardial infarction.
In the present study, no severe clinical
manifestations in the patients with CIN were detected, such as
acute renal failure requiring dialysis or mortality resulted from
CIN. In general, levels of serum creatinine typically peaked at 3–5
days following exposure to contrast agents, and returned to the
baseline, or near baseline, level within 1–3 weeks following
adequate hydration (46).
Several limitations of the present study should be
noted, firstly that it is a retrospective study. Secondly, the
renal function of patients was only assessed based on the increase
in serum creatinine; no other indicators, such as glomerular
filtration rate, were used. Thirdly, the present study included
patients with multi-vessel and single coronary artery diseases, and
the former may necessitate the use of higher volumes of contrast
media. Finally, the hemoglobin level in populations is known to
vary with altitude (47). The
current study was performed in Southeast China, a region of low
altitude. Thus, the results of the present study should be reviewed
with caution.
In conclusion, patients with low levels of
hemoglobin, including those with normal renal function, are at a
higher risk of developing CIN. Therefore, the level of hemoglobin
should be closely monitored in patients with low hemoglobin prior
to administration of contrast media, particularly in those with
hemoglobin levels below the cut-off point and at risk of developing
CIN.
Acknowledgements
The present study was supported by grants from the
Wenling Foundation of Science and Technology (no. 2011WLCB0109 and
2014C311051), the Natural Science Foundation of China (no. 81100993
and 81300311), the Zhejiang Natural Science Foundation (no.
LY12H03001 and LQ13H280002) and the Research Development Fund of
Wenzhou Medical University (no. QTJ15001).
Glossary
Abbreviations
Abbreviations:
|
CAG
|
coronary angiography
|
|
CIN
|
contrast-induced nephropathy
|
|
PCI
|
percutaneous coronary intervention
|
References
|
1
|
Parfrey P, Griffiths S, Barrett B, Paul
MD, Genge M, Withers J, Farid N and McManamon PJ: Contrast
material-induced renal failure in patients with diabetes mellitus,
renal insufficiency or both: A prospective controlled study. N Engl
J Med. 320:143–149. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gandhi S, Mosleh W, Abdel-Qadir H and
Farkouh ME: Statins and contrast-induced acute kidney injury with
coronary angiography. Am J Med. 127:987–1000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chertow GM, Christiansen CL, Cleary PD,
Munro C and Lazarus JM: Prognostic stratification in critically ill
patients with acute renal failure requiring dialysis. Arch Intern
Med. 155:1505–1511. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rihal CS, Textor SC, Grill DE, Berger PB,
Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, et al:
Incidence and prognostic importance of acute renal failure after
percutaneous coronary intervention. Circulation. 105:2259–2264.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palli E, Makris D, Papanikolaou J,
Garoufalis G and Zakynthinos E: Contrast-induced nephropathy in
aged critically ill patients. Oxid Med Cell Longev.
2014:7564692014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwasa EA, Vinayak S and Armstrong R: The
role of inflammation in contrast-induced nephropathy. Br J Radiol.
87:201307382014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pucelikova T, Dangas G and Mehran R:
Contrast-induced nephropathy. Catheter Cardiovasc Interv. 71:62–72.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toprak O, Cirit M, Yesil M, Bayata S,
Tanrisev M, Varol U, Ersoy R and Esi E: Impact of diabetic and
pre-diabetic state on development of contrast-induced nephropathy
in patients with chronic kidney disease. Nephrol Dial Transplant.
22:819–826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehran R and Nikolsky E: Contrast-induced
nephropathy: Definition, epidemiology, and patients at risk. Kidney
Int Suppl. 69:S11–S15. 2006. View Article : Google Scholar
|
|
10
|
Lee J, Cho JY, Lee HJ, Jeong YY, Kim CK,
Park BK, Sung DJ, Kang BC, Jung SI, Lee EJ, et al: Korean Society
of Urogenital Radiology (KSUR); Korean Society of Radiology:
Contrast-induced nephropathy in patients undergoing intravenous
contrast-enhanced computed tomography in Korea: A
multi-institutional study in 101487 patients. Korean J Radiol.
15:456–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikolsky E, Mehran R, Lasic Z, Mintz GS,
Lansky AJ, Na Y, Pocock S, Negoita M, Moussa I, Stone GW, et al:
Low hematocrit predicts contrast-induced nephropathy after
percutaneous coronary interventions. Kidney Int. 67:706–713. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Celsing F, Svedenhag J, Pihlstedt P and
Ekblom B: Effects of anaemia and stepwise-induced polycythaemia on
maximal aerobic power in individuals with high and low haemoglobin
concentrations. Acta Physiol Scand. 129:47–54. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SJ, Salem MR, Joseph NJ, Madayag MA,
Cavallino RP and Crystal GJ: Contrast media adversely affect
oxyhemoglobin dissociation. Anesth Analg. 71:73–76. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heyman SN, Evans RG, Rosen S and
Rosenberger C: Cellular adaptive changes in AKI: Mitigating renal
hypoxic injury. Nephrol Dial Transplant. 27:1721–1728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cigarroa RG, Lange RA, Williams RH and
Hillis LD: Dosing of contrast material to prevent contrast
nephropathy in patients with renal disease. Am J Med. 86:649–652.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Task Force on the management of ST-segment
elevation acute myocardial infarction of the European Society of
Cardiology (ESC). Steg PG, James SK, Atar D, Badano LP,
Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq
G, Fernandez-Aviles F, et al: ESC Guidelines for the management of
acute myocardial infarction in patients presenting with ST-segment
elevation. Eur Heart J. 33:2569–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamm CW, Bassand JP, Agewall S, Bax J,
Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, et al: ESC
Committee for Practice Guidelines: ESC Guidelines for the
management of acute coronary syndromes in patients presenting
without persistent ST-segment elevation: The Task Force for the
management of acute coronary syndromes (ACS) in patients presenting
without persistent ST-segment elevation of the European Society of
Cardiology (ESC). Eur Heart J. 32:2999–3054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu LS: Writing Group of 2010 Chinese
Guidelines for the Management of Hypertension: 2010 Chinese
guidelines for the management of hypertension. Zhonghua Xin Xue
Guan Bing Za Zhi. 39:579–615. 2011.(In Chinese). PubMed/NCBI
|
|
19
|
Chinese Diabetes Society: 2010 Chinese
Guidelines for the Management of Diabetes. Zhonghua Xin Xue Guan
Bing Za Zhi. 20:S1–S36. 2012.(In Chinese).
|
|
20
|
Ruilope LM, Salvetti A, Jamerson K,
Hansson L, Warnold I, Wedel H and Zanchetti A: Renal function and
intensive lowering of blood pressure in hypertensive participants
of the hypertension optimal treatment (HOT) study. J Am Soc
Nephrol. 12:218–225. 2001.PubMed/NCBI
|
|
21
|
Guo J, Zheng C, Xiao Q, Gong S, Zhao Q,
Wang L, He J, Yang W, Shi X, Sun X and Liu J: Impact of anaemia on
lung function and exercise capacity in patients with stable severe
chronic obstructive pulmonary disease. BMJ Open. 5:e0082952015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chinese Society of Cardiology; Editorial
Committee of Chinese Journal of Cardiology: Chinese expert
consensus on the clinical application of iodinated contrast media
in cardiovascular diseases. Zhonghua Xin Xue Guan Bing Za Zhi.
41:94–98. 2013.(In Chinese). PubMed/NCBI
|
|
23
|
Rich MW and Crecelius CA: Incidence, risk
factors, and clinical course of acute renal insufficiency after
cardiac catheterization in patients 70 years of age or older. A
prospective study. Arch Intern Med. 150:1237–1242. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kini AS, Mitre CA, Kim M, Kamran M, Reich
D and Sharma SK: A protocol for prevention of radiographic contrast
nephropathy during percutaneous coronary intervention: Effect of
selective dopamine receptor agonist fenoldopam. Catheter Cardiovasc
Interv. 55:169–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Solomon R: The role of osmolality in the
incidence of contrast-induced nephropathy: A systematic review of
angiographic contrast media in high risk patients. Kidney Int.
68:2256–2263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barrett BJ and Carlisle EJ: Metaanalysis
of the relative nephrotoxicity of high- and low-osmolality
iodinated contrast media. Radiology. 188:171–178. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nozue T, Michishita I, Iwaki T, Mizuguchi
I and Miura M: Contrast medium volume to estimated glomerular
filtration rate ratio as a predictor of contrast-induced
nephropathy developing after elective percutaneous coronary
intervention. J Cardiol. 54:214–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldenberg I and Matetzky S: Nephropathy
induced by contrast media: Pathogenesis, risk factors and
preventive strategies. CMAJ. 172:1461–1471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chinese society of cardiology, Editorial
committee of Chinese journal of cardiology: Chinese expert
consensus on the clinical application of iodinated contrast media
in cardiovascular diseases. Chinese Journal of Cardiology.
41:94–98. 2013.PubMed/NCBI
|
|
30
|
Alamartine E, Phayphet M, Thibaudin D,
Barral FG and Veyret C: Contrast medium-induced acute renal failure
and cholesterol embolism after radiological procedures: Incidence,
risk factors, and compliance with recommendations. Eur J Intern
Med. 14:426–431. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho KM and Power BM: Benefits and risks of
furosemide in acute kidney injury. Anaesthesia. 65:283–293. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gu GQ, Lu R, Cui W, Liu F, Zhang Y, Yang
XH, Chen XF and Jia WM: Low-dose furosemide administered with
adequate hydration reduces contrast-induced nephropathy in patients
undergoing coronary angiography. Cardiology. 125:69–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toprak O and Cirit M: Risk factors for
contrast-induced nephropathy. Kidney Blood Press Res. 29:84–93.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toprak O, Cirit M, Yesil M, Byrne DW,
Postaci N, Bayata S, Majchrzak KM and Esi E: Metabolic syndrome as
a risk factor for contrast-induced nephropathy in non-diabetic
elderly patients with renal impairment. Kidney Blood Press Res.
29:2–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Tan N, Zhou YL, He PC, Luo JF and
Chen JY: The contrast medium volume to estimated glomerular
filtration rate ratio as a predictor of contrast-induced
nephropathy after primary percutaneous coronary intervention. Int
Urol Nephrol. 44:221–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Macciò A, Madeddu C, Gramignano G, Mulas
C, Tanca L, Cherchi MC, Floris C, Omoto I, Barracca A and Ganz T:
The role of inflammation, iron, and nutritional status in
cancer-related anemia: Results of a large, prospective,
observational study. Haematologica. 100:124–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weiss G and Goodnough LT: Anemia of
chronic disease. N Engl J Med. 352:1011–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Waters JS, O'Brien ME and Ashley S:
Management of anemia in patients receiving chemotherapy. J Clin
Oncol. 20:601–603. 2002.PubMed/NCBI
|
|
39
|
Hsu CY, McCulloch CE and Curhan GC:
Epidemiology of anemia associated with chronic renal insufficiency
among adults in the United States: Results from the Third National
Health and Nutrition Examination Survey. J Am Soc Nephrol.
13:504–510. 2002.PubMed/NCBI
|
|
40
|
Nurko S: Anemia in chronic kidney disease:
Causes, diagnosis, treatment. Cleve Clin J Med. 73:289–297. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Astor BC, Coresh J, Heiss G, Pettitt D and
Sarnak MJ: Kidney function and anemia as risk factors for coronary
heart disease and mortality: The Atherosclerosis Risk in
Communities (ARIC) Study. Am Heart J. 151:492–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shah AD, Nicholas O, Timmis AD, Feder G,
Abrams KR, Chen R, Hingorani AD and Hemingway H: Threshold
haemoglobin levels and the prognosis of stable coronary disease:
Two new cohorts and a systematic review and meta-analysis. PLoS
Med. 8:e10004392011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carson JL, Noveck H, Berlin JA and Gould
SA: Mortality and morbidity in patients with very low postoperative
Hb levels who decline blood transfusion. Transfusion. 42:812–818.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barbarova I, Klempfner R, Rapoport A,
Wasserstrum Y, Goren I, Kats A and Segal G: Avoidance of Blood
Transfusion to Patients Suffering From Myocardial Injury and Severe
Anemia Is Associated With Increased Long-Term Mortality: A
Retrospective Cohort Analysis. Medicine (Baltimore). 29:e16352015.
View Article : Google Scholar
|
|
45
|
Klein MJ, Carter TI, Smith MC, Wong J and
Sugiyama G: Prophylactic hypothermia and neuromuscular blockade to
limit myocardial oxygen demand in a critically anemic Jehovah's
Witness after emergency surgery. J Surg Case Rep. 12:rju1352014.
View Article : Google Scholar
|
|
46
|
McCullough PA and Sandberg KR:
Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med.
4(Suppl 5): S3–S9. 2003.
|
|
47
|
World Health Organization: Haemoglobin
concentrations for the diagnosis of anaemia and assessment of
severity. Vitamin and Mineral Nutrition Information System. WHO.
(Geneva). 1–6. 2011.
|