Introduction
Intervertebral disc (IVD) degenerative disease is a
common disease in clinic which is also the major factor of lumbago
(1,2). Currently, the main methods used to
treat these diseases in clinic are nucleus pulposus removal, spinal
canal decompression and degenerative spinal segmental fusion.
However, these methods only relieve patient symptoms for a short
period of time and their long-term efficiency is unsatisfactory,
and they may cause multiple complications (3–5).
Therefore, identifying a new target for the treatment of
intervertebral disc degenerative diseases in order to perfect the
clinical treatment strategy for the IVD degeneration is
required.
Previous findings showed that the Shh-signaling
pathway plays an important role in the process of the
intervertebral disc development, differentiation and degeneration
(6). Furthermore, the P27
gene is a significant gene in the regulation of the Shh-signal
pathway (7,8). Shimura-Miura et al (9) cultured a 13-week-old male Sprague
Dawley (SD) rat at fasting status for 6 and 48 h after the
intervertebral disc nucleus pulposus cell, to induce cell
senescence, and found that P27kip1 expression was elevated in
nucleus pulposus cell, and cell percentage was significantly
increased at G0/G1 stage, while apparently decreased at S stage,
indicating that P27kip1 may be involved in the development process
of IVD by regulating the cell cycle. To the best of our knowledge,
there is currently no study showing that the P27 gene plays
a role via the Shh-signaling pathway. On the basis of the
successful establishment of mouse P27 gene knockout
(P27−/−) of the IVD degeneration model, this experiment
primarily investigates the role of P27 gene in the
development process of IVD and determines whether P27 gene
played a role via the Shh-signaling pathway. Subsequently, the
pathogenesis and possible related molecular mechanism of IVD were
further revealed genetically to provide an experimental and
theoretical basis for the early prevention and treatment of IVD
degeneration.
Materials and methods
Experimental animal
P27−/− IVD degeneration model of mice was
established by the Animal Experiment Center of Shanghai General
Hospital of Nanjing Medical University. Male and female mice were
taken out for mating, and genotype identification was performed for
their offspring. The 4-week-old wild-type (WT) mice (WT group,
n=36) and P27−/− mice (P27−/− group, n=36) in
the same brood were obtained. Mice were housed in a temperature
controlled room (21±2°C) on a 12:12-h light/dark cycle (lights on
at 06:00 a.m.). All mice had free access to water and food. This
study was approved by the Animal Ethics Committee of Nanjing
Medical University Animal Center.
Material drawing
The mice were sacrificed by the cervical dislocation
when they were 4 weeks old. IVD degenerative vertebral bodies of
mice were dissected, separated and 4% of paraformaldehyde was
utilized to fix them overnight. Conventional dehydration and
paraffin embedding were employed. The bodies were sectioned for
standby application.
X-ray examination
Prior to sacrifice, at the 4th week, X-ray
examination was conducted. The experimental mice inhaled isoflurane
and were anesthetized transitorily, and an X-ray examination was
carried out using a metal needle to guide by positioning the
location of IVD in the mouses tail. The changes of the IVD height
were observed, and then the intervertebral disc height index (DHI)
was calculated.
Immunohistochemical staining
First conventional deparaffinage was performed for
paraffin sections and then immunohistochemical staining was carried
out for them, followed by observation under a light microscope
(BX-42; Olympus, Tokyo, Japan).
Alkaline phosphatase (ALP)
histochemical staining
After paraffin sections were processed by
conventional deparaffinage and hydration, 1% MgCl2
Tris-HCl buffer solution was used to culture them at room
temperature overnight, and then they were cultured for 2 h under
the environment of ALP staining solution at room temperature in the
dark. Water was used to wash them for a few minutes, and then
methyl green was employed to restain and conventional water was
then used to seal the sections.
Western blot analysis
Lysate and 1% (V/V) phenylmethylsulfonyl fluoride,
respectively, were added into the IVD vertebral bodies of
4-week-old WT and P27−/− mice [vertebral body weight:
lysate and 1% (V/V) phenylmethylsulfonyl fluoride weight =1:20],
and the solutions were mixed well, followed by centrifugation at
10,050 × g for 15 min at 4°C. The supernatant was absorbed and
full-automatic microplate reader was used to detect the protein
concentration. Additionally, 30 mg protein sample was separated by
lauryl sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE)
electrophoresis. The protein was then transferred from the gel onto
nitrocellulose membrane and developed via enhanced
chemiluminescence, followed by X-ray film exposure and
film-developing in a dark room. β-actin was served as internal
control.
Statistical analysis
Statistical software, SPSS 20.0 (IBM, SPSS, Armonk,
NY, USA), was employed for analysis. The measurement data were
presented as mean ± SD. The Chi-square test was used for label data
and the paired sampled-test was utilized for the measurement data.
For all the tests, a 5% level of significance was used to draw the
conclusions.
Results
X-ray examination
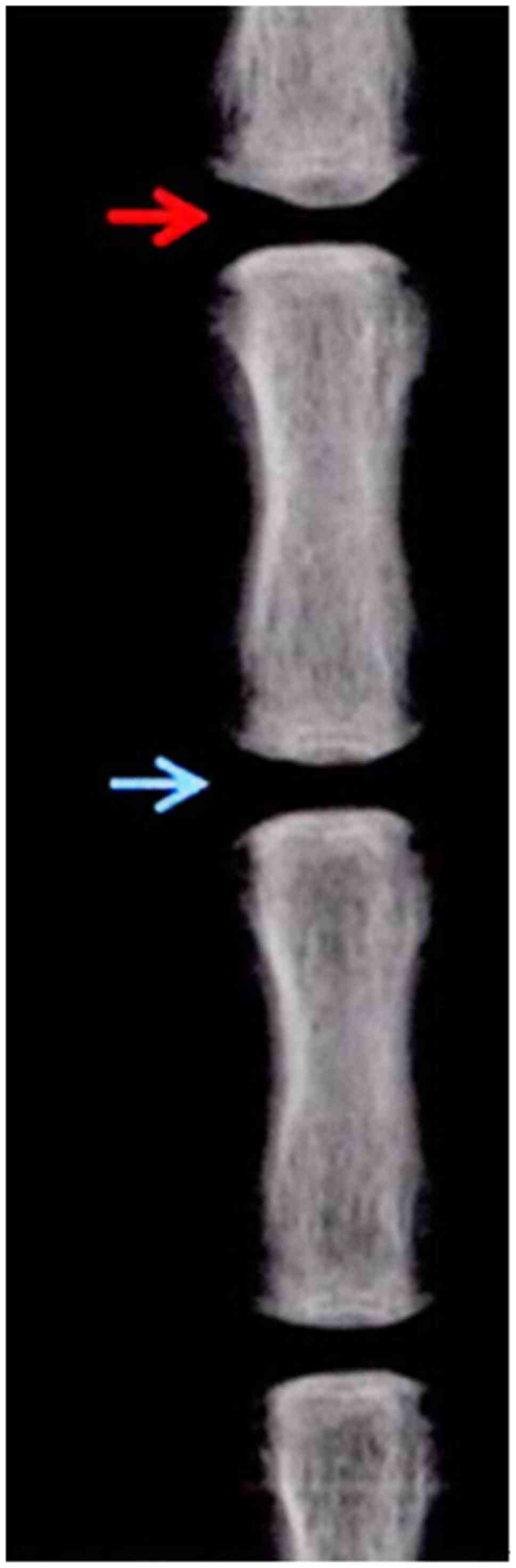
At the end of the fourth week of the model
establishment, the average DHI% of the WT group was 0.83±0.06 but
it was not statistically significant when compared with that at 0
week of the model establishment (P>0.05) (Fig. 1). At the end of the 4th week since
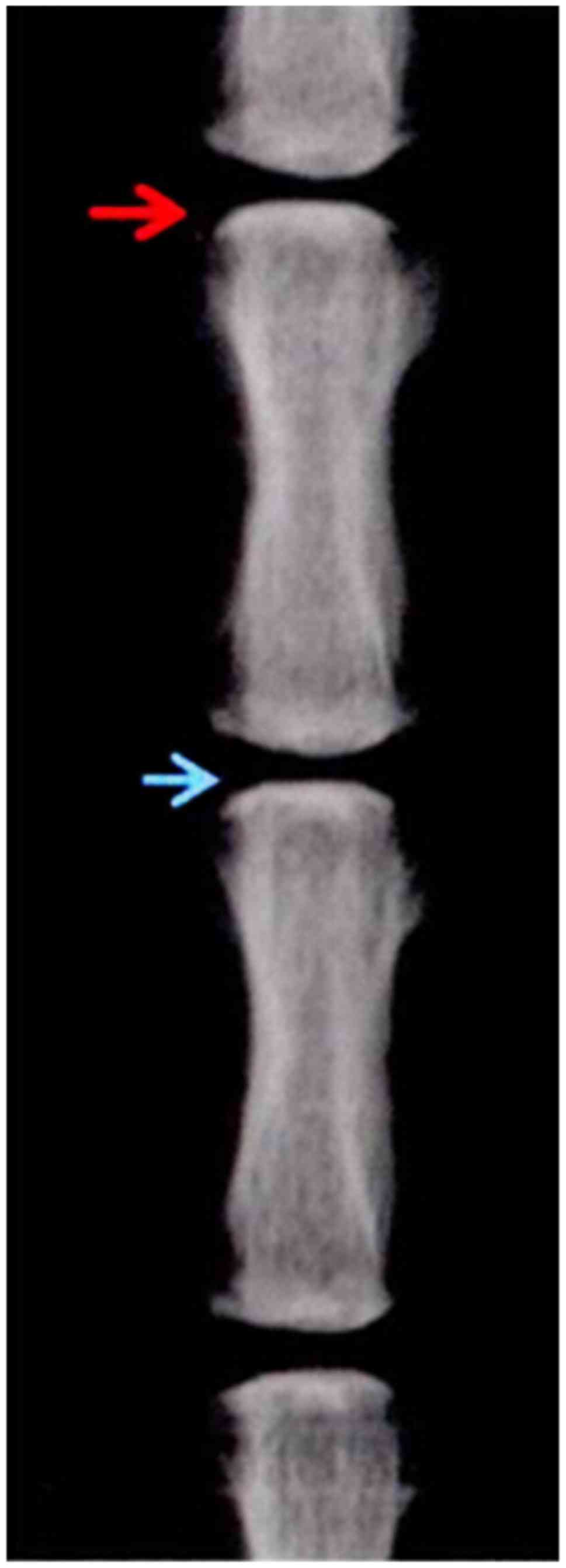
the model was established, the average DHI% of the
P27−/− group was 0.53±0.03, indicating a significant
difference when compared with the average DHI% of P27−/−
at 0 week since model establishment (P>0.05) (Fig. 2).
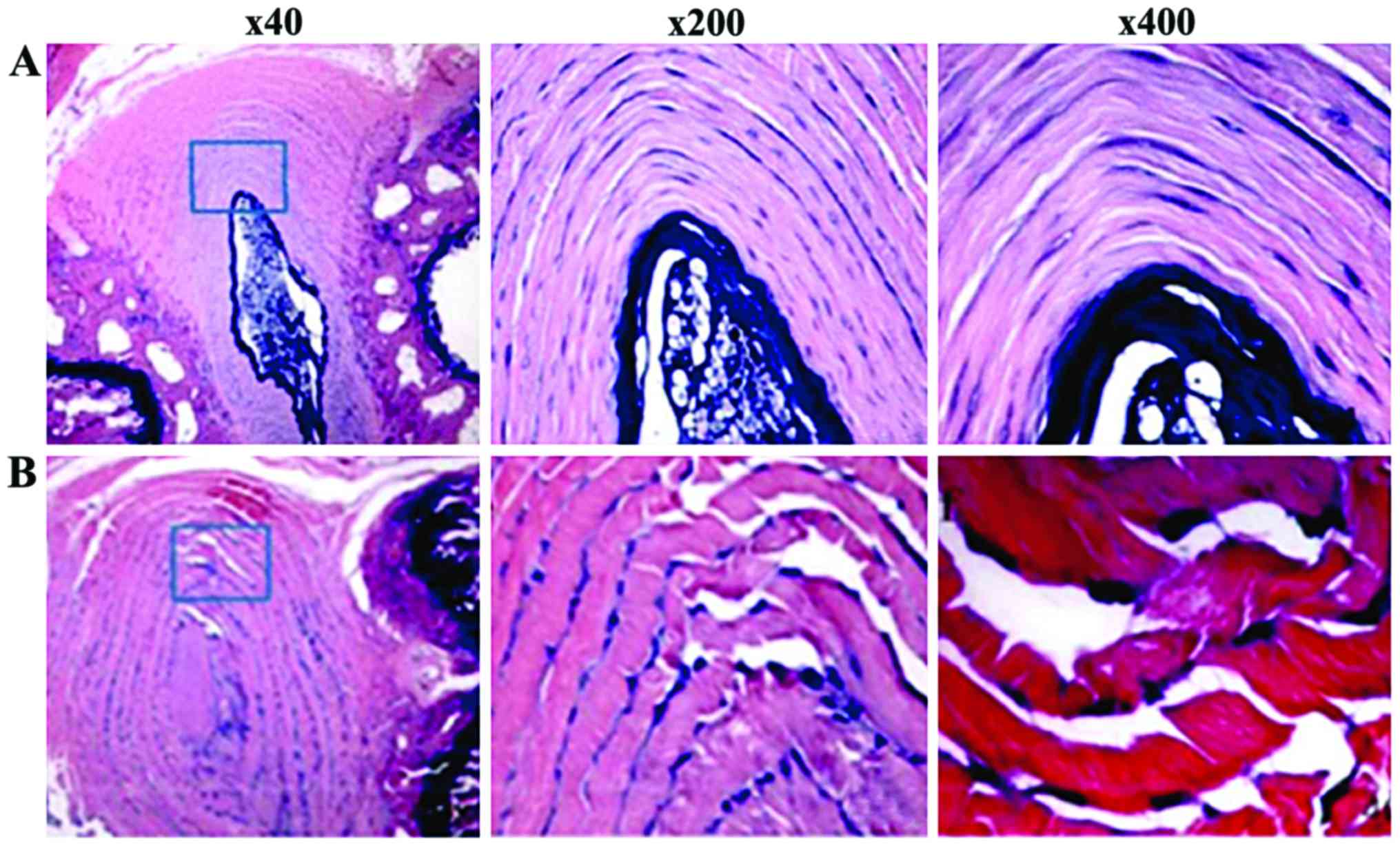
Result of histology
In the WT group, normal IVD staining indicated that
round or elliptic nucleus pulposus tissue was evident. The collagen
lamellae arrangement was normal and the boundary between nucleus
pulposus and annulus fibrosus was clear. In addition, nucleus
pulposus cell showed a star-like shape, and annulus fibrosus cells
were fibroblast-like, which was located in the collagen fiberboard
room (Fig. 3).
In the P27−/− group, mouse IVD staining
indicated that puncturing side annulus fibrosus was fractured with
needle-tip puncture trace. Furthermore, the interlamellar
architecture of annulus fibrosus distributed in disorder, showing
wavy-like shape and radial direction and concentric circle-like
fracture image was evident. IVD nucleus pulposus was irregularly
reduced to the small volume, extracellular matrix staining in
nucleus pulposus became thin, and its boundary with annulus
fibrosus was unclear (Fig. 4).
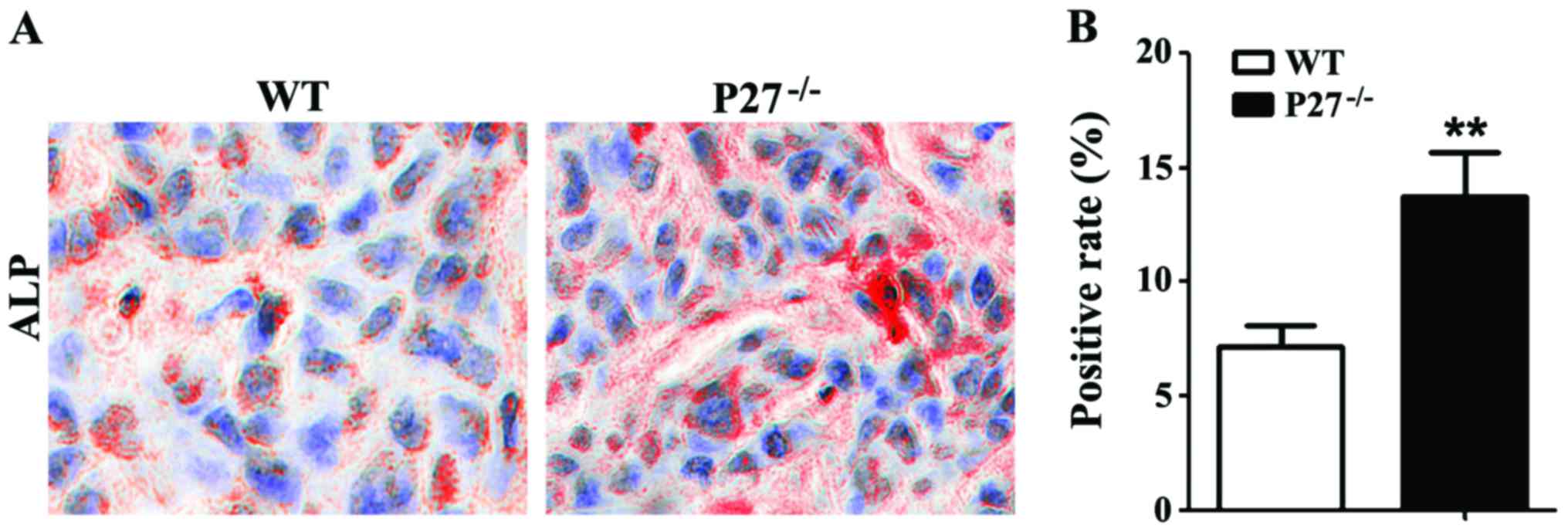
Influence of P27 deficiency on mouse
IVD bone mass
ALP histochemical staining revealed that the
ALP-positive area of mice in the P27−/− group was
significantly increased compared with that of mice in the WT group
(Fig. 4A and B). In addition, where
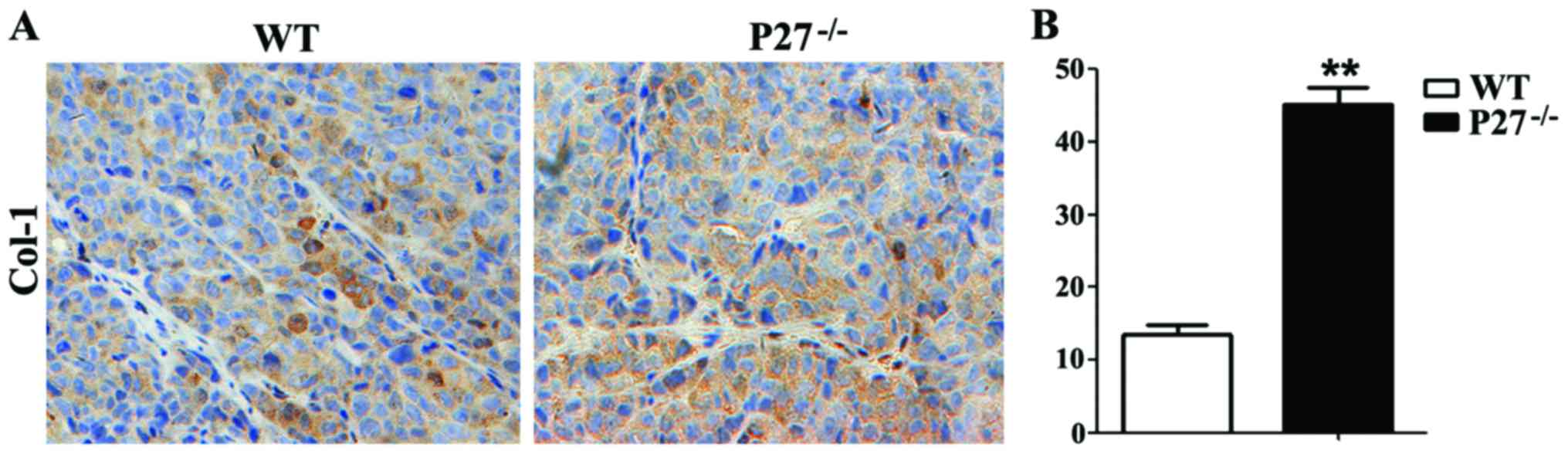
Col-I immumohistochemical staining revealed that Col-I-positive
area of mice in the P27−/− group was apparently
increased compared with that of mice in the WT group (Fig. 5A and B).
Influence of P27 deficiency on the
Shh-signal pathway
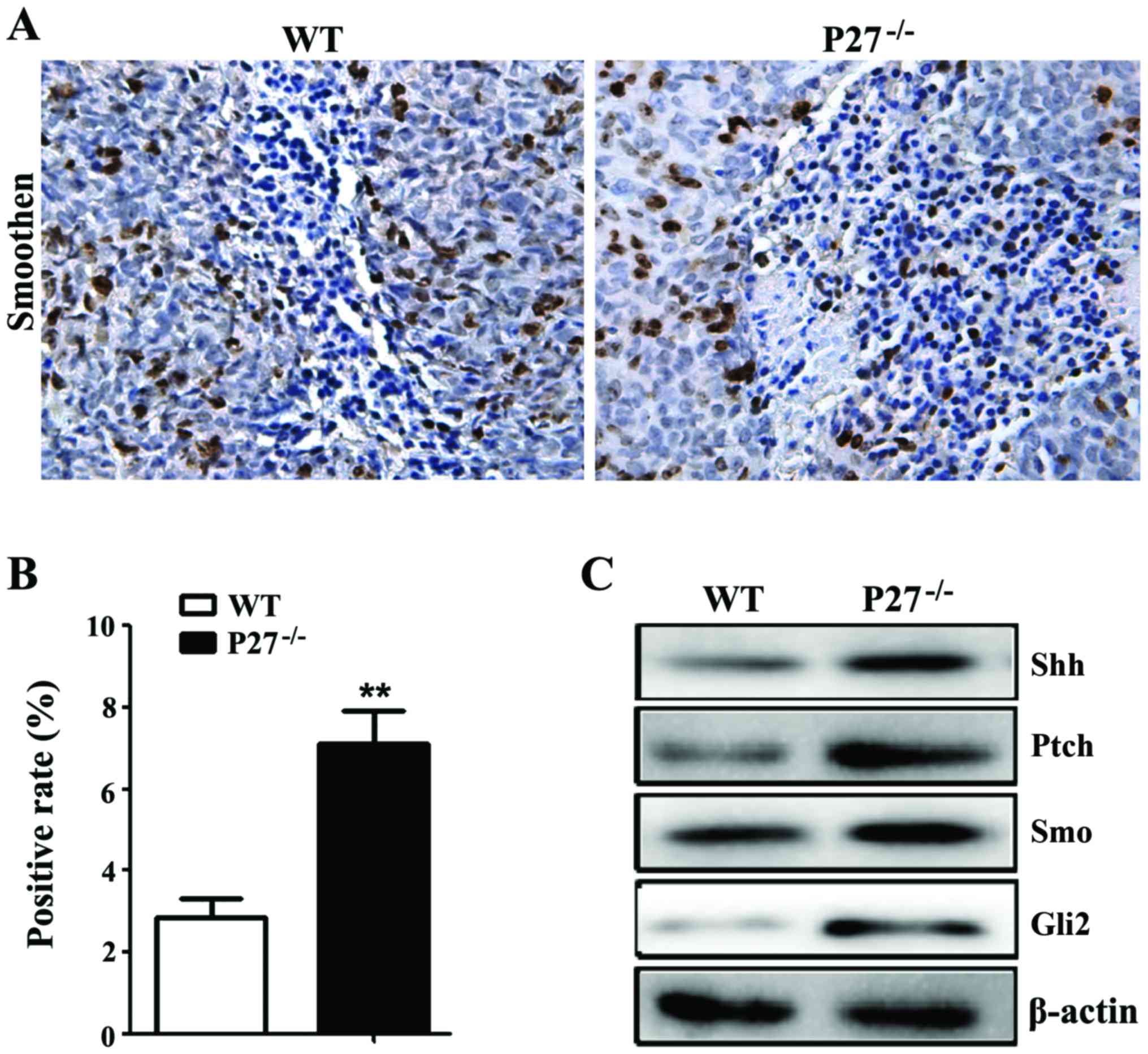
The statistical significant increase in Smo-positive
cell rate of mice in the P27−/− group was found compared
to mice in the WT group (Fig. 6A and
B). In order to further observe the influence of P27 deficiency
on the changes of Shh-signal pathway, western blot analysis was
used to detect the changes in the protein expression levels of Shh,
Patched, Smoothened and Gli2 in IVD. It was found that the protein
expression levels of Shh, Patched, Smoothened and Gli2 of mice in
the P27−/− group were markedly increased compared to
mice in the WT group (Fig. 6C).
Discussion
The Shh-signal pathway is one of the Hedgehog
pathways that regulate the development and differentiation of
vertebrate entoderm, which is a highly conservative morphogenesis
pathway of the medial axis organ development existing in both
Drosophila melanogaster and vertebrate (10–13).
Membrane proteins Ptch and Smo control Shh-signal transmission
towards the cell, and receptor Ptch negatively regulates the
Shh-signaling pathway (14–16). Receptor Smo is coded by
proto-oncogene Smoothened which is homologous with
G-protein-coupled receptor and consists of a single peptide chain
of seven transmembrane regions, whereas the Ptch affects its
function. Transcription factors of Shh-signal pathway belong to the
Gli gene family, of which Glil, Gli2 and G1i3, with zinc
finger, have been identified (17–20).
Previous findings have shown that Shh can induce
notochord and apical plate cells into the IVD and bony vertebral
body in the process of mouse embryonic development. However, when
Shh is deficient, notochord and apical plate cells cannot normally
develop into the IVD and bony vertebral body (21). This shows that Shh-signal pathway
plays a key role in the development process of the IVD. It has been
shown that the IVD nucleus pulposus cell can also excrete Shh in a
4-day-old mouse, and the activated Shh-signal pathway can thus
interact with signal pathways, including TGF-β, BMP and Wnt, as
well as regulate the expression of transcription factors, such as
P27, SOX9, type-I collagen protein, type-II collagen protein and
chondroitin sulfate, and as a result, regulate the growth and
development of IVD (22). This
finding indicates that the Shh-signal pathway is involved in the
whole process of IVD occurrence and may play a crucial role in it.
We also found that the protein expression levels of Shh, Patched,
Smoothened and Gli2 of mice in the P27−/− group were
increased compared to those of mice in the WT group, suggesting
that P27 deficiency can activate the Shh-signaling pathway.
ALP belongs to a critical enzyme in the process of
osteoblast differentiation and can be used to measure osteoblast
activity, whereas type-I collagen belongs to osteoblast product,
and can be used to evaluate the osteoblast-differentiated degree.
In the present study, ALP histochemical staining demonstrated that
the ALP-positive area of mice in the P27−/− group was
significantly increased compared to mice in the WT group.
Furthermore, the Col-I immunohistochemical staining showed that the
Col-I-positive area of mice in the P27−/− group was
significantly increased compared to mice in the WT group. These
results indicate that P27 deficiency can induce an increase in
osteoblast bone formation in the intervertebral disc.
In conclusion, P27 deficiency activates the
expression of the Shh-signal pathway and promotes the proliferation
of osteoblast. This plays a role in promoting IVD degeneration,
which provides a scientific and reliable experimental basis for the
treatment of IVD degeneration-related diseases in clinical
practice.
References
|
1
|
DeLucca JF, Cortes DH, Jacobs NT,
Vresilovic EJ, Duncan RL and Elliott DM: Human cartilage endplate
permeability varies with degeneration and intervertebral disc site.
J Biomech. 49:550–557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen HT, Huang AB, He YL, Bian J and Li
HJ: Wnt11 overexpression promote adipose-derived stem cells
differentiating to the nucleus pulposus-like phenotype. Eur Rev Med
Pharmacol Sci. 21:1462–1470. 2017.PubMed/NCBI
|
|
3
|
Cui YZ, Yang XH, Liu PF, Wang B and Chen
WJ: Preliminary study on diagnosis of lumbar disc degeneration with
magnetic resonance T1p, T2 mapping and DWI quantitative detection
technologies. Eur Rev Med Pharmacol Sci. 20:3344–3350.
2016.PubMed/NCBI
|
|
4
|
Ma T, Guo CJ, Zhao X, Wu L, Sun SX and Jin
QH: The effect of curcumin on NF-κB expression in rat with lumbar
intervertebral disc degeneration. Eur Rev Med Pharmacol Sci.
19:1305–1314. 2015.PubMed/NCBI
|
|
5
|
Liu C, Fei HD, Sun ZY and Tian JW:
Bioinformatic analysis of the microarray gene expression profile in
degenerative intervertebral disc cells exposed to TNF-α. Eur Rev
Med Pharmacol Sci. 19:3332–3339. 2015.PubMed/NCBI
|
|
6
|
Baranto A, Ekström L, Holm S, Hellström M,
Hansson HA and Swärd L: Vertebral fractures and separations of
endplates after traumatic loading of adolescent porcine spines with
experimentally-induced disc degeneration. Clin Biomech (Bristol,
Avon). 20:1046–1054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lundin O, Ekström L, Hellström M, Holm S
and Swärd L: Exposure of the porcine spine to mechanical
compression: Differences in injury pattern between adolescents and
adults. Eur Spine J. 9:466–471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mackiewicz Z, Salo J, Konttinen YT, Holm A
Kaigle, Indahl A, Pajarinen J and Holm S: Receptor activator of
nuclear factor kappa B ligand in an experimental intervertebral
disc degeneration. Clin Exp Rheumatol. 27:299–306. 2009.PubMed/NCBI
|
|
9
|
Shimura-Miura H, Hattori N, Kang D, Miyako
K, Nakabeppu Y and Mizuno Y: Increased 8-oxo-dGTPase in the
mitochondria of substantia nigral neurons in Parkinsons disease.
Ann Neurol. 46:920–924. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bach FC, Zhang Y, Miranda-Bedate A,
Verdonschot LC, Bergknut N, Creemers LB, Ito K, Sakai D, Chan D,
Meij BP and Tryfonidou MA: Increased caveolin-1 in intervertebral
disc degeneration facilitates repair. Arthritis Res Ther.
18:592016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thoreson O, Baranto A, Ekström L, Holm S,
Hellström M and Swärd L: The immediate effect of repeated loading
on the compressive strength of young porcine lumbar spine. Knee
Surg Sports Traumatol Arthrosc. 18:694–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arpinar VE, Rand SD, Klein AP, Maiman DJ
and Muftuler LT: Changes in perfusion and diffusion in the endplate
regions of degenerating intervertebral discs: A DCE-MRI study. Eur
Spine J. 24:2458–2467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Wang D, Yan T and Yuan H:
MiR-138-5p promotes TNF-α-induced apoptosis in human intervertebral
disc degeneration by targeting SIRT1 through PTEN/PI3K/Akt
signaling. Exp Cell Res. 345:199–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jang TW, Ahn YS, Byun J, Lee JI, Kim KH,
Kim Y, Song HS, Lee CG, Kwon YJ, Yoon JH and Jeong K: Lumbar
intervertebral disc degeneration and related factors in Korean
firefighters. BMJ Open. 6:e0115872016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin C, Zhang B, Zhang L, Zhang Z, Wang L,
Tang L, Li S, Yang Y, Yang F, Zhang P and Yang B: MyD88-dependent
Toll-like receptor 4 signal pathway in intervertebral disc
degeneration. Exp Ther Med. 12:611–618. 2016.PubMed/NCBI
|
|
16
|
Yang H, Yuan C, Wu C, Qian J, Shi Q, Li X,
Zhu X and Zou J: The role of TGF-β1/Smad2/3 pathway in
platelet-rich plasma in retarding intervertebral disc degeneration.
J Cell Mol Med. 20:1542–1549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicole W, Tellegen AR, Niklas B, Creemers
LB, Jeannette W, Christian F, Benz K, Grinwis GCM, Tryfonidou MA
and Meij BP: Inflammatory profiles in canine intervertebral disc
degeneration. BMC Vet Res. 12:1–12. 2016.PubMed/NCBI
|
|
18
|
Zhang F, Zhao X, Shen H and Zhang C:
Molecular mechanisms of cell death in intervertebral disc
degeneration (Review). Int J Mol Med. 37:1439–1448. 2016.PubMed/NCBI
|
|
19
|
Lv FJ, Peng Y, Lim FL, Sun Y, Lv M, Zhou
L, Wang H, Zheng Z, Cheung KM and Leung VY: Matrix
metalloproteinase 12 is an indicator of intervertebral disc
degeneration co-expressed with fibrotic markers. Osteoarthritis
Cartilage. 24:1826–1836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Y, Zhang L, Wang WY, Hu QF, Song HP
and Zhang YZ: The inhibitory effect of salmon calcitonin on
intervertebral disc degeneration in an ovariectomized rat model.
Eur Spine J. 24:1691–1701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi KS, Cohn MJ and Harfe BD:
Identification of nucleus pulposus precursor cells and notochordal
remnants in the mouse: Implications for disk degeneration and
chordoma formation. Dev Dyn. 237:3953–3958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dahia CL, Mahoney E and Wylie C: Shh
signaling from the nucleus pulposus is required for the postnatal
growth and differentiation of the mouse intervertebral disc. PLoS
One. 7:e359442012. View Article : Google Scholar : PubMed/NCBI
|