Introduction
Recent advances have indicated that there is a
notable involvement of macrophages in the progression of malignant
tumors, including lymphoma and glioma, and macrophages are now of
interest as target cells for therapy against malignant tumors
(1,2). Circulating monocytes infiltrate tumor
tissues following their attraction via tumor cell-derived
chemokines, such as chemokine (CC motif) ligand 2 [CCL2; also known
as monocyte chemoattractant protein-1 (MCP)]. Activated macrophages
are known to secrete a number of cytokines that are associated with
cell growth, angiogenesis, matrix remodeling and immune suppression
(3,4). Macrophages that infiltrate into the
tumor microenvironment are referred to as tumor-associated
macrophages (TAMs). Since TAMs have pro-tumor functions in a number
of malignant tumor types, TAMs are also considered as target cells
for anti-tumor therapy. Previously, different materials, such as
nanoparticles and nanocarriers, have been reported to improve
anti-tumor therapy (5,6).
TAM activation is induced by direct contact of the
TAMs with tumor cells in the tumor microenvironment (1–4).
Intracellular adhesion molecule-1 and membrane type
macrophage-colony stimulating factor (M-CSF) are known to be
involved in this direct cell-cell contact (7,8). It has
previously been demonstrated that the growth of a number of tumor
cells, including glioma and lymphoma cells, was upregulated by
direct co-culture with macrophages, and that signal transducer and
activator of transcription (STAT) 3 activation was involved in this
tumor cell activation (9,10). However, the significance of cell-cell
contact between macrophages and tumor cells remains unknown.
Previous studies attempted to separate macrophages from tumor cells
following direct co-culture experiments; however, it proved too
difficult to separate the two types of cells (8,11).
Sorting methods using flow cytometry were not suitable for gene
expression analysis because of RNA degradation during the procedure
(11). Although anti-cluster of
differentiation (CD) 14 or CD11b antibody-labeled magnet beads are
commonly available to rapidly isolate monocytes/macrophages,
downregulation of CD14/CD11b has been observed on activated
macrophages when using these beads (12,13). It
is therefore necessary to develop novel and simple methods to
separate macrophages from tumor cells following direct co-culture
experiments.
The present study developed magnetite nanoparticles
modified with gelatin that are specifically engulfed by
macrophages, in addition to methods to deplete these macrophages in
direct co-culture experiments by means of a magnet. By using these
nanoparticles, the present study revealed that the expression of
pro-tumor genes, including CCL2, interleukin (IL)-6, and M-CSF
receptor (M-CSFR) were significantly upregulated in T98G glioma
cells by direct co-culture with macrophages.
Materials and methods
Synthesis of magnetite
nanoparticles
Fe(III) acetylacetonate (1.09 g), 1,8-octanediol
(0.09 g), 1-octadecene (21 ml), and oleylamine (2.1 ml; all Wako
Pure Chemical Industries, Ltd., Osaka, Japan) were loaded into a
three-necked flask. The mixture was heated to 110°C for 30 min
under vacuum. The temperature was then increased to 200°C under a
99.9% argon atmosphere and incubated for 2 h to produce magnetite
nanoparticles. Following the reaction, the nanoparticles were
further heated to 280°C and incubated for 1 h under an argon
atmosphere. The resulting magnetite nanoparticles were washed 3
times by repeated precipitation from 100% hexane by adding excess
99% ethanol to remove any impurities from the magnetite surface.
The synthesized magnetite nanoparticles were dispersed in hexane
for storage.
Gelatin coating of magnetite
nanoparticles
Following the removal of the hexane and dispersal of
the magnetite nanoparticles by centrifugation at 1,200 × g for 30
min, the magnetite nanoparticles were dried in vacuo. Gelatin (0.13
g; Nitta Gelatin Inc., Osaka, Japan) was added to 0.35 ml distilled
water and incubated for 2 h at room temperature. Following
hydration, the gelatin was heated to 60°C and dissolved. The
magnetite nanoparticles (0.20 g) were mixed with the gelatin
solution at 60°C to produce a gel formation of the gelatin. This
gel was dried following cooling, in vacuo, and the resultant black
block was rubbed on an inkstone and gelatin-coated magnetite
nanoparticles were obtained. The morphology of the gelatin-coated
magnetite nanoparticles was observed using transmission electron
microscopy (TEM; JEM-1400 plus; JEOL, Ltd., Tokyo, Japan). Samples
were placed on carbon-coat copper grids (Okenshoji Co., Ltd.,
Tokyo, Japan), allowed to dry in the open air and then dried in
vacuo. The size distribution of the magnetite nanoparticles was
measured using dynamic light scattering analysis with the Zetasizer
Nano ZS™ (Malvern Instruments, Ltd., Malvern, UK).
Macrophages
Peripheral blood mononuclear cells were obtained
from healthy volunteer donors (six male healthy donors recruited
from Kumamoto University; Kumamoto, Japan; 25–40 years old)
recruited between April 2015 and February 2016 whom had all
provided written informed consent for the use of their cells in
accordance with the study protocols approved by the Kumamoto
University Hospital Review Board (no. G309; Kumamoto, Japan).
CD14+ monocytes were isolated using CD14-microbeads
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and the cells
were then cultured in 2% human serum, 1 ng/ml granulocyte
macrophage-colony stimulating factor (GM-CSF) and 50 ng/ml M-CSF
(all Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 7 days
at 37°C to induce macrophages.
Glioma cell line
The human glioma cell line, T98G, was purchased from
the American Type Culture Collection (Manassas, VA, USA), and was
maintained in Dulbecco's modified Eagle medium (DMEM)/Ham's F-12
supplemented with 10% fetal bovine serum (FBS; all Wako Pure
Chemical Industries, Ltd.). T98G cells were infected with a
lentivirus encoding green fluorescent protein (GFP; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and T98G GFP-expressing
(T98G-GFP) cells were selected using puromycin, as described in the
manufacturer's protocol (Santa Cruz Biotechnology, Inc.).
Uptake of magnetite nanoparticles by
human macrophages and T98G cells
The gelatin-coated magnetite nanoparticles (10 µl;
7.3 mg/ml) were added to human macrophages obtained from healthy
volunteers and to the human glioblastoma T98G cells, which were
then cultured for up to 2 h at 37°C in 100 µl DMEM/Ham's F-12
supplemented with 10% FBS in LabTech Chamber Slides (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Uptake of the nanoparticles
was observed using an optical microscope (BX51; Olympus Corp.,
Tokyo, Japan) following hematoxylin staining for 1 min at room
temperature.
Separation of human macrophages from
the co-culture of macrophages and T98G cells
Human macrophages and T98G cells (2×105
and 1×105 cells/well in a 6-well culture plate,
respectively) were mixed and co-cultured at 37°C under 5%
CO2. After 2 days, the magnetite nanoparticles were
added to the culture medium. Following a 1-h incubation at 37°C,
the cells were collected into a centrifuge tube using cell
dissociating buffer (Thermo Fisher Scientific, Inc.). Cells that
had taken up the magnetite nanoparticles were collected using a
magnet (MPC-S, DYNAL®; Invitrogen; Thermo Fisher Scientific, Inc.).
All cells, magnet-collected or not, were attached to glass slides
using Cytospin (800 × g for 5 min ar room temperatyre; Thermo
Fisher Scientific, Inc.).
Immunostaining
All procedures were performed at 37°C. Cells were
fixed with cold acetone (100%) and subsequently incubated with 1%
FBS in PBS for blocking (10 min) and then anti-CD204 antibody
(1:100; clone no. SRA-E5; MAB1710, Cosmo Bio, Tokyo, Japan) for 1 h
in order to detect TAMs. Following this, cells were incubated with
secondary antibody (1:2; 414131; Nichirei Biosciences, Inc., Tokyo,
Japan) for 30 min. Reactions were visualized under light microscope
using a diaminobenzidine (DAB) substrate kit as described in
manufacture's protocol (Nichirei Biosciences, Inc.).
Magnet beads
Anti-human CD14 antibody-labeled microbeads and a
magnetic column were purchased from Miltenyi Biotec GmbH were used,
according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression in T98G cells was evaluated
using RT-qPCR, as previously described (14). Total RNA was extracted with RNAiso
Plus (Takara Bio, Inc., Otsu, Japan), following the manufacturers
protocol. RNA was reverse transcribed using a PrimeScript RT
Reagent kit, according to the manufacturer's instructions, and
DNase from Takara Bio, Inc. The complementary DNA product (25 µl)
was amplified as follows: 94°C for 5 min followed by 40 cycles of
94°C for 30 sec and 60°C for 30 sec. qPCR was performed using
TaqMan polymerase with SYBR-Green fluorescence (Takara Bio, Inc.)
with an ABI PRISM® 7300 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The sequences of the
primers were designed using the Primer3 website (bioinfo.ut.ee/primer3-0.4.0/) and were
synthesized by Hokkaido System Science Co., Ltd., (Tokyo, Japan).
The primer sequences are presented in Table I. The internal control gene used was
β-actin, and three parallel wells were set up for each DNA sample
(25 µl/well). The relative expression level was determined using
the 2−ΔΔCq normalization method (15).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence (5′-3′) |
|---|
| Chemokine (CC motif)
ligand 2 | F |
CATAGCAGCCACCTTCATTCC |
|
| R |
TGCACTGAGATCTTCCTATTGGTG |
| Interleukin-6 | F |
ATGTGTGAAAGCAGCAAAGAGG |
|
| R |
GTGATGATTTTCACCAGGCAAG |
| O(6)-methylguanine-DNA methyltansferase | F |
GGCCGAAACTGAGTATGTGC |
|
| R |
CCTTTAATACAGCGGTGCCT |
| Transforming growth
factor-β | F |
AGCAACAATTCCTGGCGATAC |
|
| R |
GCGAAAGCCCTCAATTTCC |
| Vascular endothelial
growth factor-A | F |
CAGGAGTACCCTGATGAGATCG |
|
| R |
TCTGCATGGTGATGTTGGAC |
| β-actin | F |
ATTCCTATGTGGGCGACGAG |
|
| R |
AAGGTGTGGTGCCAGATTTTC |
Flow cytometry
Cells were detached using cell dissociation buffer
(Thermo Fisher Scientific, Inc.) and incubated with blocking
solution (422302; BioLegend, San Diego, CA, USA) for 10 min at room
temperature. Cells were then stained using anti-M-CSFR antibody
(1:1,000; rabbit polyclonal; LS-C167079; LifeSpan Bioscience, Inc.,
Seattle, WA, USA) or control rabbit immunoglobulin (Ig)G (AB-105C;
Santa Cruz Biotechnology, Inc.) with Fc receptor blocking solution
(BioLegend, Inc.). The cells were then incubated for 30 min at room
temperature with allophycocianin (APC)-labeled anti-rabbit antibody
(1:1; 406414; BioLegend, Inc.), and staining signals were evaluated
using FACSverse™ (BD Biosciences, Franklin Lakes, NJ, USA) and
FACSuite software 21 (BD Biosciences).
Western blot analysis
Cells were lysed in ice-cold lysis buffer [50 mM
Tris (pH 8.0), 1 mM EDTA, 150 mM NaCl and 1% NP-40] and a protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Following electrophoresis of 10 µg/lane protein using 10% SDS-PAGE,
the polyvinylidene difluoride membrane was incubated for 1 h at
room temperature with anti-M-CSFR antibody (1:1,000; rabbit
polyclonal; 3152; Cell Signaling Technology, Inc., Danvers, MA,
USA) or anti-β-actin antibody (1:2,000; mouse monoclonal; sc47778;
Santa Cruz Biotechnology, Inc.) Horseradish peroxidase-goat
anti-mouse or rabbit IgG (1:1,000; 31430; 31460; Thermo Fisher
Scientific, Inc.) were used as the secondary antibody and incubated
for 30 min at room temperature. Immunoreactive bands were
visualized using the Pierce Western Blotting Substrate Plus kit
(Pierce; Thermo Fisher Scientific, Inc.) and ImageQuant™ LAS-4000
mini (GE Healthcare Life Sciences, Uppsala, Sweden).
Statistical analysis
Statistical analysis of PCR data was completed using
Student's t-tests using EXEL 2016 software (Redmond, WA, USA). Data
are presented as the mean ± standard deviation of triplicate
results. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of magnetite
nanoparticles
Magnetite nanoparticles were prepared by reducing
Fe(III) in the presence of 1,8-octanediol, 1-octadecene and
oleylamine by heating under an argon atmosphere, followed by the
use of previously reported methods (16). The magnetite nanoparticles were mixed
with a gelatin solution at 60°C and, following cooling, a gelatin
gel containing the magnetite nanoparticles was prepared. The gel
was dried in vacuo and the solid block was rubbed on an inkstone
with water. This method is the same as the traditional method used
to prepare Chinese gelatin-coated carbon black ink. Nanoparticles
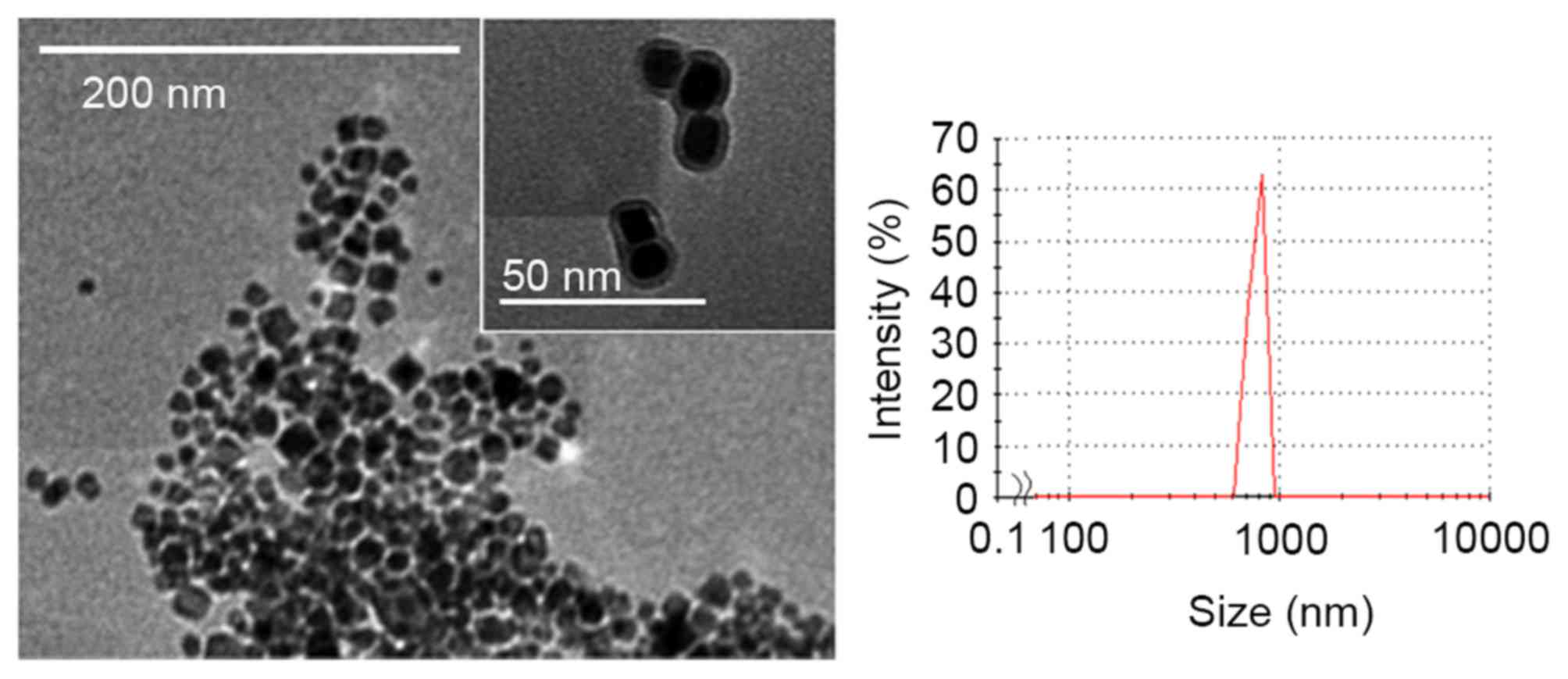
of ~20 nm in diameter were observed under TEM and the particles
formed aggregates (Fig. 1). Dynamic
light scattering analysis indicated that the mean diameter of the
aggregates was 800 nm. The TEM image indicated a shadow on the
surface of these particles, confirming that the magnetite
nanoparticles were coated with a gelatin coating that was ~4 nm
thick (Fig. 1).
Uptake of magnetite by macrophages and
T98G cells
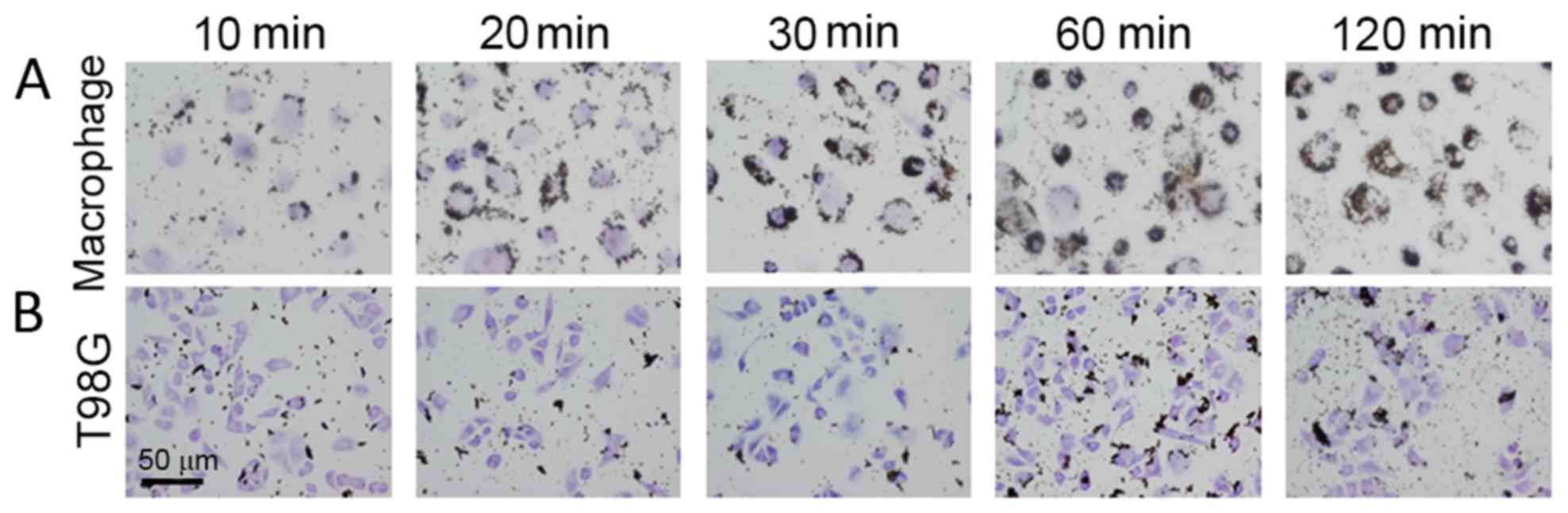
The gelatin-coated magnetite nanoparticles were
added to human macrophages and glioblastoma T98G cells. Uptake of
the nanoparticles by macrophage cells was observed at 10 min, and
the amount of nanoparticles taken up increased with the incubation
time (Fig. 2A). Since the
nanoparticles were observed as dots, it is possible that they were
internalized by an endocytic pathway. T98G cells also internalized
the nanoparticles; however, the amount internalized was lower than
that in the human macrophages (Fig.
2B).
Separation of human macrophages from
co-culture of macrophages and T98G cells
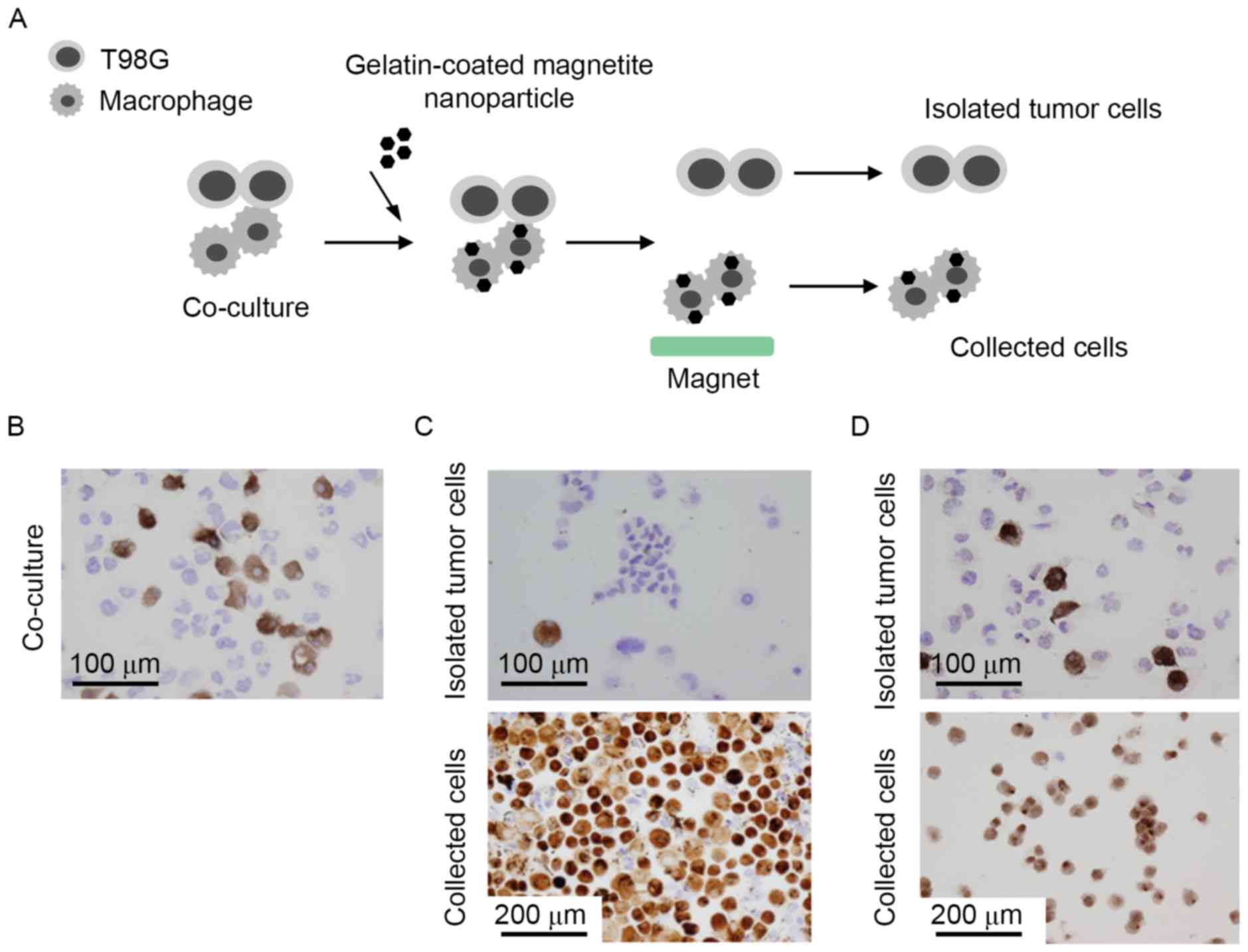
In order to determine whether the magnetite
nanoparticles may be used to separate macrophages from co-cultures
with T98G cells, the nanoparticles were added to a mixture of these
cells. Once the nanoparticles were taken up by the cells, any cells
with magnetite nanoparticles were collected using a magnet and
analyzed (Fig. 3A). CD204 is a
specific marker of macrophages and, based on the expression of this
marker, ~30% of the co-cultured cells were macrophages (Fig. 3B). As presented in Fig. 3C, the majority of the collected cells
were stained with CD204, indicating that they were macrophages.
However, certain CD204-negative T98G cells were also detected in
the collected cells, and the percentage of contaminating T98G cells
was >5%. By contrast, the vast majority of the cells not
collected with the magnet were not stained with CD204, and
contamination with CD204-positive macrophages was <1% (Fig. 3C). These results indicated that,
while the purity of the collected macrophages was not high, the
purity of the T98G cells in the uncollected fraction was high
enough that the cells could be used for further analysis. In
addition, the results obtained using magnetite nanoparticles were
compared with those obtained using commercially available anti-CD14
antibody-labeled microbeads and a magnet column. The purity of the
separated tumor cells using commercially available magnet beads was
12–14%, which was higher than that obtained using magnetite
nanoparticles (Fig. 3D).
Enhanced expression of CCL2, IL-6 and
M-CSFR by T98G cells co-cultured with macrophages
It has been previously demonstrated that co-culture
with macrophages induces activation of T98G cells; however, the
gene expression profile of T98G cells was not evaluated (8). Therefore, in the present study, the
gene expression of CCL2, IL-6, O(6)-methylguanine-DNA methyltansferase
(MGMT), transforming growth factor-β (TGF-β), vascular endothelial
growth factor-A (VEGF-A) and M-CSFR in control T98G cells and in
the T98G cells isolated from a co-culture with macrophages was
investigated by RT-qPCR. Upregulation of CCL2, IL-6, and M-CSFR
mRNA expression was demonstrated in T98G cells that had been
co-cultured with macrophages compared with control cells
(P<0.01; Fig. 4A). No significant
differences in the mRNA expression levels of MGMT, TGF-β or VEGF-A
were observed (data not shown). M-CSFR expression in the cells was
further evaluated by flow cytometry to confirm the increased
expression of M-CSFR in T98G cells co-cultured with macrophages
compared with control cells. In this experiment, T98G-GFP cells and
macrophages were directly mixed and co-cultured for 2 days,
following which the cells were stained with an anti-M-CSFR
antibody. As demonstrated in Fig.
4B, overexpression of M-CSFR was observed on T98G-GFP cells
when they were co-cultured with macrophages. In addition to
analysis using flow cytometry, the effect of the conditioned medium
of a co-culture of T98G cells and macrophages on M-CSFR expression
by T98G cells was evaluated using western blot analysis. T98G cells
were cultured with the conditioned medium of co-cultured (T98G and
macrophages) cells for 6, 24, or 48 h following which cell lysates
were analyzed by western blot analysis. M-CSFR expression in T98G
cells was markedly increased in a time dependent manner by
stimulation of the cells with the conditioned medium compared with
control cells (Fig. 4C).
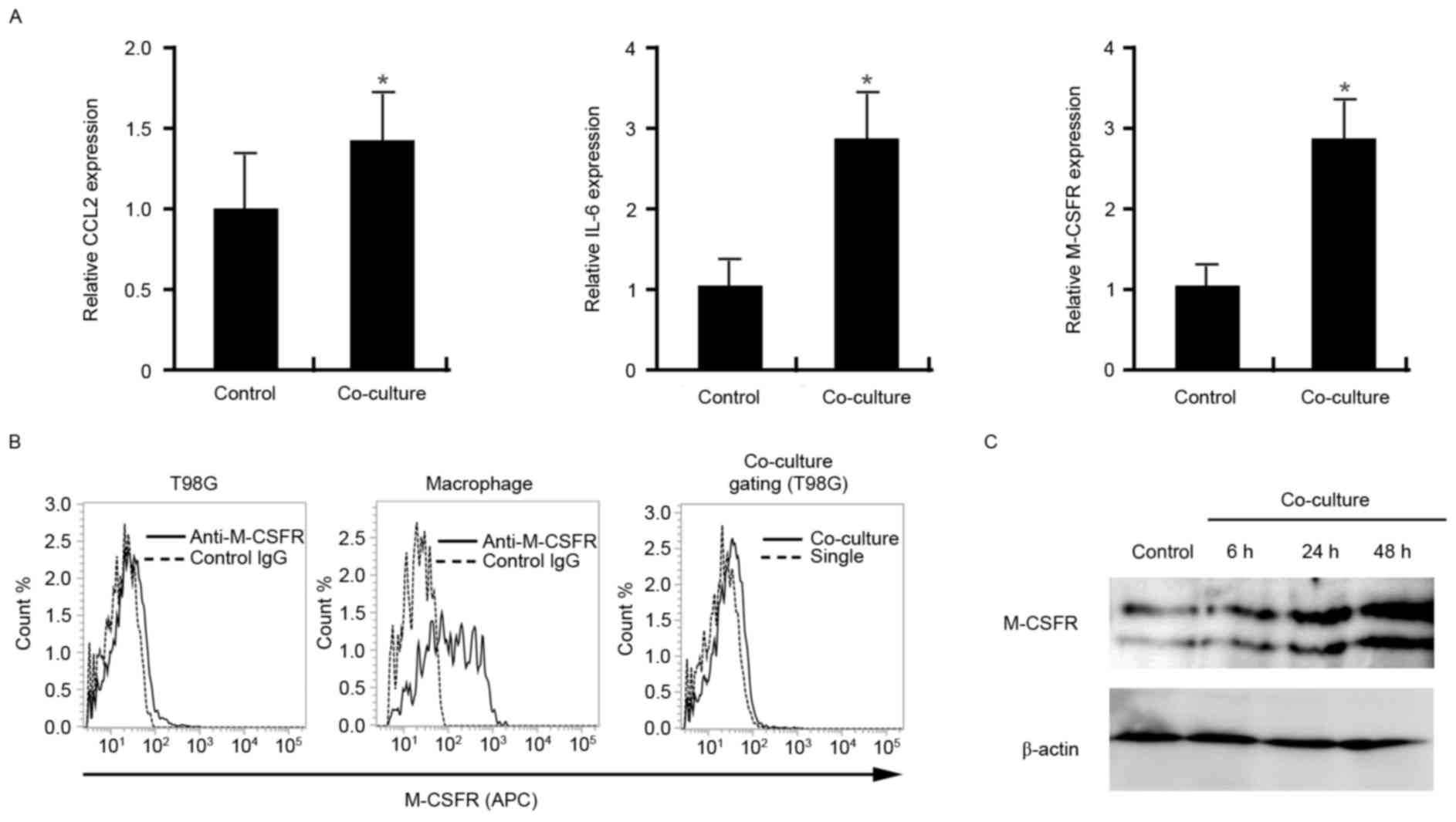 | Figure 4.Expression of pro-tumor molecules by
T98G cells following co-culture with macrophages. (A) mRNA
expression of CCL2, IL-6 and M-CSFR, normalized to β-actin, in
control T98G cells and T98G cells following their isolation from
co-culture with macrophages by depletion of the macrophages using
gelatin-coated magnetite nanoparticles. (B) M-CSFR expression on
the cell surface of T98G-GFP cells and macrophages was analyzed
using flow cytometry. M-CSFR expression on macrophages and T98G-GFP
cells was distinguished by gating the green fluorescence of GFP.
(C) T98G cells were cultured with the conditioned medium of a
co-culture of T98G cells and macrophages, following which M-CSFR
expression was evaluated by western blot analysis. β-actin
expression was analyzed as a loading control. The data are
representative of at least two or three independent experiments.
*P<0.01 vs. the control. CCL2, chemokine (CC motif) ligand 2;
IL-6, interleukin-6; M-CSFR, macrophage-colony stimulating factor
receptor; GFP, green fluorescence protein; IgG, immunoglobulin G;
APC, allophycocyanin. |
Discussion
The present study developed a simple and easy method
for separating macrophages from tumor cells in a co-culture system.
The purity of the tumor cells was <90% following a previous
attempt to separate macrophages from tumor cells in a co-culture
system using commercially available anti-CD14 microbeads and a
magnet column (unpublished data). Downregulation of the CD14
antigen in macrophages is considered to be the reason for this
result. As the cost of preparing magnetite nanoparticles is
markedly lower than that of commercially available antibody-labeled
microbeads, the methods presented in the present study may be more
acceptable or more convenient than such microbeads for separating
macrophages from tumor cells in a co-culture system. Although the
purity of the obtained tumor cells is sufficient for further
experiments aimed at evaluating mRNA expression, the purity of the
obtained macrophages was affected by tumor cell contamination.
Tumor cell contamination was considered to be due to non-specific
binding of magnetite nanoparticles to tumor cells, and further
studies are necessary to reduce the non-specific binding of
magnetite nanoparticles by modulating the coating materials or
methods.
In the present study, magnetite nanoparticles were
coated with gelatin because of its low cost and specific binding
affinity to macrophages (17).
Nanoparticles or nanocarriers targeting macrophages have been
developed and have been demonstrated to be useful for anti-tumor
therapy targeting TAMs (18,19). The present study initially aimed to
develop the magnetite nanoparticles used in the present study for
use as nanoparticles for targeting TAMs. These magnetite
nanoparticles were assessed previously by the authors of the
present study in a murine tumor implantation model. However,
magnetite nanoparticles that were injected into a vein mainly
localized to the liver, spleen and lung, and not to tumor tissues
(unpublished data). Therefore, it may be necessary to develop
additional methods to specifically deliver the magnetite
nanoparticles to tumor tissues in vivo.
In the present study, pro-tumor molecules including,
CCL2, IL-6 and M-CSFR were significantly upregulated in T98G cells
following co-culture with macrophages. CCL2 is also known as MCP-1
and was first identified in glioblastoma cell lines (20). CCL2 was then identified to be
expressed by different types of tumor cells (20). CCL2 is closely involved in the
chemotaxis of TAMs in the tumor microenvironment (20). TAMs are believed to accelerate tumor
progression and development, therefore CCL2/CC receptor type 2
binding is considered a promising target for additional anti-tumor
therapy (21). IL-6 has a well-known
association with tumor cell proliferation, survival and
tumorigenesis via activation of Janus kinases and STATs (22). IL-6 secreted by mesenchymal cells and
tumor cells activates the STAT3 signal, which is involved in the
maintenance and tumorigenicity of glioma stem cells in glioma
(23). Therefore, STAT3 is
considered to be a therapeutic target for glioma. M-CSFR, also
known as c-fms, is not only a well-known oncogene that is involved
in tumor proliferation and survival, but is expressed in
macrophages and is associated with macrophage activation (24). M-CSFR inhibition abrogates glioma
progression by inhibiting the pro-tumor activation of TAMs
(25). Although there have been a
number of research studies regarding M-CSF production from glioma
cells (26–28), to the best of our knowledge, there is
no published research assessing M-CSFR expression in glioma cells,
other than a previous study that reported the activation of M-CSFR
in both glioma cells and TAMs in human glioma samples (8). The observations indicated that M-CSFR
expression in cultured glioma cell lines is markedly lower than
that in macrophages, and may suggest that M-CSFR expression in
glioma cells may not be of particular interest for further
research. However, the present study demonstrated that M-CSFR
expression in T98G glioma cells was markedly upregulated by
cell-cell interaction with TAMs. M-CSFR is known to be closely
involved in tumor progression in other malignant tumors (29) and therefore, the function of the
observed M-CSFR activation in glioma cells may be potentially
associated with glioma progression.
In conclusion, the present study developed
gelatin-coated magnetite nanoparticles for use in separating
macrophages from tumor cells in a co-culture system, and
demonstrated that certain pro-tumor molecules are induced in glioma
cells by cell-cell interaction with macrophages. Although the
methods used to coat the magnetite nanoparticles require
improvement in future studies to inhibit their non-specific binding
to tumor cells, magnetite nanoparticles are cheaper and easier to
handle than antibody-labeled microbeads. Magnetite nanoparticles
may be a novel tool not only for targeting TAMs, but also for
investigation of the unique activation status of tumor cells in
co-culture conditions.
Acknowledgements
The authors of the present study would like to thank
Ms. Emi Kiyota, Mr. Osamu Nakamura, Ms. Yui Hayashida and Mr.
Takenobu Nakagawa (Department of Cell Pathology, Kumamoto
University, Kumamoto, Japan) for their technical assistance. The
present study was supported by a grant from the Ministry of
Education, Culture, Sports, Science and Technology of Japan (grant
no. 25460497).
References
|
1
|
Casey SC, Amedei A, Aquilano K, Azmi AS,
Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR,
et al: Cancer prevention and therapy through the modulation of the
tumor microenvironment. Semin Cancer Biol. 35 Suppl:S199–S223.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Komohara Y, Fujiwara Y, Ohnishi K and
Takeya M: Tumor-associated macrophages: Potential therapeutic
targets for anti-cancer therapy. Adv Drug Deliv Rev. 99:180–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitamura T, Qian BZ and Pollard JW: Immune
cell promotion of metastasis. Nat Rev Immunol. 15:73–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naguib YW and Cui Z: Nanomedicine: The
promise and challenges in cancer chemotherapy. Adv Exp Med Biol.
811:207–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amoozgar Z and Goldberg MS: Targeting
myeloid cells using nanoparticles to improve cancer immunotherapy.
Adv Drug Deliv Rev. 91:38–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komohara Y, Jinushi M and Takeya M:
Clinical significance of macrophage heterogeneity in human
malignant tumors. Cancer Sci. 105:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komohara Y, Horlad H, Ohnishi K, Fujiwara
Y, Bai B, Nakagawa T, Suzu S, Nakamura H, Kuratsu J and Takeya M:
Importance of direct macrophage-tumor cell interaction on
progression of human glioma. Cancer Sci. 103:2165–2172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Usami Y, Ishida K, Sato S, Kishino M,
Kiryu M, Ogawa Y, Okura M, Fukuda Y and Toyosawa S: Intercellular
adhesion molecule-1 (ICAM-1) expression correlates with oral cancer
progression and induces macrophage/cancer cell adhesion. Int J
Cancer. 133:568–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komohara Y, Niino D, Ohnishi K, Ohshima K
and Takeya M: Role of tumor-associated macrophages in hematological
malignancies. Pathol Int. 65:170–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Russell JN, Clements JE and Gama L:
Quantitation of gene expression in formaldehyde-fixed and
fluorescence-activated sorted cells. PLoS One. 8:e738492013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruppert J, Schütt C, Ostermeier D and
Peters JH: Down-regulation and release of CD14 on human monocytes
by IL-4 depends on the presence of serum or GM-CSF. Adv Exp Med
Biol. 329:281–286. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prieto J, Eklund A and Patarroyo M:
Regulated expression of integrins and other adhesion molecules
during differentiation of monocytes into macrophages. Cell Immunol.
156:191–211. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motoshima T, Komohara Y, Horlad H,
Tsukamoto H, Fujita M, Saito Y, Tanoue K, Kasejima Y, Sugiyama Y,
Kawano Y, et al: CXCL10 and CCL2 mRNA expression in monocytes is
inversely correlated with the HLA-DR lower fraction of monocytes in
patients with renal cell carcinoma. Oncol Lett. 11:1911–1916.
2016.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun S and Zeng H: Size-controlled
synthesis of magnetite nanoparticles. J Am Chem Soc. 124:8204–8205.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gudewicz PW, Molnar J, Lai MZ, Beezhold
DW, Siefring GE Jr, Credo RB and Lorand L: Fibronectin-mediated
uptake of gelatin-coated latex particles by peritoneal macrophages.
J Cell Biol. 87:427–433. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niu M, Naguib YW, Aldayel AM, Shi YC,
Hursting SD, Hersh MA and Cui Z: Biodistribution and in vivo
activities of tumor-associated macrophage-targeting nanoparticles
incorporated with doxorubicin. Mol Pharm. 11:4425–4436. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu S, Niu M, O'Mary H and Cui Z:
Targeting of tumor-associated macrophages made possible by
PEG-sheddable, mannose-modified nanoparticles. Mol Pharm.
10:3525–3530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshimura T, Robinson EA, Tanaka S,
Appella E, Kuratsu J and Leonard EJ: Purification and amino acid
analysis of two human glioma-derived monocyte chemoattractants. J
Exp Med. 169:1449–1459. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunol.
26:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hossain A, Gumin J, Gao F, Figueroa J,
Shinojima N, Takezaki T, Priebe W, Villarreal D, Kang SG, Joyce C,
et al: Mesenchymal stem cells isolated from human gliomas increase
proliferation and maintain stemness of glioma stem cells through
the IL-6/gp130/STAT3 pathway. Stem Cells. 33:2400–2415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamilton JA: Colony-stimulating factors in
inflammation and autoimmunity. Nat Rev Immunol. 8:533–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pyonteck SM, Akkari L, Schuhmacher AJ,
Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT,
Teijeiro V, et al: CSF-1R inhibition alters macrophage polarization
and blocks glioma progression. Nat Med. 19:1264–1272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De I, Steffen MD, Clark PA, Patros CJ,
Sokn E, Bishop SM, Litscher S, Maklakova VI, Kuo JS, Rodriguez FJ
and Collier LS: CSF1 overexpression promotes high-grade glioma
formation without impacting the polarization status of
glioma-associated microglia and macrophages. Cancer Res.
76:2552–2560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sielska M, Przanowski P, Wylot B,
Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J,
Vinnakota K, Kettenmann H, et al: Distinct roles of CSF family
cytokines in macrophage infiltration and activation in glioma
progression and injury response. J Pathol. 230:310–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bender AM, Collier LS, Rodriguez FJ, Tieu
C, Larson JD, Halder C, Mahlum E, Kollmeyer TM, Akagi K, Sarkar G,
et al: Sleeping beauty-mediated somatic mutagenesis implicates CSF1
in the formation of high-grade astrocytomas. Cancer Res.
70:3557–3565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patsialou A, Wyckoff J, Wang Y, Goswami S,
Stanley ER and Condeelis JS: Invasion of human breast cancer cells
in vivo requires both paracrine and autocrine loops involving the
colony-stimulating factor-1 receptor. Cancer Res. 69:9498–9506.
2009. View Article : Google Scholar : PubMed/NCBI
|