Introduction
Autoimmune hepatitis (AIH) is a chronic inflammatory
liver disease characterized by interface hepatitis, increased
levels of autoantibodies and immunoglobulins (Igs), as well as
liver dysfunction (1–4). The disease has a universal distribution
affecting all age groups and both genders, irrespective of the
ethnicity of the affected individual (5–10).
Although much progress has been made in the understanding of the
pathogenesis of AIH in the past few years, the immunological
mechanism of AIH remain to be elucidated.
The tumor necrosis factor-alpha (TNF-α)-induced
protein-8 like-2 (TIPE2) is a newly identified negative regulator
of immunity (11,12). It is highly expressed in
hematopoietic cells (13,14), and negatively regulates inflammation
through T cell receptor (TCR), Toll-like receptor (TLR), nuclear
factor-kappa-B, c-Jun N-terminal kinase and p38 signaling pathways
(11,13,15,16–18). The
association between TIPE2 and liver diseases has been widely
studied (19–24). It has been reported that TIPE2 has
important roles in suppressing Hepatitis B virus (HBV) -induced
hepatic inflammation. Patients with chronic hepatitis B had
significantly reduced expression levels of TIPE2 in their
peripheral blood mononuclear cells (PBMCs) compared with the
healthy controls (22). Kong et
al (23) reported that the TIPE2
mRNA expression was significantly downregulated in chronic
hepatitis C patients, and HCV may promote chronic hepatitis by
inhibiting TIPE2 expression. In patients with acute-on- chronic
hepatitis B liver failure (ACHBLF) (24), the levels of TIPE2 mRNA correlated
with serum total bilirubin and model for end-stage liver disease
scores. TIPE2 mRNA levels were significantly upregulated in
patients with ACHBLF compared with those in the patients with
chronic infection or the healthy controls. Cytotoxic T
cell-mediated killing of virus-infected hepatocytes is an important
pathogenic process of hepatitis B. Recently, Zhang et al
(21) reported that TIPE2 protein
negatively regulates HBV-specific CD8 (+) T-cell functions in
patients with hepatitis B. This suggests that TIPE2 may activate
the destruction of hepatocytes by certain types of blood cell.
However, to the best of our knowledge, the expression and the role
of TIPE2 in AIH has not yet been reported.
In the present study, a murine model of concanavalin
A (ConA)-induced AIH was established, and the expression of TIPE
family members was assessed in the PBMCs of these mice.
Furthermore, liver function, pro-inflammatory cytokine production
and hepatic histopathology were assessed in TIPE2-deficient mice to
evaluate whether TIPE2 is involved in the pathogenesis of AIH.
Materials and methods
Experimental animals
A total of 72 wild-type (WT) C57BL/6 mice were
purchased from Shanghai Slac Laboratory Animal, Co., Ltd.
(Shanghai, China). The TIPE2-knockout (TIPE2−/−)
mice with C57BL/6J background were generated as described
previously (11). All mice used were
male, 8–10 weeks old and weighed 22–25 g. The mice were maintained
in individual cages under controlled conditions (22±2°C; 55%
humidity; 12-h light/dark cycle), with free access to food and
fresh water. All animal experiments were approved by the Ethics
Committee of Hebei Province Chinese Medicine Hospital
(Shijiazhuang, China) following the Guide for the Care and Use of
Laboratory Animals published by the National Institutes of Health
(NIH Publication no. 86–23, revised 1986). To induce AIH, the mice
were injected with 2 mg/kg ConA (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) through the tail vein. Mice in the control
group were injected with an identical volume of phosphate-buffered
saline (PBS). Each group contained 6 mice.
Collection of PBMCs
Blood samples (~200 µl) were obtained via the tail
vein at 6, 12 and 18 h after ConA-induced AIH. At 24 h, the mice
were sacrificed via cervical dislocation and ~1 ml blood samples
were harvested. Peripheral blood mononuclear cells (PBMCs) were
isolated using Lymphocyte Separation Solution (Sigma-Aldrich; Merck
Millipore) and stored at −80°C.
Western blot analysis
The PBMCs were lysed with radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Shanghai, China)
and the total protein concentration was determined using a Bradford
Protein Concentration Assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. A
total of 30 µg protein was separated by 10% SDS-PAGE and
transferred onto a nitrocellulose membrane (Millipore, Billerica,
MA, USA). After blocking with 5% non-fat milk in Tris-buffered
saline containing Tween 20 (TBST) at 4°C overnight, the membranes
were incubated with the following primary antibodies at 4°C
overnight: Rabbit polyclonal to TIPE2 (dilution, 1:500; cat. no.,
ab170258; Abcam, Cambridge, MA, USA), rabbit polyclonal to GAPDH
(dilution, 1:2,000; cat. no. ab9485; Abcam). GAPDH antibody was
used as an internal control. After washing with TBST, the membranes
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (dilution, 1:4,000; cat. no. ab6721; Abcam) at room
temperature for 2 h. Detection of the signals was performed using
an ECL Western Blotting kit (Pierce Biotechnolgy, Inc., Rockford,
IL, USA) by exposure to X-ray films (Phenix Research Products,
Candler, NC, USA) for 5 min. Protein was quantified using a ScanJet
4C Flatbed Scanner (Hewlett-Packard, Palo Alto, CA, USA) with NIH
Image v1.52 software (rsb.info.nih.gov/nih-image/).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the PBMCs obtained at
12 h after ConA injection by using TRIzol (Invitrogen, Carlsbad,
CA, USA) according to the manufacturer's instructions. Reverse
transcription was performed with a First Strand cDNA Synthesis kit
(Fermentas, Vilnius, Lithuania). Real-time PCR was performed in an
ABI 7300 Sequence Detection System with a SYBR-Green PCR kit (both
Applied Biosystems; Thermo Fisher Scientific, Inc.). All primers
used in this study were purchased from Sangon Biotech Co., Ltd.,
(Shanghai, China), and the sequences were as follows: TIPE, forward
5′-ACATGATGCGGACAGTGGTA-3 and reverse 5′-AGCTCCTGCCTCTAGGTTCC-3′;
TIPE1, forward 5′-TGACCCCAAACACCCTATGT-3′ and reverse
5′-TCTCCCAATTCTCCACAACC-3′; TIPE2, forward
5′-TGAAACTCAGGTCCGCTTCT-3′ and reverse 5′-tCCTAGTGCTGCCTCCAACT-3′;
TIPE3, forward 5′-GGCATTCTCTACCGGAACAA-3′ and reverse
5′-GTCCTTGCACTCATGCAGAA-3′. For qPCR, 1 µl cDNA, 1 nM primers and
12.5 µl 2X SYBR Green were mixed to obtain a final volume of 25 µl.
Thermal cycling was performed as follows: 40 cycles at 95°C for 30
sec, 56°C for 30 sec and 72°C for 30 sec. Relative levels of gene
expression were determined using the comparative Cq method
(25) with GAPDH as the control.
Measurement of alanine amiotransferase
(ALT) and aspartate aminotransferase (AST)
The blood samples were centrifuged at 1,000 ×
g at 4°C for 15 min, and the serum was extracted and stored
at −20°C. The levels of ALT and AST in the serum were measured
using the ALT/AST Assay Reagent kit (Sichuan Maker Science
Technology Co., Ltd, Chengdu, China) according to the
manufacturer's instructions with colorimetric evaluation on an
Automated Chemical Analyzer (7600; Hitachi, Tokyo, Japan).
Cytokine measurement
The levels of TNF-α, interleukin (IL)-6 and IL-12 in
the serum were measured using commercial kits (R&D Systems,
Inc., Minneapolis, MN, USA) following the manufacturer's
instructions.
Hematoxylin and eosin (HE)
staining
The mice were sacrificed at 24 h after ConA-induced
AIH using cervical dislocation method. Liver tissues were fixed in
4% paraformaldehyde (Beijing Dingguo Changsheng Biotechnology Co.,
Ltd, Beijing, China) for 24 h and embedded in paraffin. Tissue
blocks were sectioned at 4 µm thickness and slices were baked at
60°C for 4 h. After removal of the paraffin by using xylene and a
graded ethanol series, the sections were incubated with hematoxylin
(Beyotime Institute of Biotechnology) for 5 min, followed by
washing with water and staining with eosin (Beyotime Institute of
Biotechnology) for 1 min. The slides were viewed under a microscope
(E200; Nikon Corp., Tokyo, Japan).
Statistical analyses
Statistical analyses were performed using SPSS 19.0
software (International Business Machines, Armonk, NY, USA). Values
are expressed as the mean ± standard deviation. Student's
t-test was used to evaluate the statistical significance of
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of TIPE genes in the PBMCs
of mice with AIH
To induce AIH, the WT mice were injected with ConA.
12 h later, the mRNA expression of TIPE genes in the PBMCs was
determined by RT-qPCR analysis. The results revealed that TIPE,
TIPE1, TIPE2 and TIPE3 mRNA was detectable in the PBMCs of normal
mice. The mRNA expression levels of TIPE2 were significantly
decreased in the PBMCs of mice with AIH, while the mRNA expression
levels of TIPE, TIPE1 and TIPE3 were not affected (Fig. 1).
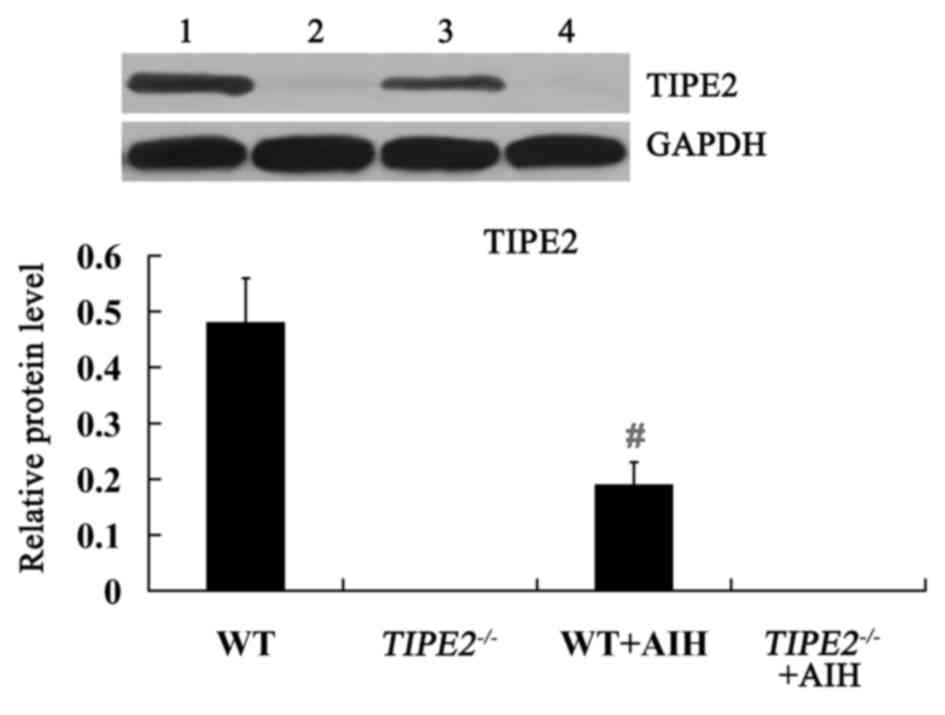
Expression of TIPE2 protein in the
PBMCs of TIPE2-deficient mice with AIH
The WT and TIPE2−/− mice were
injected with ConA to induce AIH. 12 h later, the expression of
TIPE2 protein in the PBMCs wsd examined by western blot analysis.
As shown in Fig. 2, TIPE2 protein
was not detectable in the PBMCs of TIPE2−/− mice,
and that the relative protein levels of TIPE2 were significantly
downregulated in the mice with AIH compared with the WT mice.
Expression of ALT and AST in
TIPE2-deficient mice with AIH
The levels of ALT and AST in the serum were
determined in order to evaluate liver function in TIPE2-deficient
mice with AIH. As shown in Fig. 3,
the levels of ALT and AST were significantly increased from 6 to 24
h post-ConA injection in the WT+AIH group compared with the WT
group. Under normal conditions, there was no difference in ALT and
AST levels between the WT and the TIPE2−/− mice.
However, following ConA-induced AIH, the levels of ALT and AST in
TIPE2−/− mice were higher compared with those in
WT mice.
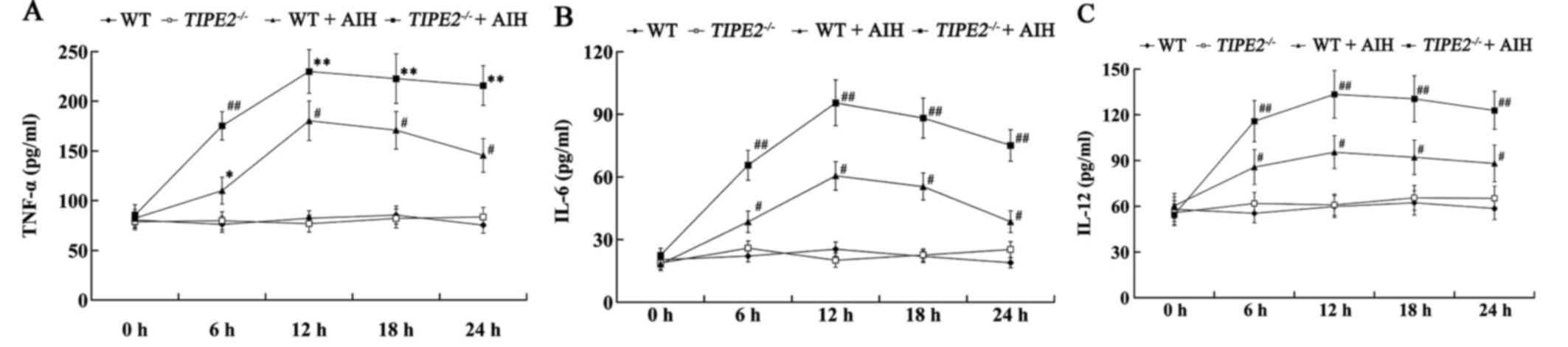
Expression of pro-inflammatory
cytokines in TIPE2-deficient mice with AIH
The expression of pro-inflammatory cytokines,
including TNF-α, IL-6 and IL-12 in the serum were measured using
ELISA. The expression of these pro-inflammatory cytokines was not
significantly different between the WT and the
TIPE2−/− mice prior to ConA-induced AIH. However,
cytokine levels were significantly increased in WT mice following
ConA-induced AIH from 6 to 24 h post-ConA injection. Furthermore,
the levels of TNF-α, IL-6 and IL-12 were significantly increased in
the TIPE2−/−+AIH group compared with those in the
WT+AIH group (Fig. 4).
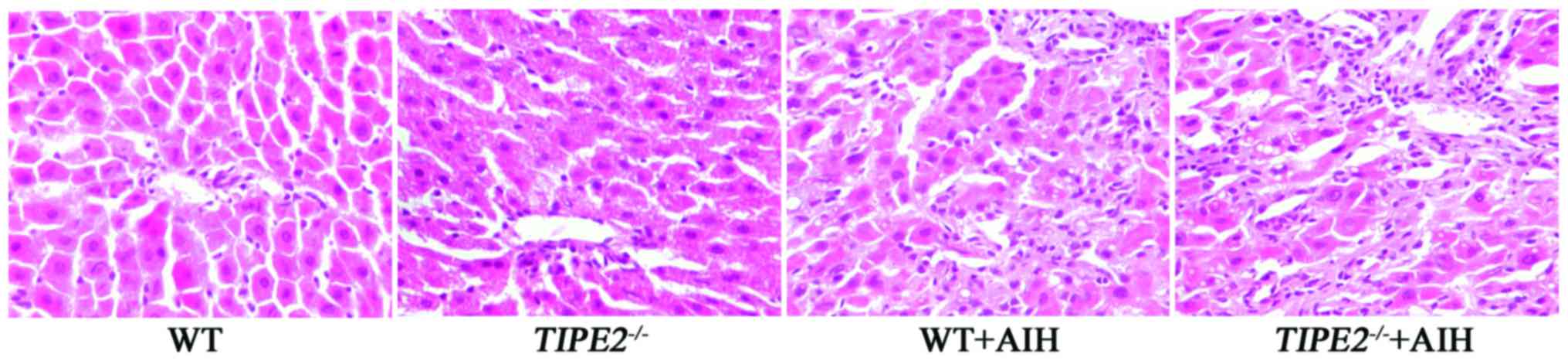
Hepatic histopathology of
TIPE2-deficient mice with AIH
The HE staining of paraffin-embedded liver sections
of the experimental mice is shown in Fig. 5. The liver structures of the WT and
the TIPE2−/− mice appeared normal. Following
ConA-induced AIH, hepatic injury was shown in the liver tissues.
Mononuclear cell infiltration into the parenchyma, portal
inflammation and hepatocellular necrosis were identified in the WT
and the TIPE2−/− mice with AIH. The hepatic
inflammation was more severe in the TIPE2−/−+AIH
group than that in the WT+AIH group.
Discussion
The family of TIPE proteins have been recently
identified and consists of four members: TIPE, TIPE1, TIPE2 and
TIPE3 (26), which have high degrees
of sequence homology. TIPE, the first identified member of this
family, acts as a negative mediator of apoptosis and may have a
role in tumor progression (12). The
biological functions of TIPE1 and TIPE3 in physiological and
pathological conditions have remained largely elusive. TIPE1 has
been indicated to regulate cell death, and loss of TIPE1 may
represent a novel prognostic indicator for hepatocellular carcinoma
(27). TIPE3 is regarded as a
transfer protein of lipid second messenger that promotes cancer
(28). In the present study, the
expression of TIPE, TIPE1 and TIPE3 in PMBCs was found to not be
affected by ConA-induced AIH in experimental mice. These results
indicated that TIPE, TIPE1 and TIPE3 may not be associated with
AIH.
TIPE2 is the most widely studied protein of the TIPE
family. It has essential roles in maintaining immune homeostasis
(11,15). TIPE2 is down-regulated in patients
with chronic inflammatory diseases, and TIPE2 deficiency in mice
causes fetal inflammatory diseases (11). TIPE2 is also associated with the
development of hepatic inflammatory diseases, including as
HBV-induced hepatitis, HCV infection and ACHBLF (22–24).
However, to the best of our knowledge, the role of TIPE2 in AIH has
not been reported to date. In the present study, the expression of
TIPE2 was found to be significantly decreased in mice with AIH.
These data suggested that TIPE2 may have an important role in the
pathogenesis of AIH.
ConA-induced hepatitis is a well-established model
for the study of human AIH (29,30), as
it mimics numerous aspects of this disease, including liver injury,
pro-inflammatory cytokine overproduction (31). In the present study, to further
investigate the role of TIPE2 in AIH, TIPE2-deficient mice were
treated with ConA. Liver function, pro-inflammatory cytokine
production and hepatic histopathology were then examined, revealing
that TIPE2-deficient mice had significantly increased serum ALT and
AST levels, and enhanced the production of pro-inflammatory
cytokines, as well as more severe hepatic inflammation compared
with the WT mice, indicating that TIPE2 has a protective effect on
liver function after AIH, and that TIPE2 may inhibit
pro-inflammatory cytokine overproduction after AIH.
In conclusion, these findings of the present study
demonstrated, for the first time, that TIPE2 is involved in the
pathogenesis of AIH. The results suggested that TIPE2 is a
regulator that prevents liver dysfunction and inhibits a
deleterious inflammatory immune response after AIH. The present
study suggested that TIPE2 may be a novel target for AIH treatment.
Future studies should assess the role of TIPE2 in AIH in clinical
studies, comparing the expression of TIPE2 between healthy controls
and patients with AIH, and investigating the correlation between
TIPE2 expression and clinical indices of patients with AIH.
References
|
1
|
Vergani D and Mieli-Vergani G:
Aetiopathogenesis of autoimmune hepatitis. World J Gastroenterol.
14:3306–3312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verma S, Maheshwari A and Thuluvath P:
Liver failure as initial presentation of autoimmune hepatitis:
Clinical characteristics, predictors of response to steroid
therapy, and outcomes. Hepatology. 49:1396–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strassburg CP: Autoimmune hepatitis. Dig
Dis. 31:155–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lapierre P, Béland K, Yang R and Alvarez
F: Adoptive transfer of ex vivo expanded regulatory T cells in an
autoimmune hepatitis murine model restores peripheral tolerance.
Hepatology. 57:217–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong RJ, Gish R, Frederick T, Bzowej N and
Frenette C: The impact of race/ethnicity on the clinical
epidemiology of autoimmune hepatitis. J Clin Gastroenterol.
46:155–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schramm C, Kanzler S, zum Büschenfelde KH,
Galle PR and Lohse AW: Autoimmune hepatitis in the elderly. Am J
Gastroenterol. 96:1587–1591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abe M, Mashiba T, Zeniya M, Yamamoto K,
Onji M and Tsubouchi H: Autoimmune Hepatitis Study Group-Subgroup
of the Intractable Hepato-Biliary Disease Study Group in Japan:
Present status of autoimmune hepatitis in Japan: A nationwide
survey. J Gastroenterol. 46:1136–1141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng M, Li Y, Zhang M, Jiang Y, Xu Y, Tian
Y, Peng F and Gong G: Clinical features in different age groups of
patients with autoimmune hepatitis. Exp Ther Med. 7:145–148.
2014.PubMed/NCBI
|
|
9
|
Muratori P, Granito A, Quarneti C, Ferri
S, Menichella R, Cassani F, Pappas G, Bianchi FB, Lenzi M and
Muratori L: Autoimmune hepatitis in Italy: The Bologna experience.
J Hepatol. 50:1210–1218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Eslick GD and Weltman M:
Systematic review with meta-analysis: Clinical manifestations and
management of autoimmune hepatitis in the elderly. Aliment
Pharmacol Ther. 39:117–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity, and cancer. Mol Immunol. 49:4–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang
Y, Qu Z, Guo C, Chen Y, Zhang Y and Liu S: Tissue-specific
expression of TIPE2 provides insights into its function. Mol
Immunol. 47:2435–2442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma
C, Chen YH and Zhang L: The unique expression profile of human
TIPE2 suggests new functions beyond its role in immune regulation.
Mol Immunol. 48:1209–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Wang J, Fan C, Li H, Sun H, Gong
S, Chen YH and Shi Y: Crystal structure of TIPE2 provides insights
into immune homeostasis. Nat Struct Mol Biol. 16:89–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H, Zhuang G, Chai L, Wang Z, Johnson
D, Ma Y and Chen YH: TIPE2 controls innate immunity to RNA by
targeting the phosphatidylinositol 3-kinase-Rac pathway. J Immunol.
189:2768–2773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Fayngerts S, Wang P, Sun H,
Johnson DS, Ruan Q, Guo W and Chen YH: TIPE2 protein serves as a
negative regulator of phagocytosis and oxidative burst during
infection. Proc Natl Acad Sci USA. 109:15413–15418. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou Y, Liu S, Zhang C, Zhang G, Li J, Ni
M, An G, Dong M, Liu X, Zhu F, et al: Enhanced atherosclerosis in
TIPE2-deficient mice is associated with increased macrophage
responses to oxidized low-density lipoprotein. J Immunol.
191:4849–4857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)-alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YH, Yan HQ, Wang F, Wang YY, Jiang
YN, Wang YN and Gao FG: TIPE2 inhibits TNF-α-induced hepatocellular
carcinoma cell metastasis via Erk1/2 downregulation and NF-κB
activation. Int J Oncol. 46:254–264. 2015.PubMed/NCBI
|
|
21
|
Zhang W, Zhang J, Zhao L, Shao J, Cui J,
Guo C, Zhu F, Chen YH and Liu S: TIPE2 protein negatively regulates
HBV-specific CD8+ T lymphocyte functions in humans. Mol
Immunol. 64:204–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y,
Qu Z, Cui J, Zhang G, Liang X, et al: Roles of TIPE2 in hepatitis B
virus-induced hepatic inflammation in humans and mice. Mol Immunol.
48:1203–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong L, Liu K, Zhang YZ, Jin M, Wu BR,
Wang WZ, Li W, Nan YM and Chen YH: Downregulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
chronic hepatitis C. Hepatol Int. 7:844–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang LY, Fan YC, Zhao J, Gao S, Sun FK,
Han J, Yang Y and Wang K: Elevated expression of tumour necrosis
factor-α-induced protein 8 (TNFAIP8)-like 2 mRNA in peripheral
blood mononuclear cells is associated with disease progression of
acute-on-chronic hepatitis B liver failure. J Viral Hepat.
21:64–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Liang X, Gao L, Ma H, Liu X, Pan
Y, Yan W, Shan H, Wang Z, Chen YH and Ma C: TIPE1 induces apoptosis
by negatively regulating Rac1 activation in hepatocellular
carcinoma cells. Oncogene. 34:2566–2574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fayngerts SA, Wu J, Oxley CL, Liu X,
Vourekas A, Cathopoulis T, Wang Z, Cui J, Liu S, Sun H, et al:
TIPE3 is the transfer protein of lipid second messengers that
promote cancer. Cancer Cell. 26:465–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tiegs G, Hentschel J and Wendel A: A T
cell-dependent experimental liver injury in mice inducible by
concanavalin A. J Clin Invest. 90:196–203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao X, Qian Y, Xu C, Hong B, Xu W, Shen
L, Jin C, Wu Z, Tong X and Yao H: The protective effect of
intrasplenic transplantation of Ad-IL-18BP/IL-4 gene-modified fetal
hepatocytes on ConA-induced hepatitis in mice. PLoS One.
8:e588362013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ichiki Y, Aoki CA, Bowlus CL, Shimoda S,
Ishibashi H and Gershwin ME: T cell immunity in autoimmune
hepatitis. Autoimmun Rev. 4:315–321. 2005. View Article : Google Scholar : PubMed/NCBI
|