Introduction
Sodium ferulate extracted from Angelica
sinensis and Oxymatrine, an alkaloid extracted from Sophorae
flavescentis, have been reported to possess anti-inflammatory
and anti-oxidant effects (1,2). Previous studies revealed that a
combination of sodium ferulate and oxymatrine exerted synergistic
anti-inflammatory effects (3–6). The
studies demonstrated that sodium ferulate and oxymatrine
combination significantly inhibited xylene-induced edema in mouse
ears and carrageenan-induced edema in rat paws. A mechanistic study
revealed that the anti-inflammatory effects of sodium ferulate and
oxymatrine combination was mainly associated with its modulatory
effect on interleukin-6 (IL-6), IL-11, C-reactive protein (CRP) and
interferon-γ (IFN-γ) in a RAW 264.7 cell model stimulated by
lipopolysaccharides (4), which had
also been verified in mouse models of cecal ligation and
puncture-induced sepsis and enthanol-induced liver damage as well
as in a RAW 264.7 cell model of lipopolysaccharide-stimulated
damage (5,6).
Inflammation is a defensive response of the body to
pathogenic agents, which is a common and important basic
pathological process in the development of diseases (7,8). An
appropriate inflammatory response is beneficial for the body to
eliminate the invading pathogens, but an exaggerated inflammatory
response, such as that to lung edema, peritonitis and sepsis, is
harmful for the body (9). Therefore,
anti-inflammatory drugs are widely used in the clinic. Study of the
inhibition of cyclooxygenase activity is an important route for the
screening of non-steroidal anti-inflammatory drugs. However,
preliminary experiments by our group found that the combination of
sodium ferulate and oxymatrine did not exhibit any synergistic
inhibitory effect on cyclooxygenase (data not shown). The present
study further explored the anti-inflammatory effects of this drug
combination and focused on the underlying mechanism.
Aquaporins (AQPs) are cell-membrane proteins that
have been reported to have a vital role in transporting water
across cell membranes (10). The
present study hypothesized that sodium ferulate and oxymatrine may
exert their anti-inflammatory exudation effect to reduce vascular
endothelial cellular edema by affecting AQPs. In the present study,
a mouse model of acetic acid-induced peritonitis was used to
evaluate the effects of sodium ferulate and oxymatrine on
anti-inflammatory exudation. By observation of the cell volume and
AQP1 expression by omentum majus vascular endothelial cells and
human umbilical vein endothelial cells (HUVECs), an approach was
made to explain the anti-inflammatory exudation mechanism.
Materials and methods
Materials
Sodium ferulate [molecular formula,
C10H9NaO4 × 2H2O;
molecular weight, 252.20; Chemical Abstracts Service (CAS) no.
24276-84-4; high-performance liquid chromatography (HPLC) purity,
>99%], oxymatrine (molecular formula,
C15H24N2O2 ×
H2O; molecular weight, 282.38; CAS no. 16837-52-8; HPLC
purity, >98%) were provided by Beijing SL Pharmaceutical Co.
Ltd. (Beijing, China). Sodium ferulate and oxymatrine were combined
at a molar ratio of 1:2, which was determined by preliminary
pharmaceutical and pharmacological tests (data not shown). At this
molar ratio, the solution system was most stable with a pH value of
7.0, and the pharmacological activity was also the best in
vivo and in vitro, (unpublished data). The primary AQP1
antibody was purchased from Santa Cruz Biotechnology, Inc. (cat.
no. SC-20810; Dallas, TX, USA). The horseradish
peroxidase-conjugated secondary antibody was obtained from
Zhongshan Golden Bridge Biotechnology Co. Ltd. (cat. no. SPN-9001;
Beijing, China). ELISA kits for measurement of the levels of IL-6,
CRP and IFN-γ were purchased from Groundwork Biotechnology
Diagnosticate Ltd. (San Diego, CA, USA). Evans blue was purchased
from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Dexamethasone was purchased from Shandong Lukang Pharmaceutical
Co., Ltd. (Jining, China). Bovine serum albumin was obtained from
Bai Qian Biotechnology Co., Ltd. (Shanghai, China).
Diaminobenzidine was purchased from Zhongshan Golden Bridge
Biotechnology Co., Ltd. RPMI-1640 medium, fetal bovine serume and
trypsin were produced by Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). HUVECs were purchased from the Insitute of
Biochemistry and Cell Biology (Shanghai Institutes for Biological
Sciences, Shanghai, China).
Animals
Male Swiss mice (400 mice, at 4 weeks of age;
Shandong Luye Pharmaceutical Co. Ltd., Yantai, China; quality
certificate no. Lu 20090009) were used. The animals were kept under
standard conditions (12-h light/dark cycle; temperature, 23±2°C;
humidity, 55±5%) and adapted to the surrounding environment for 3
days prior to the experiments. In order to reduce the animal
individual differences, the healthy animals, weighting 18–22 g were
selected for use in the subsequent experiments, because the drugs
were administered on the basis of the animal weight (mg/kg). Other
animals were returned to the animal department. The animals fasted,
with access to water only for 12 h prior to the experiment, and
following administration of drugs to the animals, food and water
were provided ad libitum. The Experimental Animal Management
Center of Yantai University (Yantai, China) approved all animal
procedures of this study in accordance with the NIH Guidelines
(11) for the Care and Use of
Laboratory Animals.
Exudation inhibition ratio of Evans
blue in mice with acetic acid-induced peritonitis
In order to investigate the anti-exudation activity
of sodium ferulate and oxymatrine combination, the mice were
injected with Evans blue and peritonitis was induced with acetic
acid according to a previously described method (12). Mice used in the experiment were
randomly divided into a normal control group (saline), a model
group (saline and acetic acid), 5 sodium ferulate groups (15.6,
31.3, 62.5, 125 and 250 mg/kg), 5 oxymatrine groups (15.6, 31.3,
62.5, 125 and 250 mg/kg) and 5 sodium ferulate + oxymatrine
combination groups (4.8+10.8, 9.7+21.6, 19.4+43.1, 38.8+86.2 and
77.5+172.5 mg/kg, respectively). Each group contained 10 animals.
The animals were intraperitoneally injected with the corresponding
drug or drug combination in saline at a volume of 10 ml/kg. One
hour later, 0.5% Evans blue solution (0.2 ml/mouse) was
intravenously injected, followed by intraperitoneal injection of
0.9% acetic acid solution (0.2 ml/mouse). After 20 min, the animals
were sacrificed and the peritoneal cavity was washed with 1 ml
saline. Subsequently, the peritoneal lavage fluid was collected and
centrifuged at 1,000 × g for 15 min at 4°C. The supernatant was
isolated and the optical density (OD) value of Evans blue was
measured at 590 nm. The inhibition ratio of Evans blue was
calculated using following formula: Exudation inhibition ratio = (1
- OD2/OD1) × 100%, with OD1 being
the optical density of Evans blue in the peritoneal lavage fluid of
the model group and OD2 being the optical density of
Evans blue in the peritoneal lavage fluid of the respective drug
treatment group.
Number of leukocytes and the levels of
IL-6, CRP and IFN-γ in peritoneal lavage fluid
The animals were randomly divided into a normal
control group (saline), a model group (saline and acetic acid),
dexamethasone group (DEX, 1 mg/kg), sodium ferulate group (19.4
mg/kg), oxymatrine group (43.1 mg/kg) and 3 sodium ferulate +
oxymatrine combination groups (9.7+21.6, 19.4+43.1 and 38.8+86.2
mg/kg). Each group contained 10 animals. One hour after
intraperitoneal injection with the corresponding drug or drug
combination in saline (10 ml/kg), 0.9% acetic acid solution (0.2
ml/mouse) was intraperitoneally injected. After 6 h, the animals
were sacrificed and the peritoneal cavity was washed with 1 ml
saline. The peritoneal lavage fluid was collected and centrifuged
at 1,000 × g for 15 min at 4°C. The supernatant and sediment were
isolated for the measurement of IL-6, CRP and INF-γ by ELISA in
accordance with the manufacturers instructions, and analysis of the
number of leukocytes by a hematocyte counter, respectively.
Pathological and immunohistological
analysis of omentum majus tissue
The animals were randomly divided into a normal
control group (saline), a model group (saline and acetic acid),
sodium ferulate group (19.4 mg/kg), oxymatrine group (43.1 mg/kg)
and 3 sodium ferulate and oxymatrine combination groups (9.7+21.6,
19.4+43.1 and 38.8+86.2 mg/kg). Each group contained 10 animals.
Treatment with drugs and acetic acid was performed as above. At 20
min after the last injection, the animals were sacrificed.
The omentum majus tissues were rapidly isolated and
fixed with 10% formalin for 24 h for pathological analysis. The
specimens were cut into 4-µm sections and stained with hematoxylin
and eosin. The damage degree of omentum majus was evaluated by an
independent observer (Pathological Department, Shandong Luye Drug
Safety Evaluation Center Yantai, China; Good Laboratory Practice
Lab as certified by the Chinese State Food and Drug
Administration), who was blinded to the grouping. The damage score
were obtained on the basis of vascular endothelial cellular
morphology and blood capillary morphology. The scoring criteria for
the surface morphology of vascular endothelial cells were as
follows: 0, no significant change of cell surface morphology; 1,
large number of villi and few cellular edema; 2, loss of
presudopodium and edema of various cells; 3, significant protrusion
into the lumen and formation of a cell gap (13). The scoring criteria of damaged
capillaries were as follows: 0, integrated capillaries with no
significant damage; 1, mild damage but hardly any shedding of
capillaries; 2, moderate damage with a small amount of capillaries
shedding; 3, a large number of capillaries shedding (14).
For immunohistochemical analysis, omentum majus
tissue samples were washed with physiological saline and fixed in
4% paraformaldehyde for 24 h. The specimens were then dehydrated
and embedded in paraffin. The tissues were cut into 4-µm sections,
deparaffinized and hydrated in PBS (pH 7.4). The sections were
treated with 3% H2O2 at room temperature for
15 min to block endogenous peroxidase activity and blocked with 1%
bovine serum albumin (BSA) for 20 min. The sections were
sequentially incubated overnight at 4°C with goat polyclonal
antibody against AQP1 (dilution, 1:200 in PBS). The sections were
then washed with PBS and incubated with horseradish
peroxidase-conjugated secondary antibody (1:100) at room
temperature for 15 min. After washing, the sections were incubated
with diaminobenzidine for 3 min at room temperature. Finally,
sections were counterstained with Mayer's hematoxylin. In the
negative control, staining was performed using 0.01M PBS instead of
the primary antibody. Following initial examination of hematoxylin
and eosin-stained slides, the most appropriate sections were
selected for semi-quantitative immunohistochemical analysis. AQP1
expression was determined using a semi-quantitative
immunohistochemical method (15).
Cell culture
HUVECs were maintained in RPMI-1640 medium
supplemented with 10% (v/v) fetal bovine serum, 100 U/ml of
penicillin and 100 µg/ml streptomycin. Cells were incubated at 37°C
in a humidified atmosphere containing 5% CO2. The medium
was routinely changed every two days.
Assessment of the volume change of
HUVECs
HUVECs were seeded in a 6-well plate at a density of
1×104 cells/ml in 3 ml and incubated for 24 h. The cells
were treated with sodium ferulate (100 µmol/l) or oxymatrine (200
µmol/l), or sodium ferulate + oxymatrine combination (50+100,
100+200 or 200+400 µmol/l). After incubation for 1 h, the cells
were stimulated with acetic acid (8 mmol/l), except for the cells
in the control well. After incubation for 20 min, the cells were
diluted with PBS and collected by trypsinization. In a suspension
with a density of 1×109 cells/l, the volume of the cells
was detected by flow cytometry (BD FACS AriaII; BD Biosciences, San
Jose, CA, USA).
Immunocytochemical staining and
microscopic examination
HUVECs were seeded onto coverslips in a 24-well
plate (1×105 per well) and incubated in complete medium,
containing fresh RPMI-1640 medium supplemented with 10% (v/v) fetal
bovine serum at 37°C under a 5% CO2 for 24 h. The cells
were treated with different concentrations of sodium ferulate (25,
50, 100, 200 or 400 µmol/l in medium) or oxymatrine (50, 100, 200,
400 or 800 µmol/l in medium), or sodium ferulate and oxymatrine
combination (25+50, 50+100, 100+200, 200+400 or 400+800 µmol/l in
medium), and incubated for 1 h. Acetic acid was then added to the
corresponding wells at a concentration of 8 mmol/l. The same amount
of solvent was added to the control wells. After 20 min of
incubation, the cells were washed with PBS.
For immunocytochemical staining, the cells were
fixed with 4% paraformaldehyde for 15 min and dried in air for 5
min (16). Subsequently, cells on
coverslips were blocked using 3% H2O2 for 15
min at room temperature and rinsed with PBS as described previously
(17). After blocking, cells on
coverslips were blocked with 1% BSA in PBS for 15 min at 37°C.
Next, the cells on coverslips were incubated with goat polyclonal
AQP1 antibody (1:200) overnight at 4°C. After rinsing with PBS, the
horseradish peroxidase-conjugated secondary antibody (1:100) were
used at room temperature for 15 min (18). Following a further wash with PBS, the
cells on coverslips were incubated with diaminobenzidine for 3 min
at room temperature. Finally, samples were washed and stained with
Mayer's hematoxylin. Coverslips were mounted on microscope slides.
Semi-quantitative assessment was performed to evaluate the
expression of AQP1 (19,20). The optical density was determined
with Image-pro plus version 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Differences among groups were analyzed using one-way
analysis of variance followed by the unpaired Student's t-test with
equal variance. SPSS 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of sodium ferulate and
oxymatrine administered alone or in combination on exudation of
Evans blue in mice with acetic acid-induced peritonitis
The anti-exudation effects of the drugs were
assessed in a mouse model of acetic acid-induced peritonitis. The
results demonstrated that treatment with sodium ferulate or
oxymatrine alone inhibited Evans blue leakage when the dose was
>62.5 mg/kg (Fig. 1A and B). The
highest inhibition rate was 37.71% for sodium ferulate and 41.85%
for oxymatrine. The concentration leading to 50% inhibition
(ID50 value) was 372.0 mg/kg for sodium ferulate and
310.3 mg/kg for oxymatrine. However, treatment with sodium ferulate
and oxymatrine combination at >9.7+21.6 mg/kg significantly
increased the exudation inhibition ratio of Evans blue in an
obvious dose-dependent manner (Fig.
1C). The highest inhibition rate was 59.74%. The
ID50 value was 46.5+104.1 (mg/kg), which was far less
than that of sodium ferulate or oxymatrine alone. The isobologram
analysis showed that the ID50 value of sodium ferulate
and oxymatrine combination was below the isobol of the theoretical
additive area, which indicated that the two drugs exerted a
synergistic anti-exudation effect (Fig.
2).
 | Figure 1.Inhibitory effects of SF and OMT
administrated alone or in combination on exudation in mice with
acetic acid-induced peritonitis as detected via staining of the
peritoneal lavage fluid following perfusion with Evans blue
solution. Values are expressed as the mean ± standard error of the
mean (n=10). The inhibition ratio compared with the peritonitis
model group was detected in (A) the SF and (B) the OMT at
concentrations of 15.6, 31.3, 62.5, 125 and 250 mg/kg and (C) the
SF + OMT group at different concentrations of SF and OMT [4.8+10.8
(15.6), 9.7+21.6 (31.3), 19.4+43.1 (62.5), 38.8+86.2 (125) and
77.5+172.5 (250) mg/kg]. *P<0.05, **P<0.01, compared with the
model group. SF, sodium ferulate; OMT, oxymatrine; ID50,
dose at which 50% inhibition was achieved. |
Effects of sodium ferulate and
oxymatrine administered alone or in combination on the number of
leukocytes in the peritoneal lavage fluid
The effects of sodium ferulate and oxymatrine
administered alone or in combination on the leukocyte number in the
peritoneal lavage fluid were determined in the mouse model of
acetic acid-induced peritonitis (Fig.
3). Treatment with sodium ferulate and oxymatrine combination
(9.7+21.6, 19.4+43.1 and 38.8+86.2 mg/kg) significantly decreased
the leukocyte number in the peritoneal lavage fluid compared with
that in the model group (P<0.05 in the low-dose group; P<0.01
in the medium- and high-dose group). The inhibition ratio was up to
72.6% in the high-dose group. In addition, the results demonstrated
that the leukocyte number in the peritoneal lavage fluid was also
decreased in the oxymatrine monotreatment group (P<0.05), but
not in the sodium ferulate monotreatment group at a dose equivalent
to the medium dose in the combination treatment (19.4 and 43.1
mg/kg, respectively). In the DEX group, the leukocyte number in the
peritoneal lavage fluid significantly decreased compared with that
in the model group (P<0.01; Fig.
3), but no statistical significance was observed when the DEX
group was compared with the combination drug treated group.
 | Figure 3.Effects of SF and OMT administered
alone or in combination on the number of leukocytes in the
peritoneal lavage fluid of mice with acetic acid-induced
peritonitis. Groups: Con, control group; HA, model group (saline
and acetic acid); DEX, model mice treated with DEX; SF, model mice
treated with SF (19.4 mg/kg); OMT, model mice treated with OMT
(43.1 mg/kg); SF + OMT, model mice treated with SF and OMT at the
indicated doses. Values are expressed as the mean ± standard error
of the mean (n=10). ##P<0.01, vs. the control group.
*P<0.05, **P<0.01, compared with the model group. DEX,
dexamethasone; SF, sodium ferulate; OMT, oxymatrine. |
Effects of sodium ferulate and
oxymatrine administered alone or in combination on the levels of
IL-6, CRP and IFN-γ in the peritoneal lavage fluid
As shown in Fig. 4,
the levels of IL-6, CRP and IFN-γ in the peritoneal lavage fluid
were significantly increased in the model group compared with those
in the control group. Compared with the model group, after
treatment with sodium ferulate and oxymatrine combination, the
levels of IL-6, CRP and IFN-γ in the peritoneal lavage fluid were
significantly decreased in a dose-dependent manner, and the effect
was better than that in the monotreatment groups. In DEX group, the
levels of IL-6, CRP and IFN-γ in the peritoneal lavage fluid also
significantly decreased compared with that in the model group
(P<0.01; Fig. 4), and no
statistical significance was observed when compared with the
combination drug treated group.
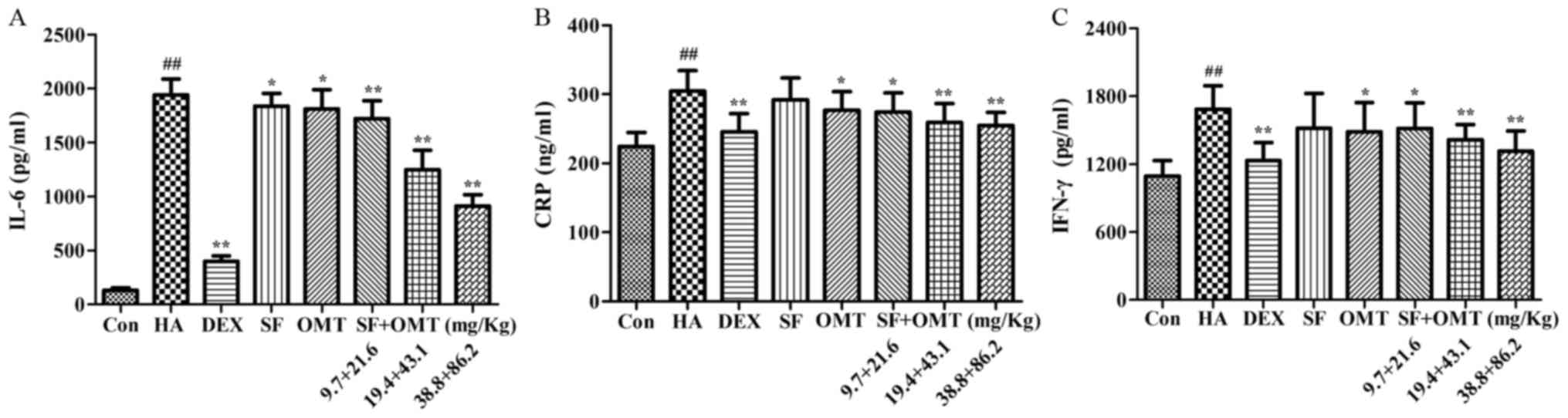 | Figure 4.Effects of sodium ferulate and
oxymatrine administered alone or in combination on the levels of
(A) IL-6, (B) CRP and (C) IFN-γ in the peritoneal lavage fluid.
Groups: Con, control group; HA, model group (saline and acetic
acid); DEX, model mice treated with DEX; SF, model mice treated
with SF (19.4 mg/kg); OMT, model mice treated with OMT (43.1
mg/kg); SF + OMT, model mice treated with SF and OMT at the
indicated doses. Values are expressed as the mean ± standard error
of the mean (n=10). ##P<0.01, vs. the control group.
*P<0.05, **P<0.01, compared with the model group. DEX,
dexamethasone; SF, sodium ferulate; OMT, oxymatrine; IL,
interleukin; CRP, C-reactive protein; IFN, interferon. |
Effects of sodium ferulate and
oxymatrine administered alone or in combination on vascular
endothelial cell morphology, capillary construction and the
expression of AQP1 in omentum majus
Pathological analysis of omentum majus revealed that
compared to the control group, the vascular endothelial cell
morphology and capillary construction were severely damaged in the
model group, including increased inter-cell gaps, protrusion into
the lumen or exfoliation leading to capillary loss (Fig. 5A and B). After treatment with sodium
ferulate and oxymatrine combination, the damage was significantly
alleviated in a dose-dependent manner. The damage was also
alleviated in the oxymatrine monotreatment group, but the effect
was less than that of sodium ferulate and oxymatrine combined
(Fig. 5C-G). The omentum majus
damage score were significantly and dose-dependently improved in
the sodium ferulate and oxymatrine combination treatment groups,
and a significant improvement was also seen in the oxymatrine
monotreatment group (Fig. 6).
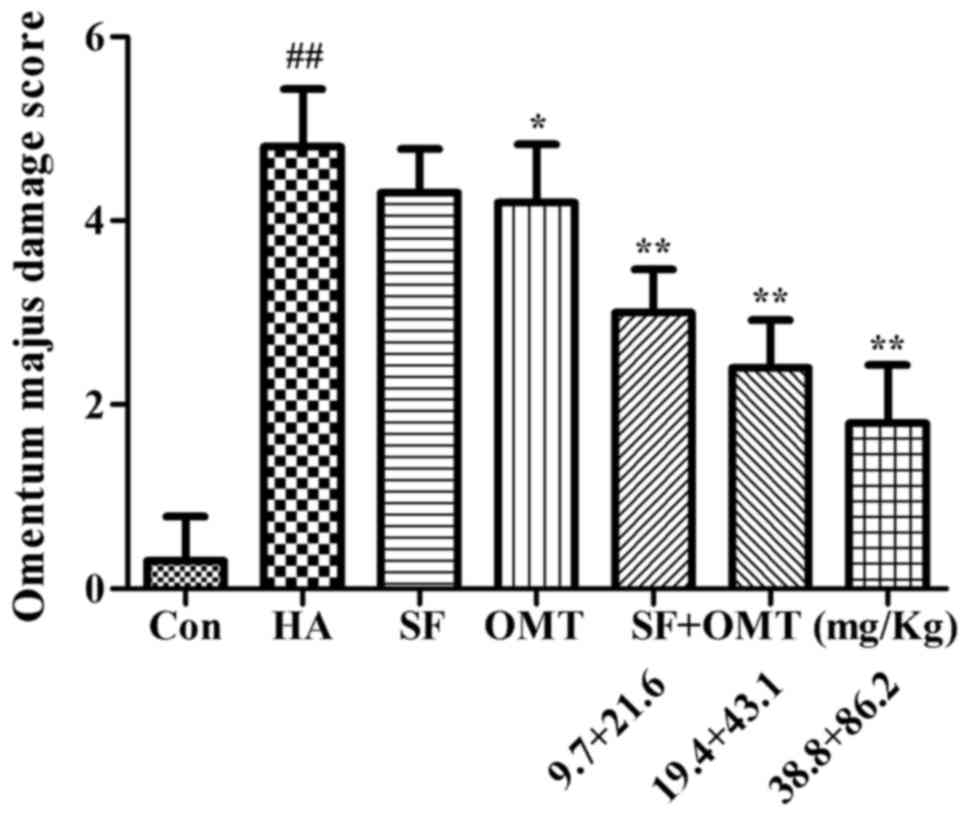 | Figure 6.Effects of SF and OMT administered
alone or in combination on pathological changes of omentum majus in
a mouse model of acetic acid-induced peritonitis. The omentum majus
damage score is shown. Groups: Con, control group; HA, model group
(saline and acetic acid); SF, model mice treated with SF (19.4
mg/kg); OMT, model mice treated with OMT (43.1 mg/kg); SF + OMT,
model mice treated with SF and OMT at the indicated doses. Values
are expressed as the mean ± standard error of the mean (n=10).
##P<0.01, vs. the control group. *P<0.05,
**P<0.01, compared with the model group. SF, sodium ferulate;
OMT, oxymatrine. |
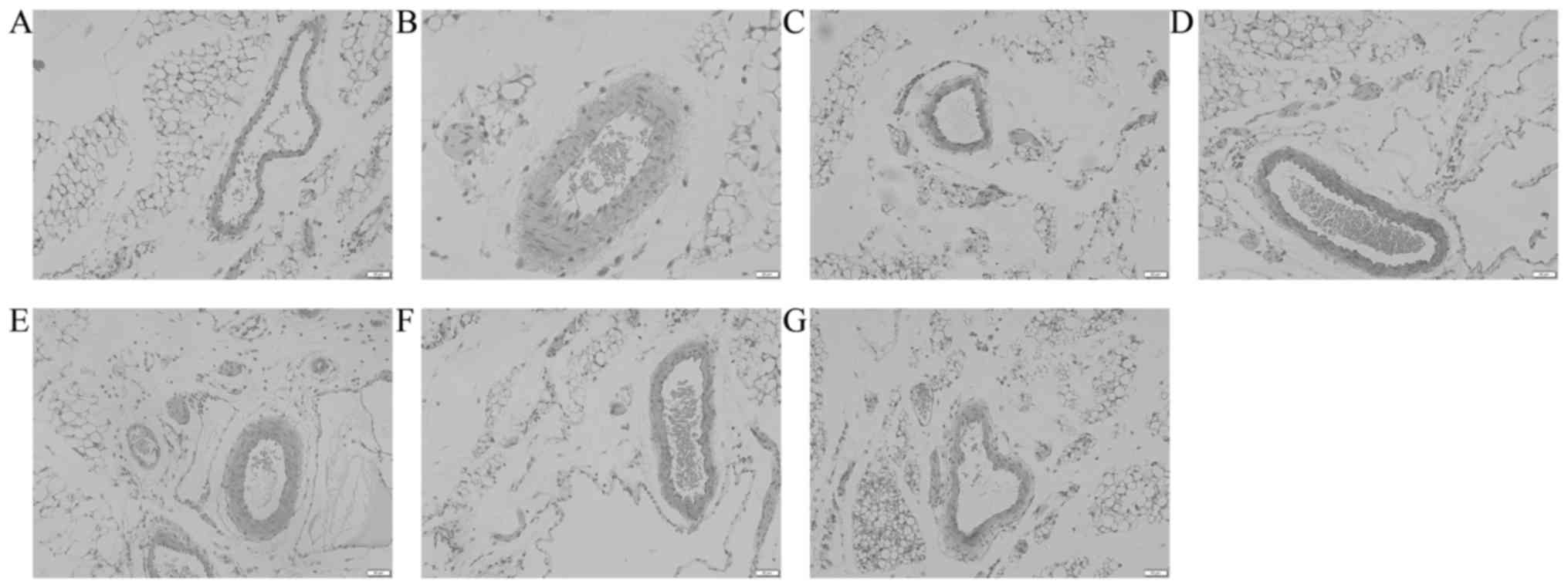
The expression of AQP1 in vascular endothelial cells
of the omentum majus was evaluated by immunohistochemistry
(Fig. 7). The results showed that
the OD of AQP1 in the model group was significantly decreased
compared with that in the control group. However, the OD was
significantly higher in the sodium ferulate and oxymatrine
combination groups compared with that in the model group (P<0.05
in the low-dose combination group; P<0.01 in the medium- and
high-dose group). Sodium ferulate or oxymatrine monotreatment group
did not significantly increase the OD of AQP1.
 | Figure 7.Effects of SF and OMT administered
alone or in combination on the expression of AQP1 in vascular
endothelial cells of the omentum majus. The optical density was
used to represent the strength of expression of AQP1. Groups: Con,
control group; HA, model group (saline and acetic acid); SF, model
mice treated with SF (19.4 mg/kg); OMT, model mice treated with OMT
(43.1 mg/kg); SF + OMT, model mice treated with SF and OMT at the
indicated doses. Values are expressed as the mean ± standard error
of the mean (n=10). ##P<0.01, vs. the control group.
*P<0.05, **P<0.01, compared with the model group. SF, sodium
ferulate; OMT, oxymatrine; AQP, aquaporin. |
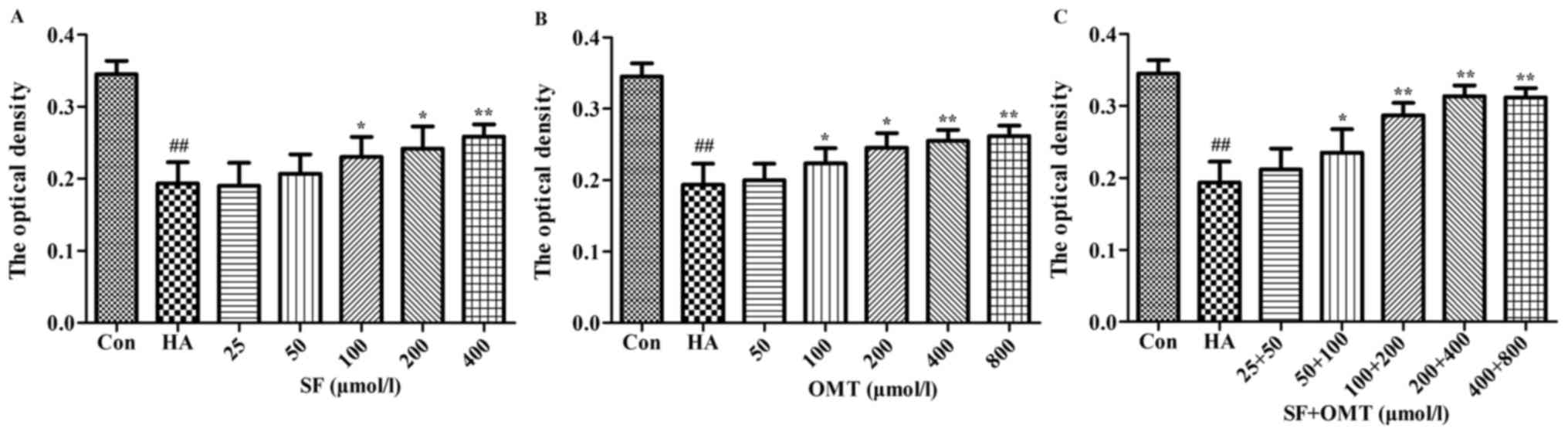
Effects of sodium ferulate and
oxymatrine administered alone or in combination on the cell volume
and the AQP1 expression of HUVEC
As shown in Table I,
the forward scatter integral area (FSC-A) and side scatter integral
area (SSC-A) were significantly increased in the model group
compared with those in the control group. However, in the
combination groups (50+100, 100+200 or 200+400 µmol/l), the FSC-A
and SSC-A were significantly reduced in a dose-dependent manner.
The expression of AQP1 was detected by immunocytochemisty (Fig. 8). The results showed that the OD of
AQP1 was significantly decreased in the model group, compared with
that in the control group. However, compared with the model group,
the OD in the sodium ferulate and oxymatrine combination treatment
groups was significantly increased when the concentration was
>50+100 µmol/l. Furthermore, the OD of AQP1 in the sodium
ferulate or oxymatrine monotreatment groups was significantly
elevated compared with that in the model group when the drug
concentration was >100 µmol/l (P<0.05).
 | Table I.Effects of sodium ferulate and
oxymatrine administrated alone or in combination on the volume of
human umbilical vein endothelial cells. |
Table I.
Effects of sodium ferulate and
oxymatrine administrated alone or in combination on the volume of
human umbilical vein endothelial cells.
|
|
| FSC-A | SSC-A |
|---|
|
|
|
|
|
|---|
| Group | Dose of SF + OMT
(µmol/l) | Value | Increase (% of
Con) | Value | Increase (% of
Con) |
|---|
| Con | – | 116,811±5493 | – | 67,967±4047 | – |
| HA | – | 137,640±8045 | 17.84
±0.04a | 96,798±9664 | 42.67
±0.15a |
| SF | 100+0 | 130,728±1086 | 11.99±0.09 | 87,912±8113 | 29.58±0.13 |
| OMT | 0+200 | 130,060±5632 |
11.397±0.03b | 87,404±6818 | 28.87
±0.11b |
| SF + OMT | 50+100 | 127,716±4853 |
9.55±0.07b | 82,389±9034 |
21.39±0.13b |
| SF + OMT | 100+200 | 122,910±4883 |
5.47±0.08c | 76,866±5579 | 13.23
±0.08c |
| SF + OMT | 200+400 | 122,213±6612 |
4.6±0.02c | 74,389±3167 |
9.65±0.06c |
Discussion
The inflammatory response, a basic pathological
process, is characterized by cell damage, inflammatory exudation
and tissue hyperplasia, including cellular edema, degeneration and
necrosis, tissue fibrosis as well as inflow of blood components and
inflammatory corpuscles to damaged tissue (21). Among them, inflammatory exudation and
cellular edema are major pathophysiological characteristics in the
early phase of the inflammatory response meaning acute
inflammation, and usually lead to vulnerability to organ damage
such as acute lung edema and peritonitis (22). Previous studies by our group found
that sodium ferulate and oxymatrine combination significantly
alleviate the acute inflammatory response (23). Based on a comprehensive analysis of
these results, it was hypothesized that the drug may have a
significant anti-exudation effect. Among the numerous animal
models, the mouse model of acetic acid-induced peritonitis is
commonly used to estimate anti-exudation effects of drugs (12), and is characterized by increased
vascular permeability, fluid exudation, leukocyte migration and
inflammatory cytokine release to the abdominal cavity (24). In this model, the degree of
inflammatory exudation is evaluated by non-subjective
quantification of these indicators.
Evans blue is a chemical dye, which easily combines
with plasma protein. Only when capillary permeability is increased,
Evans blue combined with protein inflows to the abdominal cavity
from capillaries. Following intravenous injection of Evans blue and
induction of peritonitis with acetic acid in mice, the amount of
Evans blue leakage reflecting vascular permeability may be
evaluated by measurement of its OD value at 590 nm in the
peritoneal lavage fluid and the inhibition ratio was calculated as
previously described (25). In the
present study, the OD value of Evans blue in the peritoneal lavage
fluid was markedly increased in the model group, meaning that
plasma protein leaked out of blood vessels and reflecting that the
vascular permeability was increased. Treatment with sodium feulate
and oxymatrine combination significantly relieved the acetic
acid-induced inflammatory exudation in a dose-dependent manner, as
represented by an increased exudation inhibition ratio of Evans
blue, and its effect was better than that of the each drug alone.
More importantly, the isobologram analysis demonstrated that the
experimental ID50 of sodium ferulate and oxymatrine
combination was under the isobol of the theoretical additive area,
indicating that the two drugs possessed a synergistic
anti-exudation effect.
With increasing vascular permeability and leukocyte
migration to the inflammatory site, large amounts of inflammatory
cytokines were released, which were found in the model group.
Treatment with sodium feulate and oxymatrine combination also
significantly decreased the leukocyte number as well as the levels
of IL-6, CRP and IFN-γ in the peritoneal lavage fluid induced by
acetic acid, and its effect was better than that of each drug alone
at doses equal to those in the medium combined group, which
verified the results of previous studies by our group, which
reported that sodium ferulate and oxymatrine combination
significantly reduced the levels of IL-6, CRP and IFN-γ in serum or
tissue homogenate of other mouse models (5,6,23). IL-6, a pro-inflammatory cytokine, has
a vital role in the process of inflammation and macrophage
activation (26–28). CRP is a non-specific biomarker of
inflammation, which is closely associated with tissue injury
(29). IFN-γ is also a potent
pro-inflammatory cytokine resulting in damage of tissue (30). Comprehensive analysis of the
anti-inflammatory effects of certain drugs revealed that they
reduced the permeability of blood vessels, inhibited inflammatory
corpuscle inflow to the inflammatory site and reduced the release
of inflammatory factors, which indicated that these effects may be
a cascading reaction.
A recent study found that damaged vascular
endothelial cells are among the reasons for increased vascular
permeability (31). In the present
study, the vascular endothelial cellular morphology and blood
capillary morphology were assessed. The vascular endothelial
cellular morphology and the integrity of blood capillaries were
significantly reduced in the model group. However, treatment with
sodium ferulate and oxymatrine combination significantly improved
the surface morphology of vascular endothelial cells and alleviated
the amount of damaged blood capillaries. In order to study the
effect of the drugs on vascular endothelial cell edema, volume
changes of HUVECs were assessed by flow cytometry. Flow cytometry
is a novel technology using a laser beam to measure single cells in
the flow to rapidly and accurately analyze or quantitate their
physical and chemical characteristics. FSC is a parameter measuring
light scattered by <10° as a cell passes through the laser beam
to detect the surface properties of cells. The FSC value is
proportional to cell size (32), and
its signal is expressed as FSC-A. Treatment with sodium ferulate
and oxymatrine combination significantly reduced the FSC-A value,
indicating that drugs in combination alleviate cellular edema. This
suggested that the anti-exudation effect of the combination of
sodium ferulate and oxymatrine may be associated with alleviating
vascular endothelial cellular edema. In addition, SSC, also
referred to as 90° scatter or right-angle scatter, includes light
scattered at a 90° angle as a cell passes through the laser beam
and reflects its interior properties. The SSC value is associated
with the internal granularity or complexity of a particle, and its
signal is expressed as the SSC-A. After treatment with the
combination of sodium ferulate and oxymatrine, the SSC-A value was
also decreased, indicating a decrease of intracellular particles,
which will be further elucidated in a future study.
It is well-known that increased intracellular water
is the basic characteristic of cellular edema (33). AQP1 is a water channel protein and
its function is mainly to transport water across the plasma
membrane, contributing to water homeostasis of cells (34). AQP1 is expressed in a great majority
of microvascular endothelia, as well as in endothelial cells of
tissues including the cornea and intestinal lacteals (35). Kim et al (36) found that decreased expression of AQP1
in the choroid plexus led to a decrease in cerebrospinal fluid
formation. The present study therefore hypothesized that vascular
endothelial cellular edema induced by acetic acid was associated
with increased AQP1 expression, and after the drug treatments, the
expression of AQP1 decreased in vascular endothelial cells, which
reduced vascular endothelial cellular edema, alleviated capillary
permeability and decreased the amount of peritoneal fluid induced
by acetic acid. To verify this surmise, the effects of sodium
ferulate and oxymatrine combination on AQP1 expression on the
membrane of the vascular endothelial cells of the omentum majus and
HUVECs were assessed. However, immunohistochemical analysis of
omentum majus and HUVECs revealed that the expression of AQP1 was
significantly decreased after acetic acid stimulation, while it was
significantly increased after treament with a combination of sodium
ferulate and oxymatrine, which is the opposite effect of what was
expected. The possible explanation is that the vascular endothelial
cellular edema induced by acetic acid were due to water retention
through an increase in intracellular metabolism, which was caused
by a reduction of transportation channels represented by their
decreased expression. After the drug treatments, the expression of
AQP1 increased in vascular endothelial cells, which was in parallel
with a reduction of vascular endothelial cellular edema and
alleviation of the increased capillary permeability induced by
acetic acid. Furthermore, in the present study, intracellular
particulate substance was significantly increased after acetic acid
stimulation, which was represented by an elevated SSC-A in the flow
cytometric analyses, and may be one of the major causes of cellular
edema. The type of intracellular particulates that are increased
and the underlying mechanism, as well as the mechanism of
intracellular water accumulation will be assessed in future
studies. For answering these questions, appropriate methods are to
be identified.
In conclusion, sodium ferulate and oxymatrine
combination exhibited significant and synergistic anti-exudation
effects, alleviating vascular endothelial cellular edema induced by
acetic acid. The significant effects of the drugs on AQP1 and the
underlying mechanisms require further exploration.
References
|
1
|
Lee JH, Shin H, Kim YJ, Paek SH, Jin S and
Ha UH: Pseudomonas aeruginosa-induced IL-1β production is inhibited
by Sophora flavescens via the NF-κB/inflammasome pathways. J
Microbiol. 52:1044–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao TT, Wang YY, Zhang Y, Bai CH and Shen
XC: Similar to spironolactone, oxymatrine is protective in
aldosterone-induced cardiomyocyte injury via inhibition of calpain
and apoptosis-inducing factor signaling. PLoS One. 9:e888562014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Pei X, Xu M, Sun S, Zhang C, Mu K
and Liu Z: The protective effect of sodium ferulate and oxymatrine
combination on paraquat-induced lung injury. Iran J Pharm Res.
14:573–583. 2015.PubMed/NCBI
|
|
4
|
Yuan X, Sun Y, Miao N, Sun S, Wang Y, Hu
Z, Yuan J, Xu M and Liu Z: The synergistic anti-inflammatory effect
of the combination of sodium ferulate and oxymatrine and its
modulation on inflammation-associated mediators in RAW 264.7 cells.
J Ethnopharmacol. 137:1477–1485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu M, Wang W, Pei X, Sun S, Xu M and Liu
Z: Protective effects of the combination of sodium ferulate and
oxymatrine on cecal ligation and puncture-induced sepsis in mice.
Exp Ther Med. 7:1297–1304. 2014.PubMed/NCBI
|
|
6
|
Pei X, Wang W, Miao N, Xu M, Zhang C, Sun
M, Xu M and Liu Z: The protective effects of the combination of
sodium ferulate and oxymatrine on ethanol-induced liver damage in
mice. Environ Toxicol Pharmacol. 37:423–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Da Cruz RB, Galdino PM, Penna KG, Hoffmann
K, Costa EA and Bataus LA: Acetone extract from Streptoverticillium
sp., a bacterium isolated from Brazilian Cerrado soil, induces
anti-inflammatory activity in mice. An Acad Bras Cienc. 85:595–603.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Syam S, Bustamam A, Abdullah R, Sukari MA,
Hashim NM, Mohan S, Looi CY, Wong WF, Yahayu MA and Abdelwahab SI:
β Mangostin suppress LPS-induced inflammatory response in RAW 264.7
macrophages in vitro and carrageenan-induced peritonitis in vivo. J
Ethnopharmacol. 153:435–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao C, Zhang Y, Zou P, Wang J, He W, Shi
D, Li H, Liang G and Yang S: Synthesis and biological evaluation of
a novel class of curcumin analogs as anti-inflammatory agents for
prevention and treatment of sepsis in mouse model. Drug Des Devel
Ther. 9:1663–1678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao J, Tan M, Gu M, Marshall C, Ding J, Hu
G and Xiao M: Cellular localization of aquaporin-1 in the human and
mouse trigeminal systems. PLoS One. 7:e463792012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Institutes of Health (US), .
Using Animals In Intramural Research: Guidelines for Investigators
and Guidelines for Animal Users. NIH Animal Care and Use Committee.
1994.
|
|
12
|
Barros WM, Rao VS, Silva RM, Lima JC and
Martins DT: Anti-inflammatory effect of the ethanolic extract from
Bowdichia virgilioides H.B.K stem bark. An Acad Bras Cienc.
82:609–616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen Q, Wu MH and Yuan SY: Endothelial
contractile cytoskeleton and microvascular permeability. Cell
Health Cytoskelet. 2009:43–50. 2009.PubMed/NCBI
|
|
14
|
Di Franco M, Paradiso M, Riccieri V,
Basili S, Mammarella A and Valesini G: Autonomic dysfunction and
microvascular damage in systemic sclerosis. Clin Rheumatol.
26:1278–1283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou S, Zheng F, Li Y, Gao L and Zhang J:
The protective effect of glycyrrhizic acid on renal tubular
epithelial cell injury induced by high glucose. Int J Mol Sci.
15:15026–15043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mu H, Lin KX, Zhao H, Xing S, Li C, Liu F,
Lu HZ, Zhang Z, Sun YL, Yan XY, et al: Identification of biomarkers
for hepatocellular carcinoma by semiquantitative
immunocytochemistry. World J Gastroenterol. 20:5826–5838. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park MS, Kim CS, Joo HK, Lee YR, Kang G,
Kim SJ, Choi S, Lee SD, Park JB and Jeon BH: Cytoplasmic
localization and redox cysteine residue of APE1/Ref-1 are
associated with its anti-inflammatory activity in cultured
endothelial cells. Mol Cells. 36:439–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang J, Kim HS, Kang JW and Kang HC: The
genetically modified polysialylated form of neural cell adhesion
molecule-positive cells for potential treatment of X-linked
adrenoleukodystrophy. Yonsei Med J. 54:246–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calero C, López-Campos JL, Izquierdo LG,
Sánchez-Silva R, López-Villalobos JL, Sáenz-Coronilla FJ,
Arellano-Orden E, Montes-Worboys A and Echevarría M: Expression of
aquaporins in bronchial tissue and lung parenchyma of patients with
chronic obstructive pulmonary disease. Multidiscip Respir Med.
9:292014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li SB, Yang KS and Zhang YT: Expression of
aquaporins 1 and 3 in degenerative tissue of the lumbar
intervertebral disc. Genet Mol Res. 13:8225–8233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toffoli-Kadri MC, Carollo CA, Lourenço LD,
Felipe JL, Néspoli JH, Wollf LG, Resende GM, de Lima JR, Franco VN,
Mdo C Vieira and de Siqueira JM: In vivo and in vitro
anti-inflammatory properties of Achyrocline alata (Kunth) DC. J
Ethnopharmacol. 153:461–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizuno M, Ito Y, Mizuno T, Harris CL,
Suzuki Y, Okada N, Matsuo S and Morgan BP: Membrane complement
regulators protect against fibrin exudation increases in a severe
peritoneal inflammation model in rats. Am J Physiol Renal Physiol.
302:F1245–F1251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan X, Wang Y, Du D, Hu Z, Xu M, Xu M and
Liu Z: The effects of the combination of sodium ferulate and
oxymatrine on lipopolysaccharide-induced acute lung injury in mice.
Inflammation. 35:1161–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smeding L, Plötz FB, Lamberts RR, van der
Laarse WJ, Kneyber MC and Groeneveld AB: Mechanical ventilation
with high tidal volumes attenuates myocardial dysfunction by
decreasing cardiac edema in a rat model of LPS-induced peritonitis.
Respir Res. 13:232012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu HL, Zhang F, Li YJ, Gong GH and Quan
ZS: Anti-inflammatory and antinociceptive effects of
6-(4-chlorophenoxy)-tetrazolo[5,1-a]phthalazine in mice. Pharmacol
Rep. 64:1155–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsing CH and Wang JJ: Clinical implication
of perioperative inflammatory cytokine alteration. Acta
Anaesthesiol Taiwan. 53:23–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niu X, Yao H, Li W, Mu Q, Li H, Hu H, Li Y
and Huang H: δ-Amyrone, a specific inhibitor of cyclooxygenase-2,
exhibits anti-inflammatory effects in vitro and in vivo of mice.
Int Immunopharmacol. 21:112–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Figueroa L Arteaga, Navarro L Barbosa,
Patiño Vera M and Petricevich VL: Preliminary studies of the
immunomodulator effect of the Bougainvillea xbuttiana extract in a
mouse model. Evid Based Complement Alternat Med.
2015:4794122015.PubMed/NCBI
|
|
29
|
de Oliveira TH, Amorin AT, Rezende IS,
Barbosa M Santos, Martins HB, Brito AK, Andrade EF, Gonçalves GK,
Campos GB, Silva RA, et al: Sepsis induced by Staphylococcus
aureus: Participation of biomarkers in a murine model. Med Sci
Monit. 21:345–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Lima TH, Sass N, Mattar R, Moron AF,
Torloni MR, Franchim CS and Daher S: Cytokine gene polymorphisms in
preeclampsia and eclampsia. Hypertens Res. 32:565–569. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J, Chen L, Shu B, Tang J, Zhang L,
Xie J, Liu X, Xu Y and Qi S: Granulocyte/macrophage
colony-stimulating factor attenuates endothelial hyperpermeability
after thermal injury. Am J Transl Res. 7:474–488. 2015.PubMed/NCBI
|
|
32
|
Jordan CT, Yamasaki G and Minamoto D:
High-resolution cell cycle analysis of defined phenotypic subsets
within primitive human hematopoietic cell populations. Exp Hematol.
24:1347–1355. 1996.PubMed/NCBI
|
|
33
|
Ma X, Shatil-Cohen A, Ben-Dor S, Wigoda N,
Perera IY, Im YJ, Diminshtein S, Yu L, Boss WF, Moshelion M and
Moran N: Do phosphoinositides regulate membrane water permeability
of tobacco protoplasts by enhancing the aquaporin pathway? Planta.
241:741–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moon C, Preston GM, Griffin CA, Jabs EW
and Agre P: The human aquaporin-CHIP gene. Structure, organization,
and chromosomal localization. J Biol Chem. 268:15772–15778.
1993.PubMed/NCBI
|
|
35
|
Verkman AS: Aquaporin water channels and
endothelial cell function. J Anat. 200:617–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JG, Son YJ, Yun CH, Kim YI, Nam-Goong
IS, Park JH, Park SK, Ojeda SR, D'Elia AV, Damante G and Lee BJ:
Thyroid transcription factor-1 facilitates cerebrospinal fluid
formation by regulating aquaporin-1 synthesis in the brain. J Biol
Chem. 282:14923–14931. 2007. View Article : Google Scholar : PubMed/NCBI
|