Introduction
Osteoarthritis (OA) is a common age-related
degenerative joint disease, which is caused by the breakdown of
joint cartilage and the underlying bone (1). OA is a major cause of severe joint
pain, knee swelling and physical disability in elderly patients
(2). A previous study reporting an
epidemiological survey revealed that >10% of adults >60 years
old suffer from OA, and that the incidence rate increases with age
and body mass index (3). An
understanding of the underlying mechanisms of pathogenesis in OA is
imperative to the development of novel treatments against the
disease.
Cartilage is an important component of joints and is
comprised of highly specialized chondrocyte cells (4). The principal function of chondrocytes
is to produce the extracellular matrix, which includes collagen and
proteoglycan, so as to maintain the integrity and stability of the
joint (4). OA is characterized by
the extensive loss of proteoglycan content surrounding the
chondrocytes (5). Several previous
studies have demonstrated that NO is a key mediator in the
pathogenesis of OA (6–8). Nitrite has been identified as being
increased in the synovial fluid and serum of patients with OA,
which suggests an increased rate of NO synthesis (9). In experimentally induced models of OA,
NO production was induced by the proinflammatory cytokine
interleukin (IL)-1β and was associated with chondrocyte apoptosis
(10). NO was revealed as promoting
lipocalin-2 production and decreasing chondrocyte vitality
(11). Endoplasmic reticulum stress
also contributed to NO production and chondrocyte apoptosis
(12).
For patients with early or middle stage OA, a
non-arthroplasty treatment regime is typically prescribed, which is
focused on the control of symptoms including the imbalance of
cartilage homeostasis (13).
Previous studies have demonstrated that chitosan and its
derivatives may serve a protective role in OA (14,15).
Chitosan is a linear polysaccharide and carboxymethyl (CM)-chitosan
is a soluble derivative of chitosan. Chitinous materials have been
described in many previous studies as having antioxidant,
anti-inflammatory, antibacterial, anticancer and antifungal
properties (16–18). Chitinous materials have similar
physiochemical properties to the extracellular proteoglycans
located in hyaline cartilage, and they have been used in animal
models to prevent OA (19).
CM-chitosan may inhibit matrix metalloproteinase (MMP) expression
and protect rabbit chondrocytes from IL-1β induced apoptosis
(20,21).
In the present study it has been hypothesized that
the protective role of CM-chitosan may be associated with NO
production, and the role of CM-chitosan in inducible NO synthase
(iNOS) expression has been investigated. The janus kinase
(JAK)/signal transducer and activator of transcription
(STAT)/suppressor of cytokine signaling (SOCS) signaling pathway
has been revealed to be associated with iNOS expression (22,23);
therefore, it was also investigated whether CM-chitosan was able to
attenuate an inflammatory reaction by activating the JAK/STAT/SOCS
signaling pathway.
Materials and methods
Isolation of chondrocytes
A total of 8 four-week old male Sprague-Dawley rats
(180–200 g; Shandong Lukang Record Pharmaceutical Co., Ltd.,
Jining, China) were used for the isolation of articular
chondrocytes as previously reported (24). All rats were allowed to eat and drink
freely and housed at 22–25°C with 45–75% relative humidity and a 12
h light/dark cycle. Briefly, cartilage tissue from the knee joint
was rinsed with PBS containing 100 µg/ml streptomycin and 100 U/ml
penicillin. Chondrocytes were released from cartilage tissue by
0.2% type II collagenase digestion at 37°C for 6 h and filtered
with a 150 mesh strainer. All cells were cultured in Dulbecco's
Modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) in a humidified hood with
5% CO2 at 37°C for 24 h. Primary cell cultures were used
in all experiments. The animal protocol used in the present study
was approved by the Animal Use Committee of Jining Medical
University (Jining, China) prior to the commencement of the
study.
Treatment of cells
Isolated chondrocytes were plated at a density of
1.3×105 cells/well in 6-well plates and incubated for 24
h at 37°C. Fresh DMEM with 2 µg/ml lipopolysaccharide (LPS) was
added to treat the cells for 4 h. Different concentrations (0, 50,
100 and 200 µg/ml) of CM-chitosan (Hebei Qian Sheng Biotechnology
Co., Ltd., Shijiazhuang, China) were then added at 37°C for 24 h.
Chondrocytes without LPS treatment were used as the negative
control.
Quantification of mRNA by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the harvested
chondrocytes using the RNAiso plus kit (Takara Biotechnology Co.,
Ltd., Dalian, China) according to the manufacturer's protocol. cDNA
was synthesized using the PrimeScript RT Master Mix kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol
in a 25-µl reaction volume containing 1 µg template RNA.
Subsequently qPCR was performed using the SYBR premix EX Taq kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. The amplification conditions were: Initial denaturation
at 94°C for 2 min and 40 cycles of denaturation at 94°C for 30 sec,
annealing at 56°C for 40 sec and extension at 72°C for 30 sec. The
endogenous control used in this study was GAPDH. The primer
sequences used in the PCR amplification were as follows: iNOS,
forward 5′-CCTTGTTCAGCTACGCCTTC-3′ and reverse
5′-CATGGTGAACACGTTCTTGG-3′ (565 bp); IL-10, forward
5′-AATCTGTGTTGTTTAAGCTGTTTCC-3′ and reverse
5′-TTTATTCAAAACGAGGATCTGCTAC-3′ (466 bp); JAK1, forward
5′-TGTGGCTGCTGACAAGTGGAG-3′ and reverse 5′-ATGATGGCTCGGAAGAAAGGT-3′
(215 bp); STAT3, forward 5′-ATGGGTTTCATCAGCAAGGAG-3′ and reverse
5′-GGGAATGTCAGGGTAGAGGTAGAC-3′ (279 bp); SOCS3, forward
5′-ACCAGCGCCACTTCTTCACA-3′ and reverse 5′-GTGGAGCATCATACTGATCC-3′
(450 bp); and GAPDH, forward 5′-GGCACAGTCAAGGCTGAGAATG-3′ and
reverse 5′-ATGGTGGTGAAGACGCCAGTA-3′ (143 bp). To compare the mRNA
expression levels of targeted genes, the relative expression ratio
was calculated using the 2−ΔΔCq method, as previously
reported (25) and standardized with
the control group.
Determination of protein expression by
western blotting
Total protein was extracted from the chondrocytes by
lysing the cells in a radioimmunoprecipitation assay lysis buffer
(50 mM Tris pH 7.4, 150 nM NaCl, 1% nonyl
phenoxypolyethoxylethanol-40, 0.5% sodium deoxycholate and protease
inhibitor cocktail (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). The protein concentration was determined via bicinchoninic
acid assay. Protein samples (30 µg each sample per lane) were
separated by 10% SDS-PAGE. Following electrophoresis, the proteins
were transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA) and blocked with 5% skimmed milk at
4°C overnight. The membranes were subsequently probed with the
following mouse monoclonal primary antibodies against iNOS (1:500;
sc-7271), IL-10 (1:1,000; sc-32815), JAK1 (1:1,000; sc-1677), STAT3
(1:1,000; sc-8019), SOCS3 (1:500; sc-73045) and GAPDH (1:1,000;
sc-47724; all Santa Cruz Biotechnology, Inc.) at room temperature
for 2 h. The membranes were washed three times with TBST (10 mM
Tris, 150 mM NaCl, 0.05% Tween-20) and then incubated with
horseradish peroxidase-conjugated secondary antibody against mouse
immunoglobulin G (1:2,000; sc-516102; Santa Cruz Biotechnology,
Inc.) at room temperature for 2 h. Blots were visualized by the
enhanced chemiluminescence method (P0018; Beyotime Institute of
Biotechnology, Haimen, China). The bands were analyzed by Quantity
One software v4.62 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Each experiment was repeated in triplicate and
representative results were presented. SPSS version 20.0 software
(IBM Corp., Armonk, NY, USA) was used for data analysis. Data are
presented as the mean ± standard deviation. Comparison among groups
was analyzed by one-way analysis of variance with Dunnett's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Treatment with CM-chitosan causes a
reduction in iNOS production in a dose dependent manner
Elevated NO concentration is typically observed in
patients with OA (7). NO is
synthesized by the NO synthase family in vivo, of which iNOS
is a member. Therefore, the effect of CM-chitosan on iNOS
production in chondrocytes was investigated. As revealed in
Fig. 1, LPS exposure significantly
increased the expression of iNOS mRNA in chondrocytes compared with
the control group. However, treatment with increasing
concentrations of CM-chitosan caused inhibition of iNOS mRNA in a
dose-dependent manner. Treatment with 50, 100 and 200 µg/ml
CM-chitosan caused a decline of iNOS mRNA at a fold rate of 2.79,
2.08, and 1.42 respectively, compared with the LPS group Fig. 2 demonstrates that the protein
expression of iNOS was also decreased by CM-chitosan in a dose
dependent manner.
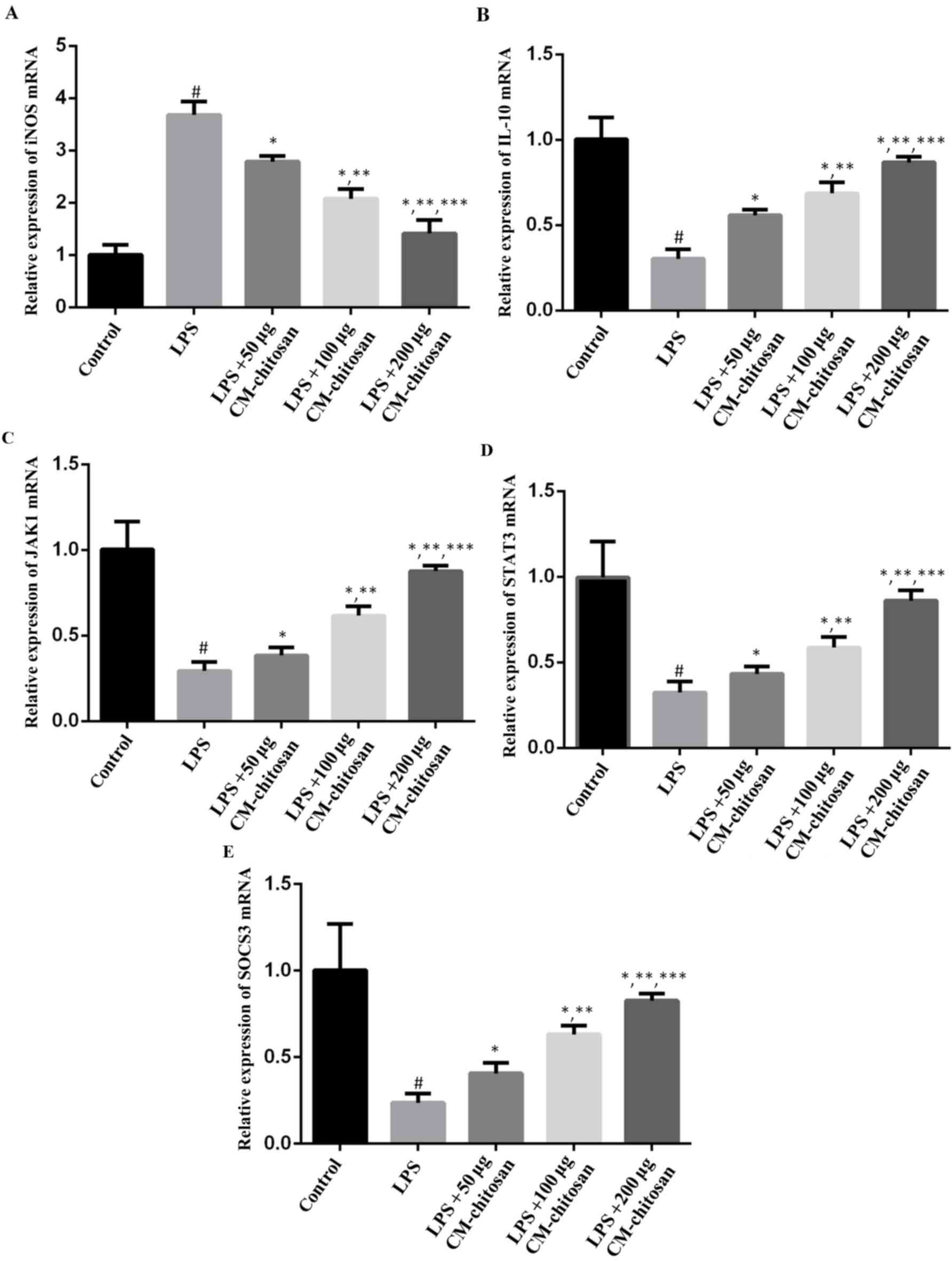 | Figure 1.Effects of CM-chitosan on the mRNA
levels of iNOS, IL-10, JAK1, STAT3 and SOCS3 in LPS-induced rat
chondrocytes. Rat chondrocytes were treated with LPS and
subsequently increasing concentrations (50, 100 and 200 µg/ml) of
CM-chitosan were added. Reverse transcription-quantitative
polymerase chain reaction was used to measure the relative mRNA
levels of (A) iNOS, (B) IL-10, (C) JAK1 (D) STAT3 and (E) SOCS3.
Data are presented as the mean ± standard deviation.
#P<0.05 vs. the control group, *P<0.05 vs. the LPS
group, **P<0.05 vs. the LPS+50 µg CM-chitosan group, and
***P<0.05 vs. the LPS+100 µg CM-chitosan group. CM,
carboxymethyl; iNOS, inducible nitric oxide synthase; IL,
interleukin; JAK, janus kinase; STAT, signal transducer and
activator of transcription; SOCS suppressor of cytokine signaling;
LPS, lipopolysaccharide. |
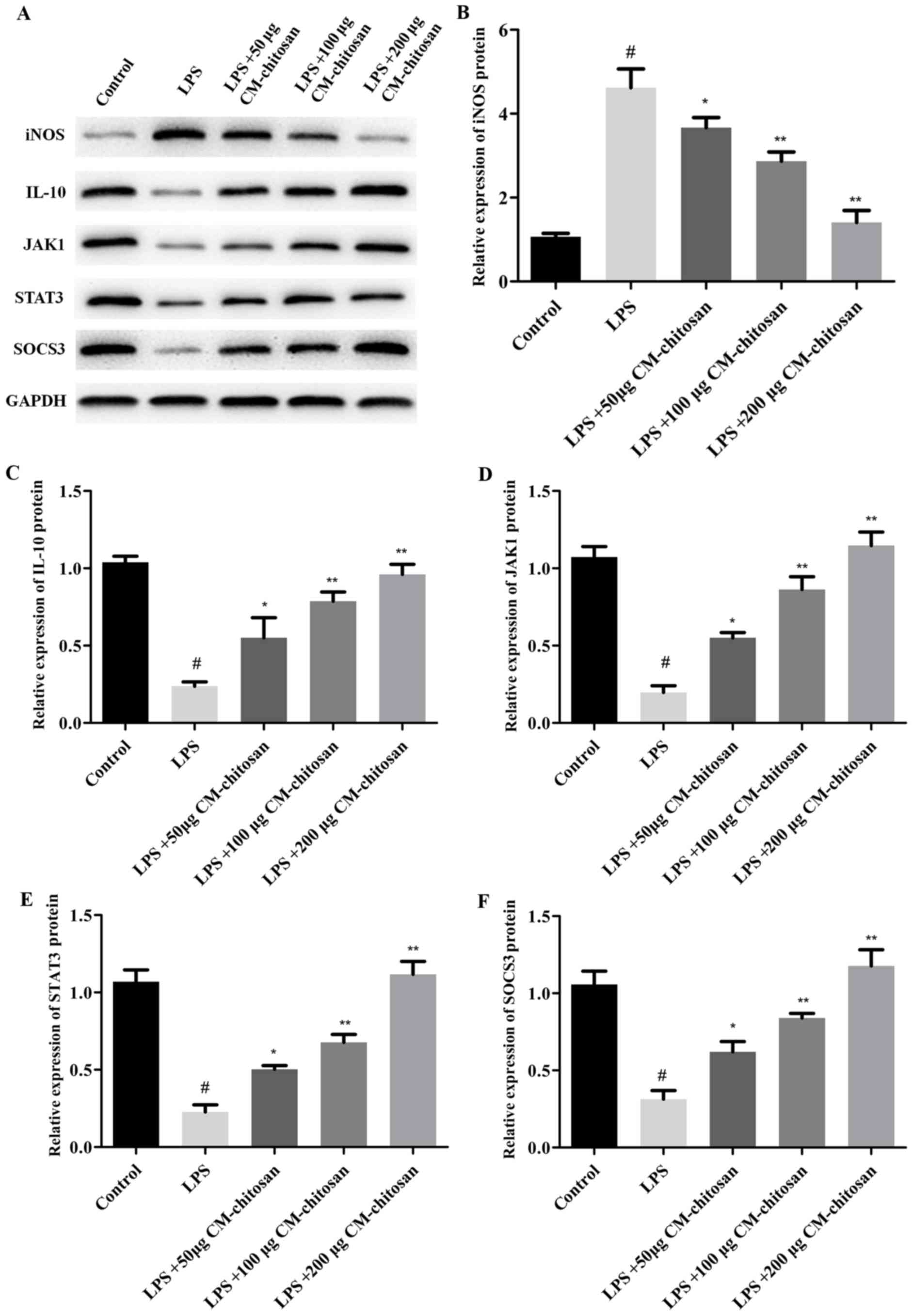 | Figure 2.Effects of CM-chitosan on the protein
levels of iNOS, IL-10, JAK1, STAT3 and SOCS3 in LPS-induced rat
chondrocytes. Rat chondrocytes were treated with LPS and
subsequently increasing concentrations (50, 100 and 200 µg/ml) of
CM-chitosan were added. (A) A western blot analysis was performed
to detect the proteins present in the rat chondrocytes. These
results were quantified and the relative protein expression levels
of (B) iNOS, (C) IL-10, (D) JAK1, (E) STAT3 and (F) SOCS3 were
presented. Data are presented as the mean ± standard deviation.
#P<0.05 vs. the control group, *P<0.05 vs. the LPS
group and **P<0.05 vs. the LPS+50 µg CM-chitosan group. CM,
carboxymethyl; iNOS, inducible nitric oxide synthase; IL,
interleukin; JAK, janus kinase; STAT, signal transducer and
activator of transcription; SOCS suppressor of cytokine signaling;
LPS, lipopolysaccharide. |
Treatment with CM-chitosan increases
IL-10 production in a dose dependent manner
IL-10 is well known as an anti-inflammatory
cytokine. A previous study has revealed that iNOS was induced in
IL-10-deficient mice (26). The
effects of CM-chitosan on IL-10 production were analyzed in the
present study. As demonstrated in Fig.
1B, IL-10 mRNA was downregulated by LPS exposure. Treatment
with increasing concentrations of CM-chitosan was revealed to
recover IL-10 production to near-control levels in a dose-dependent
manner. There was no significant difference between the IL-10 mRNA
level of the control group and the group treated with 200 µg/ml
CM-chitosan. The level of IL-10 protein demonstrated the same trend
and also increased in a dose dependent manner when treated with
CM-chitosan (Fig. 2A and C). These
results indicated that iNOS inhibition was associated with IL-10
induction.
CM-chitosan activates the
JAK/STAT/SOCS signaling pathway
The present study also investigated whether the
JAK/STAT/SOCS signaling pathway was activated by CM-chitosan. The
mRNA levels of JAK1, STAT3 and SOCS3 were all suppressed in
chondrocytes exposed to LPS (Fig.
1D-F), and promoted by CM-chitosan treatment in a dose
dependent manner. Similarly, western blot analysis demonstrated
that the protein expression of JAK1, STAT3 and SOCS3 were also
upregulated in CM-chitosan groups (Fig.
2). When treated with 200 µg/ml of CM-chitosan all three
proteins were expressed at a markedly higher level than the
control. These results indicated that the JAK/STAT/SOCS signaling
pathway was activated by CM-chitosan and this activation was
associated with NO inhibition and IL-10 induction.
Discussion
Loss of cartilage is one of the primary symptoms
identified in patients with OA (27). Articular cartilage cannot be repaired
if it is damaged as chondrocytes, which are the only cell type in
cartilage, have a very low metabolic activity. This feature of
chondrocytes makes the treatment of OA challenging (3). To the best of our knowledge there are
currently no approved disease-modifying drugs against OA.
Inflammation plays a central role in OA and Zheng et al
(28) have recently reviewed the use
of anti-cytokine approaches to improve the symptoms of OA as well
as stabilizing the tissue structure. Inhibition of NO production is
another potential strategy to treat OA as NO production is closely
associated with inflammatory reactions (10). Chemical substances including
echinocystic acid, selenomethionine and piperine have been
identified in previous studies as inhibitors of iNOS production and
therefore attenuators of inflammation (29–31). In
the present study, it was revealed that CM-chitosan inhibits iNOS
production and upregulates the anti-inflammatory cytokine IL-10
mRNA and protein production in LPS-induced chondrocytes. To the
best of our knowledge this is a novel discovery and adds to the
protective role that CM-chitosan appears to serve against OA.
Similar results were reported in a previous study by Chen et
al (20), in which CM-chitosan
was revealed to suppress the levels of NO in IL-1β induced
chondrocytes.
The anti-inflammatory properties of chitosan and its
derivatives have been described in a number of diseases (32). Although OA is conceptualized as a
non-inflammatory disease, inflammatory cytokines serve a vital role
in the breakdown of proteoglycan (33). Tumor necrosis factor (TNF)-α, IL-1β
and IL-6 are the main proinflammatory cytokines studied in
association with OA. These cytokines have complex regulatory
functions within OA (6,24,34).
IL-1β and TNF-α may induce MMP-mediated digestion of proteoglycan
in OA (33,35). IL-1β may induce the production of NO
and promote apoptosis of chondrocytes (10), whereas IL-6 may reduce the expression
of type II collagen and cartilage matrix proteins (33,36). In
the present study, the anti-inflammatory cytokine IL-10 was
studied. In previous studies the downregulation of IL-10 secretion
has been observed in patients with OA and a rat model of OA
(37,38). This downregulation of IL-10 was
associated with a reduction in T cell immunoglobulin mucin receptor
3 expression (39). In human
articular chondrocytes IL-10 may upregulate the expression of type
II collagen and promote cartilage-specific extracellular matrix
synthesis in response to TNF-α (40). In the present study, the results
suggested that IL-10 activation was correlated with inhibition of
iNOS production and was associated with the protective role of
CM-chitosan against OA.
IL-10 interacts with its high-affinity receptor
IL-10R1 and low-affinity receptor IL-10R2 (41) and leads to the activation of the
JAK/STAT/SOCS signaling pathway (42). In the present study it was revealed
that the protective effect of CM-chitosan was in part through the
activation of the JAK/STAT/SOCS signaling pathway. Previous studies
have also demonstrated that the JAK/STAT/SOCS signaling pathway was
associated with iNOS production (22,23).
In conclusion, the results of the present study
indicated that CM-chitosan inhibited iNOS production by
upregulating IL-10 and activating the JAK/STAT/SOCS signaling
pathway. However, there were some limitations to the methodology
used in the present study; the experiments were performed in a cell
model, an animal model should be considered for any future
investigations to give a better representation of OA in humans. The
expression of selected proteins was tested, but the effect of
CM-chitosan on cell proliferation and extracellular matrix
synthesis was not measured. Further studies into the effect of
CM-chitosan on cartilage formation are required to develop these
findings.
References
|
1
|
Kidd BL: Osteoarthritis and joint pain.
Pain. 123:6–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rogers MW and Wilder FV: The association
of BMI and knee pain among persons with radiographic knee
osteoarthritis: A cross-sectional study. BMC Musculoskelet Disord.
9:1632008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y and Jordan JM: Epidemiology of
osteoarthritis. Clin Geriatr Med. 26:355–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le LT, Swingler TE and Clark IM: Review:
The role of microRNAs in osteoarthritis and chondrogenesis.
Arthritis Rheum. 65:1963–1974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wheaton AJ, Borthakur A, Shapiro EM,
Regatte RR, Akella SV, Kneeland JB and Reddy R: Proteoglycan loss
in human knee cartilage: Quantitation with sodium MR
imaging-seasibility study. Radiology. 231:900–905. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clements KM, Price JS, Chambers MG, Visco
DM, Poole AR and Mason RM: Gene deletion of either
interleukin-1beta, interleukin-1beta-converting enzyme, inducible
nitric oxide synthase, or stromelysin 1 accelerates the development
of knee osteoarthritis in mice after surgical transection of the
medial collateral ligament and partial medial meniscectomy.
Arthritis Rheum. 48:3452–3463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murrell GA, Jang D and Williams RJ: Nitric
oxide activates metalloprotease enzymes in articular cartilage.
Biochem Biophys Res Commun. 206:15–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashimoto S, Takahashi K, Amiel D, Coutts
RD and Lotz M: Chondrocyte apoptosis and nitric oxide production
during experimentally induced osteoarthritis. Arthritis Rheum.
41:1266–1274. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farrell AJ, Blake DR, Palmer RM and
Moncada S: Increased concentrations of nitrite in synovial fluid
and serum samples suggest increased nitric oxide synthesis in
rheumatic diseases. Ann Rheum Dis. 51:1219–1222. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vuolteenaho K, Moilanen T, Jalonen U,
Lahti A, Nieminen R, van Beuningen HM, van der Kraan PM and
Moilanen E: TGFbeta inhibits IL-1 -induced iNOS expression and NO
production in immortalized chondrocytes. Inflamm Res. 54:420–427.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gómez R, Scotece M, Conde J, Lopez V, Pino
J, Lago F, Gómez-Reino JJ and Gualillo O: Nitric oxide boosts TLR-4
mediated lipocalin 2 expression in chondrocytes. J Orthop Res.
31:1046–1052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takada K, Hirose J, Yamabe S, Uehara Y and
Mizuta H: Endoplasmic reticulum stress mediates nitric
oxide-induced chondrocyte apoptosis. Biomed Rep. 1:315–319. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Girolamo L, Kon E, Filardo G, Marmotti
AG, Soler F, Peretti GM, Vannini F, Madry H and Chubinskaya S:
Regenerative approaches for the treatment of early OA. Knee Surg
Sports Traumatol Arthrosc. 24:1826–1835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lahiji A, Sohrabi A, Hungerford DS and
Frondoza CG: Chitosan supports the expression of extracellular
matrix proteins in human osteoblasts and chondrocytes. J Biomed
Mater Res. 51:586–595. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oprenyeszk F, Sanchez C, Dubuc JE, Maquet
V, Henrist C, Compère P and Henrotin Y: Chitosan enriched
three-dimensional matrix reduces inflammatory and catabolic
mediators production by human chondrocytes. PLoS One.
10:e01283622015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia W, Liu P, Zhang J and Chen J:
Biological activities of chitosan and chitooligosaccharides. Food
Hydrocolloid. 25:170–179. 2011. View Article : Google Scholar
|
|
17
|
Jung WJ and Park RD: Bioproduction of
chitooligosaccharides: Present and perspectives. Mar Drugs.
12:5328–5356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerch G: The potential of chitosan and its
derivatives in prevention and treatment of age-related diseases.
Mar Drugs. 13:2158–2182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Croisier F and Jérôme C: Chitosan-based
biomaterials for tissue engineering. Eur Polym J. 49:780–792. 2013.
View Article : Google Scholar
|
|
20
|
Chen Q, Liu SQ, Du YM, Peng H and Sun LP:
Carboxymethyl-chitosan protects rabbit chondrocytes from
interleukin-1beta-induced apoptosis. Eur J Pharmacol. 541:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu SQ, Qiu B, Chen LY, Peng H and Du YM:
The effects of carboxymethylated chitosan on metalloproteinase-1,
−3 and tissue inhibitor of metalloproteinase-1 gene expression in
cartilage of experimental osteoarthritis. Rheumatol Int. 26:52–57.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H, Liu Z, Zhou H, Dai W, Chen S, Shu Y
and Feng J: JAK-STAT pathway modulates the roles of iNOS and COX-2
in the cytoprotection of early phase of hydrogen peroxide
preconditioning against apoptosis induced by oxidative stress.
Neurosci Lett. 529:166–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dell'Albani P, Santangelo R, Torrisi L,
Nicoletti VG, De Vellis J and Giuffrida Stella AM: JAK/STAT
signaling pathway mediates cytokine-induced iNOS expression in
primary astroglial cell cultures. J Neurosci Res. 65:417–424. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Séguin CA and Bernier SM: TNFalpha
suppresses link protein and type II collagen expression in
chondrocytes: Role of MEK1/2 and NF-kappaB signaling pathways. J
Cell Physiol. 197:356–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goff WL, O'Rourke KI, Johnson WC, Lacy PA,
Davis WC and Wyatt CR: The role of IL-10 in iNOS and cytokine mRNA
expression during in vitro differentiation of bovine mononuclear
phagocytes. J Interf Cytok Res. 18:139–149. 1998. View Article : Google Scholar
|
|
27
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng S, Hunter DJ, Xu J and Ding C:
Monoclonal antibodies for the treatment of osteoarthritis. Expert
Opin Biol Ther. 16:1529–1540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Z, Wang Y, Piao T and Liu J:
Echinocystic acid inhibits IL-1β-induced COX-2 and iNOS expression
in human osteoarthritis chondrocytes. Inflammation. 39:543–549.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng AW, Stabler TV, Bolognesi MP and
Kraus VB: Selenomethionine inhibits IL-1β inducible nitric oxide
synthase (iNOS) and cyclooxygenase 2 (COX2) expression in primary
human chondrocytes. Osteoarthritis Cartilage. 19:118–125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ying X, Chen X, Cheng S, Shen Y, Peng L
and Xu HZ: Piperine inhibits IL-β induced expression of
inflammatory mediators in human osteoarthritis chondrocyte. Int
Immunopharmaco. 17:293–299. 2013. View Article : Google Scholar
|
|
32
|
Azuma K, Osaki T, Minami S and Okamoto Y:
Anticancer and anti-inflammatory properties of chitin and chitosan
oligosaccharides. J Funct Biomater. 6:33–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martel-Pelletier J: Pathophysiology of
osteoarthritis. Osteoarthritis Cartilage. 12 Suppl A:S31–S33. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Namba A, Aida Y, Suzuki N, Watanabe Y,
Kawato T, Motohashi M, Maeno M, Matsumura H and Matsumoto M:
Effects of IL-6 and soluble IL-6 receptor on the expression of
cartilage matrix proteins in human chondrocytes. Connect Tissue
Res. 48:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin PM, Chen CC and Torzilli PA: Increased
stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and
collagen damage in cyclically load-injured articular cartilage.
Osteoarthritis Cartilage. 12:485–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Porée B, Kypriotou M, Chadjichristos C,
Beauchef G, Renard E, Legendre F, Melin M, Gueret S, Hartmann DJ,
Malléin-Gerin F, et al: Interleukin-6 (IL-6) and/or soluble IL-6
receptor down-regulation of human type II collagen gene expression
in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and
of the binding activity of both factors to the COL2A1 promoter. J
Biol Chem. 283:4850–4865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Imamura M, Targino RA, Hsing WT, Imamura
S, Azevedo RS, Boas LS, Tozetto-Mendoza TR, Alfieri FM, Filippo TR
and Battistella LR: Concentration of cytokines in patients with
osteoarthritis of the knee and fibromyalgia. Clin Interv Aging.
9:939–944. 2014.PubMed/NCBI
|
|
38
|
Rojas-Ortega M, Cruz R, Vega-López MA,
Cabrera-González M, Hernández-Hernández JM, Lavalle-Montalvo C and
Kouri JB: Exercise modulates the expression of IL-1β and IL-10 in
the articular cartilage of normal and osteoarthritis-induced rats.
Pathol Res Pract. 211:435–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li S, Wan J, Anderson W, Sun H, Zhang H,
Peng X, Yu Z, Wang T, Yan X and Smith W: Downregulation of IL-10
secretion by Treg cells in osteoarthritis is associated with a
reduction in Tim-3 expression. Biomed Pharmacothr. 79:159–165.
2016. View Article : Google Scholar
|
|
40
|
Müller RD, John T, Kohl B, Oberholzer A,
Gust T, Hostmann A, Hellmuth M, Laface D, Hutchins B, Laube G, et
al: IL-10 overexpression differentially affects cartilage matrix
gene expression in response to TNF-alpha in human articular
chondrocytes in vitro. Cytokine. 44:377–385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Josephson K, Logsdon NJ and Walter MR:
Crystal structure of the IL-10/IL-10R1 complex reveals a shared
receptor binding site. Immunity. 15:35–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carey AJ, Tan CK and Ulett GC:
Infection-induced IL-10 and JAK-STAT: A review of the molecular
circuitry controlling immune hyperactivity in response to
pathogenic microbes. JAKSTAT. 1:159–167. 2012.PubMed/NCBI
|
















