Introduction
Immune-mediated liver disease is caused by abnormal
immune responses in the liver. Numerous cell types are attacked by
the immune system, leading to liver damage and other organ
disorders (1,2). The three major immune-mediated liver
diseases include primary biliary cirrhosis, primary sclerosing
cholangitis and autoimmune hepatitis, and these are associated with
differing autoimmune pathogenic mechanisms (3–5). The
estimated prevalence of the overlap syndrome (presentation of
clinical characteristics of a second auto-immune liver disease in
patients with an existing auto-immune liver disease) of
immune-mediated liver disease is 5–20% (6). Although the exact etiology remains
unclear, evidence has suggested that impairment of apoptotic cell
debri clearance may contribute to the development of
immune-mediated liver disease (3).
Immunosuppressive therapies, including corticosteroids, have been
effective in relieving the symptoms of autoimmune hepatitis,
however, therapeutic progress remains limited (1,7).
4-1BB is an inducible T-cell surface receptor that
belongs to the tumor necrosis factor receptor (TNFR) superfamily.
4-1BB is typically expressed at very low levels on naive T-cells;
however, its expression is induced upon T-cell activation, such
that it is expressed at relatively high levels on activated T-cells
(8–10). The interaction of 4-1BB with its
ligand (4-1BBL) regulates T-cell-mediated immune responses by
providing CD 28-independent co-stimulation for T-cell activation
(8,10). Previous studies on transgenic mice
demonstrated that 4-1BBL-deficient transgenic mice were less
responsive to the influenza virus, and that 4-1BB knockout mice had
reduced T-cell activity. These findings further supported a role
for 4-1BB in T-cell-mediated immune responses (10–13).
4-1BB and 4-1BBL have previously been associated
with the development of autoimmune disease. The expression levels
of 4-1BBL in the duct epithelial cells of salivary glands were
previously demonstrated to be markedly elevated in a mouse model of
Sjörgren's syndrome and were associated with the severity of
sialadenitis (14). In addition,
4-1BBL has been indicated to be essential for the development of
autoimmune encephalomyelitis in a mouse model of multiple sclerosis
(15). In another study, the serum
levels of the soluble forms of 4-1BB and 4-1BBL were significantly
higher in patients with rheumatoid arthritis, as compared with
those in healthy controls, and were correlated with disease
severity (16). Furthermore,
patients with multiple sclerosis exhibited increased serum levels
of soluble 4-1BBL, as compared with healthy controls (17).
The role of 4-1BB and 4-1BBL in immune-mediated
liver disease remains unclear. Therefore, the present study aimed
to analyze the expression levels of 4-1BB in the T-cells of a mouse
model of concanavalin A (Con A)-induced immune-mediated liver
injury. In addition, the effects of an anti-4-1BB monoclonal
antibody (4-1BB mAb) on Con A-induced liver injury were
investigated.
Materials and methods
Reagents
Con A was obtained from Sigma-Aldrich (St. Louis,
MO, USA). Fluorescence-conjugated anti-mouse CD3 (1:100; cat. no.
100204), anti-mouse CD4 (1:100; cat. no. 130308, anti-mouse CD25
(cat. no. 101908) and 4-1BB (LEAF purified anti-mouse CD137; cat.
no. 106107) monoclonal antibodies were purchased from BioLegend,
Inc. (San Diego, CA, USA) and diluted 1:100. Methylprednisolone
(MEP) was purchased from GE Healthcare Life Sciences (Uppsala,
Sweden).
Establishment of a mouse model of Con
A-induced immune-mediated liver injury
A total of 60 male Kunming mice (age, 6–7-weeks;
weight, 20–30 g) were purchased from the Animal Center of Shandong
Province (Jinan, China). The mice were maintained in a clean room
under a 12-h dark:light cycle at 22–26°C and 40–70% humidity, at
the Animal Care Center of Shandong University, with ad
libitum access to food and water. All experimental procedures
were approved by the ethics committee at Shandong University
(Jinan, China).
In order to establish a mouse model of
immune-mediated liver injury, the mice were injected with 0.3 ml
Con A solution (20 mg/kg) in phosphate-buffered saline (PBS) into
the tail vein. The control mice were injected with 0.3 ml of PBS
only. The mice were randomly divided into eight groups, as follows
(n=10/group): i) The control group, with PBS injection; ii) the Con
A group, Con A injection; iii) the Con A + 4-1BB mAb group, 4-1BB
mAb (100 µg) was injected into the tail vein at 2 h post-Con A
injection; iv) the Con A + MEP group, mice were intraperitoneally
injected with MEP (3 mg/kg) at 2 h post-Con A injection; v) the Con
A + 4-1BB mAb + MEP group, mice were injected with 4-1BB mAb (100
µg) and MEP (3 mg/kg) at 2 h post-Con A injection; vi) the Con A +
preventive 4-1BB mAb group, 4-1BB mAb (100 µg) was injected into
the tail vein 2 h prior to Con A injection; vii) the Con A +
control IgG (100 µg); and viii) the Con A + preventive IgG (100
µg). All mice were closely monitored following injection.
Liver function assessment
Following anesthetization by abdominal injection
with 2% pentobarbital solution (Sigma-Aldrich), blood was collected
from the heart of mice at 8 h post-Con A injection. A 50 µl aliquot
of the blood sample was analyzed using a AU5400 Chemistry Automated
Analyzer (Olympus Corporation) to assess the liver function by
determining the aspartate transaminase (AST) and alanine
transaminase (ALT) levels.
Hematoxylin and eosin (H&E)
staining of liver tissue
The mice were sacrificed by cervical dislocation 8 h
following Con A injection. The liver was dissected, fixed in 4%
formaldehyde for 24 h and embedded in paraffin (both
Sigma-Aldrich). Subsequently, the liver tissue was sectioned into
0.5 mm3 sections using a rotary microtome (Leica
Microsystems GmbH, Wetzlar, Germany). Tissue sections were
deparaffinized with xylene, followed by sequential washing with
anhydrous ethanol, 95% ethanol, 85% ethanol and finally, 75%
ethanol. Sections were then washed with water for 4 minutes prior
to staining with H&E (Sigma-Aldrich). Images were captured
using a BH2 microscope (Olympus Corporation, Tokyo, Japan) at
magnification, ×20.
Flow cytometry
In order to prevent coagulation, 50 µl
ethylenediaminetetraacetic acid (BD Biosciences, Franklin Lakes,
NJ, USA) was added to each aliquot of mouse blood, after which, the
blood samples were diluted with PBS and incubated with fluorescein
isothiocyanate (FITC-conjugated CD3 (1.25 µl), phycoerythrin
(PE)-conjugated CD4 (1.2 µl), FITC-CD25 (0.5 µl) or PE-4-1BB (1 µl)
antibodies on ice for 20 min. Subsequently, the blood samples were
incubated with 500 µl OptiLyse C Hemolysin (Beckman Coulter Inc.,
Brea, CA, USA) at 37°C for 10 min to lyse and remove erythrocytes,
followed by washing with PBS and centrifugation for 5 min at 430 ×
g. The cell pellet was resuspended in PBS, mixed and analyzed on a
FACScan flow cytometer (BD Biosciences). Data were analyzed using
the Epics XL software (version 3.0; Beckman Coulter, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were conducted using SPSS software, version
12.0 (SPSS, Inc., Chicago, IL, USA). Intergroup comparisons were
conducted using Student's t-test, whereas multigroup comparisons
were performed using analysis of variance followed by Dunnett's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Con A injection induces liver damage
in mice
Compared with the control group, the mice that were
injected with Con A appeared less active and had a poor appetite. A
histopathological examination detected a large number of necrotic
hepatocytes in the liver tissue from the Con A group, and these
necrotic hepatocytes were smaller, than normal hepatocytes,
containing condensed chromatin and exhibiting reticular
degeneration and steatosis. In addition, the liver tissue from the
Con A group exhibited marked lymphocyte and neutrophil
infiltration, in particular within the hepatic portal area.
Furthermore, severe erythrocyte sedimentation was observed in the
hepatic sinusoid (Fig. 1A and B).
The Con A-injected mice exhibited significantly increased serum
levels of ALT and AST, compared with the control mice (P<0.01;
Fig. 1C); thus suggesting that the
liver function was impaired following Con A injection. In addition,
the percentage of 4-1BB-positive T-cells was significantly
increased in the Con A-injected mice compared with the control mice
(P=0.0018; Fig. 1D). These results
suggest that Con A injection may cause liver damage in mice and
stimulate 4-1BB expression in T-cells.
4-1BB mAb exerts beneficial effects on
mice with Con A-induced liver damage
Compared with the control mice, the mice injected
with 4-1BB mAb at 2 h post-Con A injection became increasingly
active and exhibited an improved appetite at 3–4 h post-injection.
H&E staining detected a marked reduction in hepatocyte necrosis
and decreased lymphocyte and neutrophil infiltration into the
hepatic portal area in mice treated with 4-1BB, compared with the
control group mice (Fig. 2A).
Furthermore, the serum levels of ALT and AST were significantly
reduced by 4-1BB mAb injection post-ConA-induced (P<0.01;
Fig. 2B), which indicated that
treatment with 4-1BB mAb was able to preserve liver function in Con
A-injected mice. Consistent with these results, injection with
4-1BB mAb at 2 h prior to the induction of liver injury by Con A
markedly attenuated Con A-induced liver damage. In addition,
preventive treatment of the mice with 4-1BB mAb maintained physical
activity, reduced liver tissue damage (Fig. 2A) and preserved liver function in the
mice (P<0.05; Fig. 2B) compared
with the preventive control IgG.
MEP has been widely used to treat immune-mediated
liver disease in clinical practice (5). Therefore, the present study analyzed
whether a combination treatment with MEP and 4-1BB mAb would
synergistically act to reduce Con A-induced liver damage in mice.
Compared with the control, MEP treatment alone improved the
physical activity of the mice and reduced liver tissue damage
(Fig. 2A) to a similar extent as
4-1BB mAb treatment alone; however, it reduced the serum levels of
ALT and AST to a greater extent than 4-1BB mAb treatment alone
(Fig. 2C). The effects of combined
MEP and 4-1BB mAb on the physical activity of the mice and liver
tissue injury (Fig. 2A) appeared
similar to those observed for either treatment alone; however, the
serum levels of ALT and AST were further reduced following the
combined treatment (P<0.01; Fig.
2C). These results suggest that a treatment with MEP combined
with 4-1BB mAb may preserve liver function in mice with Con
A-induced liver injury.
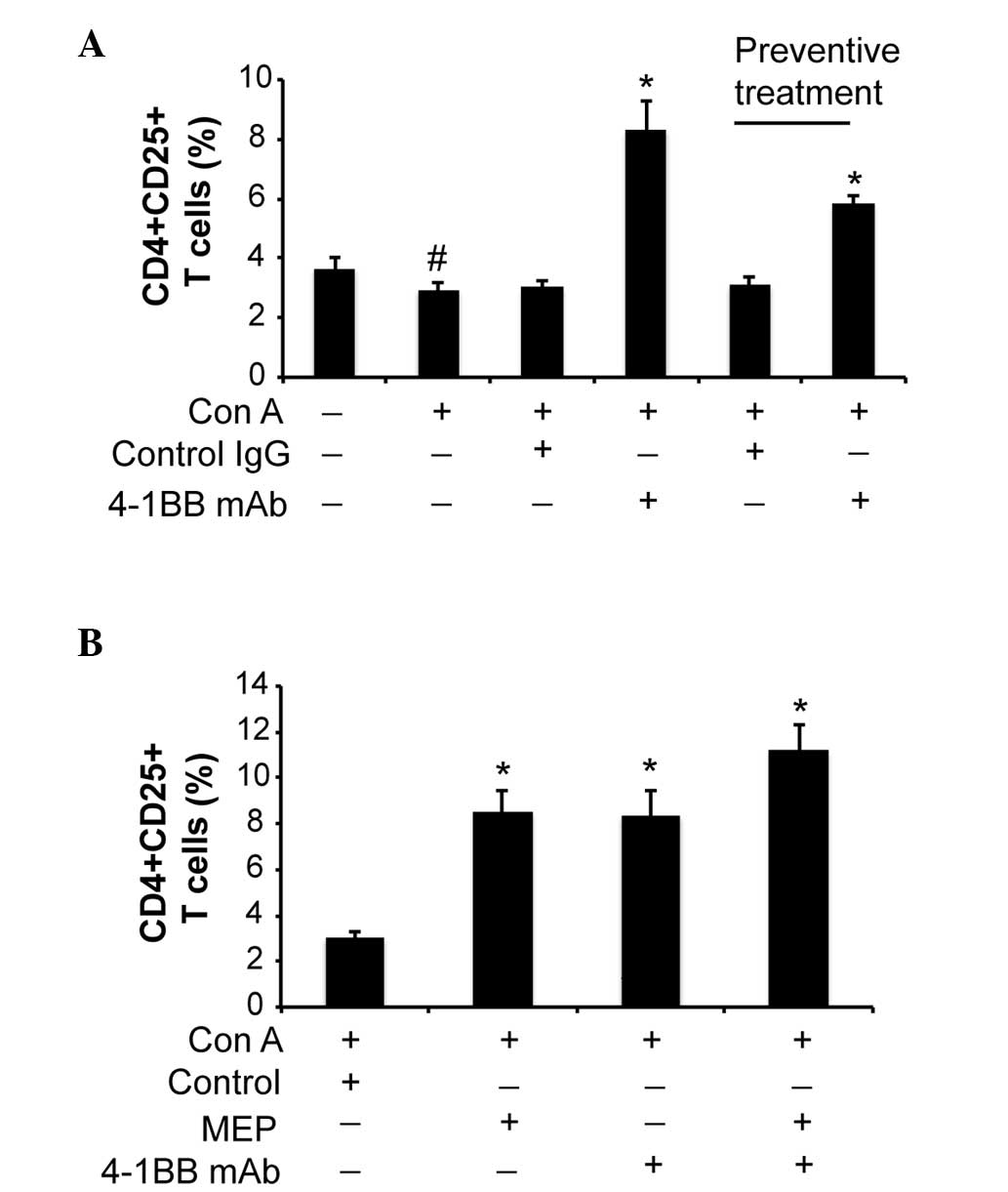
The proportion of CD4+/CD25+ T-cells
increases following 4-1BB mAb treatment
The proportion of CD4+/CD25+
T-cells was significantly reduced in the Con A-injected mice,
compared with the control group mice (P<0.05; Fig. 3A). Treatment with 4-1BB mAb following
Con A injection and preventive treatment with 4-1BB mAb prior to
Con A injection markedly increased the proportion of
CD4+/CD25+ T-cells compared with the
ConA+control IgG group (P=0.030; Fig.
3A). In addition, MEP alone significantly increased the
proportion of CD4+/CD25+ T-cells (P<0.01;
Fig. 3B), which was further
increased by combined treatment of MEP and 4-1BB mAb (P<0.01 vs.
the control; Fig. 3B).
Discussion
The present study demonstrated that 4-1BB expression
was increased on the surface of T-cells following Con A-induced
liver injury in mice. This is consistent with previous studies, in
which 4-1BB and 4-1BBL were demonstrated to be upregulated in human
autoimmune diseases, including rheumatoid arthritis and multiple
sclerosis (16,17). Therefore, 4-1BB may have a role in
the development of immune-mediated liver disease. The 4-1BB mAb has
previously been investigated in animal models of various autoimmune
disorders; Haga et al (18)
investigated the role of 4-1BB in a rat model of autoimmune
myocarditis and reported that inhibition of 4-1BB pathways by
intraperitoneal injection with 4-1BB mAb was able to attenuate the
development of disease. In addition, the mRNA expression levels of
proinflammatory cytokines in the heart tissue, including
interleukin and TNF-α, were decreased following 4-1BB mAb treatment
(18). In another study, the 4-1BB
mAb inhibited the development of a interphotoreceptor
retinoid-binding protein-induced autoimmune uveoretinitis by
increasing the proportion of CD11c+/CD8+
T-cells (19), and intraperitoneal
injection with agonistic 4-1BB mAb markedly reduced the development
and severity of experimental autoimmune encephalomyelitis in rats
(20). Furthermore, the 4-1BB mAb
markedly reduced mercury-induced autoimmunity in mice (21). In the present study, inhibition of
the 4-1BB pathway by intravenous injection with 4-1BB mAb
significantly reduced liver tissue damage and preserved liver
function in mice with Con A-induced immune-mediated liver
injury.
The molecular mechanisms underlying the beneficial
effects of the 4-1BB mAb in various animal models of autoimmune
disorders remain unclear. Haga et al (18) reported that the 4-1BB mAb reduced
heart tissue damage and preserved heart function in a rat model of
autoimmune myocarditis by suppressing the activity of the signaling
molecules c-Jun N-terminal kinase, p38 and the inhibitor of NF-κB.
Conversely, Choi et al (19)
reported that the inhibitory effects of the 4-1BB mAb on the
development of autoimmune uveoretinitis was not associated with the
inhibition of 4-1BB signaling, but with 4-1BB mAb-mediated
expansion of CD11c+/CD8+ T-cells. The present
study demonstrated that Con A injection significantly reduced the
proportion of CD4+/CD25+ T-cells, and that
this was significantly attenuated following treatment with 4-1BB
mAb. A reduction in the number of CD4+/CD25+
T-cells has been associated with various autoimmune disorders. For
example, in a previous study patients with chronic autoimmune
urticaria exhibited significantly reduced levels of
CD4+/CD25+ T-cells compared with healthy
controls (22), and the absolute
count of CD4+/CD25+ T-cells in patients with
active systemic lupus erythematosus was markedly lower compared
with healthy controls in another study (23). Therefore, Con A-mediated reduction of
CD4+/CD25+ T-cells may have contributed to
the development of immune-mediated liver injury in the mice, and
the protective effects of 4-1BB mAb may have been due to the
increase in CD4+/CD25+ T-cell levels.
The disruption of the 4-1BB/4-1BBL pathway has
previously been associated with adverse effects in animal models of
autoimmune disorders. Sytwu et al (24) demonstrated that transgenic, non-obese
diabetic mice overexpressing membrane-bound agonistic single-chain
anti-4-1BB variable fragment in pancreatic β-cells developed more
severe diabetes compared with their non-transgenic littermates. In
particular, this was associated with earlier-onset, faster diabetic
processes and a higher mortality. In the present study, the
short-term effects of 4-1BB mAb treatment in a mouse model of Con
A-induced immune-mediated liver injury appeared promising; however,
the long-term effects of 4-1BB mAb on liver function remain
unclear. Therefore, future studies are required to verify the
results of the present study.
In conclusion, the present study demonstrated that
the 4-1BB mAb was able to reduce liver tissue damage and preserve
liver function in a mouse model of Con A-induced immune-mediated
liver injury by increasing the proportion of
CD4+/CD25+ T-cells. A long-term observation
is required in order to verify these findings. In addition, the
clinical significance of the 4-1BB mAb in managing immune-mediated
liver disease remains to be determined.
Acknowledgements
Medical writing services were provided by Cactus
Communications. The authors retained full control of the manuscript
content.
References
|
1
|
Kremer AE, Rust C, Eichhorn P, Beuers U
and Holdenrieder S: Immune-mediated liver diseases: Programmed cell
death ligands and circulating apoptotic markers. Expert Rev Mol
Diagn. 9:139–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baier JL and Mattner J: Mechanisms of
autoimmune liver disease. Discov Med. 18:255–263. 2014.PubMed/NCBI
|
|
3
|
Lleo A, Invernizzi P, Mackay IR, Prince H,
Zhong RQ and Gershwin ME: Etiopathogenesis of primary biliary
cirrhosis. World J Gastroenterol. 14:3328–3337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levy C and Lindor KD: Primary sclerosing
cholangitis: Epidemiology, natural history and prognosis. Semin
Liver Dis. 26:22–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krawitt EL: Autoimmune hepatitis. N Engl J
Med. 354:54–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hagymási K and Tulassay Z: Review of
overlap syndromes in autoimmune liver diseases. Diagnostic and
therapeutic difficulties. Orv Hetil. 154:923–929. 2013.(In
Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krawitt EL: Clinical features and
management of autoimmune hepatitis. World J Gastroenterol.
14:3301–3305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vinay DS and Kwon BS: Role of 4-1BB in
immune responses. Semin Immunol. 10:481–489. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon BS and Weissman SM: cDNA sequences of
two inducible T-cell genes. Proc Natl Acad Sci USA. 86:1963–1967.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeBenedette MA, Wen T, Bachmann MF, Ohashi
PS, Barber BH, Stockling KL, Peschon JJ and Watts TH: Analysis of
4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both
4-1BBL and CD28 reveals a role for 4-1BB in skin allograft
rejection and in the cytotoxic T cell response to influenza virus.
J Immunol. 163:4833–4841. 1999.PubMed/NCBI
|
|
11
|
Tan JT, Whitmire JK, Ahmed R, Pearson TC
and Larsen CP: 4-1BB ligand, a member of the TNF family, is
important for the generation of antiviral CD8 T cell responses. J
Immunol. 163:4859–4868. 1999.PubMed/NCBI
|
|
12
|
Tan JT, Whitmire JK, Murali-Krishna K,
Ahmed R, Altman JD, Mittler RS, Sette A, Pearson TC and Larsen CP:
4-1BB costimulation is required for protective anti-viral immunity
after peptide vaccination. J Immunol. 164:2320–2325. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo
SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE and Vinay DS: Immune
responses in 4-1BB (CD137)-deficient mice. J Immunol.
168:5483–5490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito K, Mori S, Date F and Ono M:
Sjögren's syndrome-like autoimmune sialadenitis in MRL-Faslpr mice
is associated with expression of glucocorticoid-induced TNF
receptor-related protein (GITR) ligand and 4-1BB ligand.
Autoimmunity. 46:231–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martínez Gómez JM, Croxford JL, Yeo KP,
Angeli V, Schwarz H and Gasser S: Development of experimental
autoimmune encephalomyelitis critically depends on CD137 ligand
signaling. J Neurosci. 32:18246–18252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung HW, Choi SW, Choi JI and Kwon BS:
Serum concentrations of soluble 4-1BB and 4-1BB ligand correlated
with the disease severity in rheumatoid arthritis. Exp Mol Med.
36:13–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu GZ, Gomes AC, Putheti P, Karrenbauer
V, Kostulas K, Press R, Hillert J, Hjelmström P and Gao XG:
Increased soluble 4-1BB ligand (4-1BBL) levels in peripheral blood
of patients with multiple sclerosis. Scand J Immunol. 64:412–419.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haga T, Suzuki J, Kosuge H, Ogawa M, Saiki
H, Haraguchi G, Maejima Y, Isobe M and Uede T: Attenuation of
experimental autoimmune myocarditis by blocking T cell activation
through 4-1BB pathway. J Mol Cell Cardiol. 46:719–727. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi BK, Asai T, Vinay DS, Kim YH and Kwon
BS: 4-1BB-mediated amelioration of experimental autoimmune
uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent
mechanisms. Cytokine. 34:233–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK,
Chen L and Fu YX: Administration of agonistic anti-4-1BB monoclonal
antibody leads to the amelioration of experimental autoimmune
encephalomyelitis. J Immunol. 168:1457–1465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vinay DS, Kim JD and Kwon BS: Amelioration
of mercury-induced autoimmunity by 4-1BB. J Immunol. 7:5708–5717.
2006. View Article : Google Scholar
|
|
22
|
Sun RS, Sui JF, Chen XH, Ran XZ, Yang ZF,
Guan WD and Yang T: Detection of CD4+ CD25+ FOXP3+ regulatory T
cells in peripheral blood of patients with chronic autoimmune
urticaria. Australas J Dermatol. 52:e15–e18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kleczynska W, Jakiela B, Plutecka H,
Milewski M, Sanak M and Musial J: Imbalance between Th17 and
regulatory T-cells in systemic lupus erythematosus. Folia Histochem
Cytobiol. 49:646–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sytwu HK, Lin WD, Roffler SR, Hung JT,
Sung HS, Wang CH, Cheng TL, Tsou SC, Hsi SC and Shen KL:
Anti-4-1BB-based immunotherapy for autoimmune diabetes: Lessons
from a transgenic non-obese diabetic (NOD) model. J Autoimmun.
21:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|