Introduction
Hepatitis B (HBV) is a viral infectious disease that
is mainly transmitted through contact with blood and body fluids of
infected people. Currently, there are more than 350 million people
worldwide that are chronically infected with HBV, which is the
leading cause of cirrhosis and liver cancer. As many as 600,000
people die from hepatitis B each year, which is one of the most
serious health problems in the world (1). Although the prevalence of chronic
hepatitis B (CHB) has been controlled to below 0.5% in low endemic
areas, it is still more than 10% in some Asian and western pacific
countries (2). Mother-to-child
infection is the most common route of transmission areas of high
CHB prevalence. Young people are the main infected population in
low endemic areas. Although HBV infection has been reduced through
the HBV vaccination program, there are still a large number of
people that are infected with HBV or carry the HBV virus (3). Gene type, virus load and immune
response function are important factors in HBV infection and
chronicity. Therefore, the reduction of immune response function is
the main reason of continuous replication of HBV in the body and
the increasing viral load (4).
Peripheral blood mononuclear cells (PBMC) play an important role in
the process of antiviral immune response, and excessive apoptosis
of PBMC after viral infection leads to the reduction of the bodys
antiviral immune response function and an increase of virus load
(5,6). However, the apoptotic mechanism of PBMC
remains unclear. The present study aims to investigate the
expression of apoptosis-related factors in PBMC of patients with
CHB and their relationships with clinical prognosis in order to
demonstrate its significance in the chronicity of HBV
infection.
Materials and methods
Sample selection
Sixty-two patients with CHB that were admitted to
Xuzhou Hospital between March 2015 and February 2016 were enrolled
as the observation group. The inclusion criteria were as follows:
i) patients who conform to the diagnostic criteria of chronic
hepatitis B; ii) patients who had not been treated with antiviral
therapy before admission; and iii) patients who signed the informed
consent. The exclusion criteria were as follows: i) patients with
hepatitis A, C, D or E; ii) patients with malignant tumor; and iii)
women during gestation or lactation. In addition, 60 healthy
subjects who were examined in the health examination center at our
hospital and had no genetic relationship with patients in the
observation group were selected as the control group. The
differences in characteristics between subjects in the two groups
were comparable and not statistically significant (P<0.05), as
shown in Table I. The study was
approved by the Ethics Committee of Xuzhou Infectious Disease
Hospital and informed consents were signed by the patients and/or
guardians.
 | Table I.General data of research subjects. |
Table I.
General data of research subjects.
| Items | Observation group
(n=62) | Control group
(n=60) | t/χ2 | P-value |
|---|
| Sex
(male/female) | 39/23 | 37/23 | 0.020 | 0.888 |
| Age (years) | 20–59 | 20–60 |
|
|
| Average age
(years) | 48.85±7.89 | 48.37±7.48 | 0.345 | 0.731 |
| Educational
level |
|
|
|
|
| Junior
high school and below | 15 (24.19) | 13 (21.67) | 0.505 | 0.777 |
| High
school and technical secondary school | 29 (46.77) | 26 (43.33) |
|
|
| Junior
college or above | 18 (29.03) | 21 (35.00) |
|
|
Sample collection
A total of 3–5 ml of fasting peripheral venous blood
was collected from the research subjects and placed into an
ethylenediamine tetracetic acid (EDTA) anticoagulant tube. One of
the tubes was separated and stored at −20°C, and another was added
to 4 ml of lymphocyte separation solution and centrifuged at 2,961
× g for 20 min. The PBMC was taken and stored at −80°C.
Detection of mRNA expression levels of
apoptotic molecules in PBMC
The extraction of total RNA is as follows. After
PBMC was thawed, total RNA was extracted according to the strict
operation of the TRIzol reagent instructions. The concentration and
purity of RNA were detected, and the concentration was ensured to
be within 1.8–2.2. For the primer design, the experimental primers
were designed and synthesized by the Shenzhen BGI (Shenzhen, China)
and the related primer sequences are shown in Table II. The total tissue RNA was
amplified by the DNA target fragment through the access RT-PCR
system. The amplification conditions were denaturation at 42°C for
5 min, 95°C for 10 sec, 95°C for 5 sec and 60°C for 20 sec, a total
of 40 cycles, 4°C for 2 h to the end. The electrophoresis was
conducted for PCR production, and the electrophoresis result was
analyzed by GeneMapper 3.0 software.
 | Table II.FAS, CASP3, CASP8 and CASP9 primer
sequences. |
Table II.
FAS, CASP3, CASP8 and CASP9 primer
sequences.
| Microsatellite
site | Sequence |
|---|
| FAS | F:
5-TCTGGTTCTTACGTCTGTTGC-3 |
|
| R:
5-CTGTGCAGTCCCTAGCTTTCC-3 |
| CASP3 | F:
5-CAGTGGAGGCCGACTTCTTG-3 |
|
| R:
5-TGGCACAAAGCGACTGGAT-3 |
| CASP8 | F:
5-GCAAACTGGATGATGACATGAA-3 |
|
| R:
5-TCTTTTCAGGATGTCCAACTTTC-3 |
| CASP9 | F:
5-GGACATCCAGCGGGCAGG-3 |
|
| R:
5-TCTAAGCAGGAGATGAACAAAGG-3 |
Through ethidium bromide (ETBR) staining and 2%
agarose gel electrophoresis, the PCR product was observed by a
UV-2000 Ultraviolet Analyzer (Shanghai Scientific Instrument
Factory, Shanghai, China). The quantitative analysis was performed
by an HMIAS-2000 high-definition color medical graphic analysis
system, and the gray ratios of FAS, CASP3, CASP8 and CASP9 mRNA to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were adopted to
represent the relative expression levels of FAS, CASP3, CASP8 and
CASP9 mRNA.
Detection of protein expression levels
of apoptotic molecules in PBMC
The protein expression levels of FAS, CASP3, CASP8
and CASP9 in the plasma of samples were respectively detected by
ELISA, and the related kits were provided by the Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). The experimental steps
were as follows. First, for dilution and application of the sample,
the sample was thawed, and added to the washing buffer for
dilution, which was followed by placing the micropore into the
reaction plate. Next, horseradish peroxidase was added to the
sample and incubated at 37°C for 60 min. After the sample was
incubated, the plate was washed four times, and placed static for
15 sec during the interval. The chromogenic agent A and B solution
(50 µl for each) was added, respectively, and mixed uniformly.
Then, it was incubated at 20°C in the dark for 15 min, which was
followed by adding 50 µl of stop buffer. The OD value at the
wavelength 450 nm was read by a microplate reader (Jiangsu Potebio
Co., Ltd., Jiangsu, China) within 15 min, and concentration of FAS,
CASP3, CASP8 and CASP9 was calculated.
The 62 patients with CHB were administered with
interferon antiviral therapy according to their specific
conditions, depending on whether there was a response and tolerance
to drugs. The patients were grouped according to the positive
conditions of HBV DNA after treatment, and then 3–5 ml of
peripheral venous blood was collected again for the detection of
protein expression levels of FAS, CASP3, CASP8 and CASP9.
Detection of HBV DNA
The HBV DNA was detected by using real-time
immunofluorescence quantitative PCR method. The related kits were
provided by the Sansure Biotech Co., Ltd. Changsha, China), and
determined by the professional staff of the test center in Xuzhou
Hospital. The positive HBV DNA was judged as ≥5.0×102
copies/ml.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) software
was used to process data. The logarithmic conversion was adopted
for HBV DNA copy number. The measurement data were expressed as
mean ± standard deviation (SD), and enumeration data were expressed
by percentage. The chi-square test was utilized and the correlation
was assessed by the Pearson correlation coefficient analysis.
P<0.05 indicates that the difference is statistically
significant.
Results
mRNA expression levels of
apoptosis-related factors in PBMC of the two groups
The mRNA expression levels of FAS, CASP3, CASP8 and
CASP9 in the observation group were significantly higher than those
in the control group (P<0.05) (Table III).
 | Table III.Comparisons of mRNA expression levels
of apoptotic molecules in PBMC between the two groups. |
Table III.
Comparisons of mRNA expression levels
of apoptotic molecules in PBMC between the two groups.
| Group | Cases | FAS mRNA | CASP3 mRNA | CASP8 mRNA | CASP9 mRNA |
|---|
| Observation
group | 62 | 2.59±0.27 | 1.95±0.26 | 2.12±0.25 | 2.23±0.27 |
| Control group | 60 | 1.04±0.18 | 0.98±0.17 | 1.03±0.14 | 1.05±0.14 |
| t-value |
| 37.182 | 24.303 | 29.579 | 30.154 |
| P-value |
| <0.05 | <0.05 | <0.05 | <0.05 |
Protein expression levels of
apoptosis-related factors in PBMC of the two groups
The protein expression levels of FAS, CASP3, CASP8
and CASP9 in the observation group were significantly higher than
those in the control group (P<0.05) (Table IV).
 | Table IV.Comparisons of protein expression
levels of apoptotic molecules in PBMC between the two groups
(ng/ml). |
Table IV.
Comparisons of protein expression
levels of apoptotic molecules in PBMC between the two groups
(ng/ml).
| Group | Cases | FAS | CASP3 | CASP8 | CASP9 |
|---|
| Observation
group | 62 | 4.59±0.73 | 7.54±0.82 | 2.83±0.51 | 3.32±0.56 |
| Control group | 60 | 2.14±0.32 | 3.78±0.74 | 1.28±0.42 | 1.86±0.43 |
| t-value |
| 23.870 | 26.561 | 18.292 | 16.114 |
| P-value |
| <0.05 | <0.05 | <0.05 | <0.05 |
Comparisons of positive rate of serum
HBV DNA and log value of copy amount of HBV DNA between the two
groups
The positive rate of HBV DNA and the log value of
the copy amount of HBV DNA in the observation group were
significantly higher than those in the control group (P<0.05)
(Table V).
 | Table V.Comparisons of positive rate of serum
HBV DNA and log value of copy amount of HBV DNA between the two
groups. |
Table V.
Comparisons of positive rate of serum
HBV DNA and log value of copy amount of HBV DNA between the two
groups.
| Group | Cases | Positive rate of HBV
DNA | Log value of copy
amount of HBV DNA |
|---|
| Observation
group | 62 | 56 (90.32) | 5.83±0.56 |
| Control group | 60 | 1 (1.67) | 1.16±0.43 |
| t-value |
| 92.373 | 43.816 |
| P-value |
| <0.05 | <0.05 |
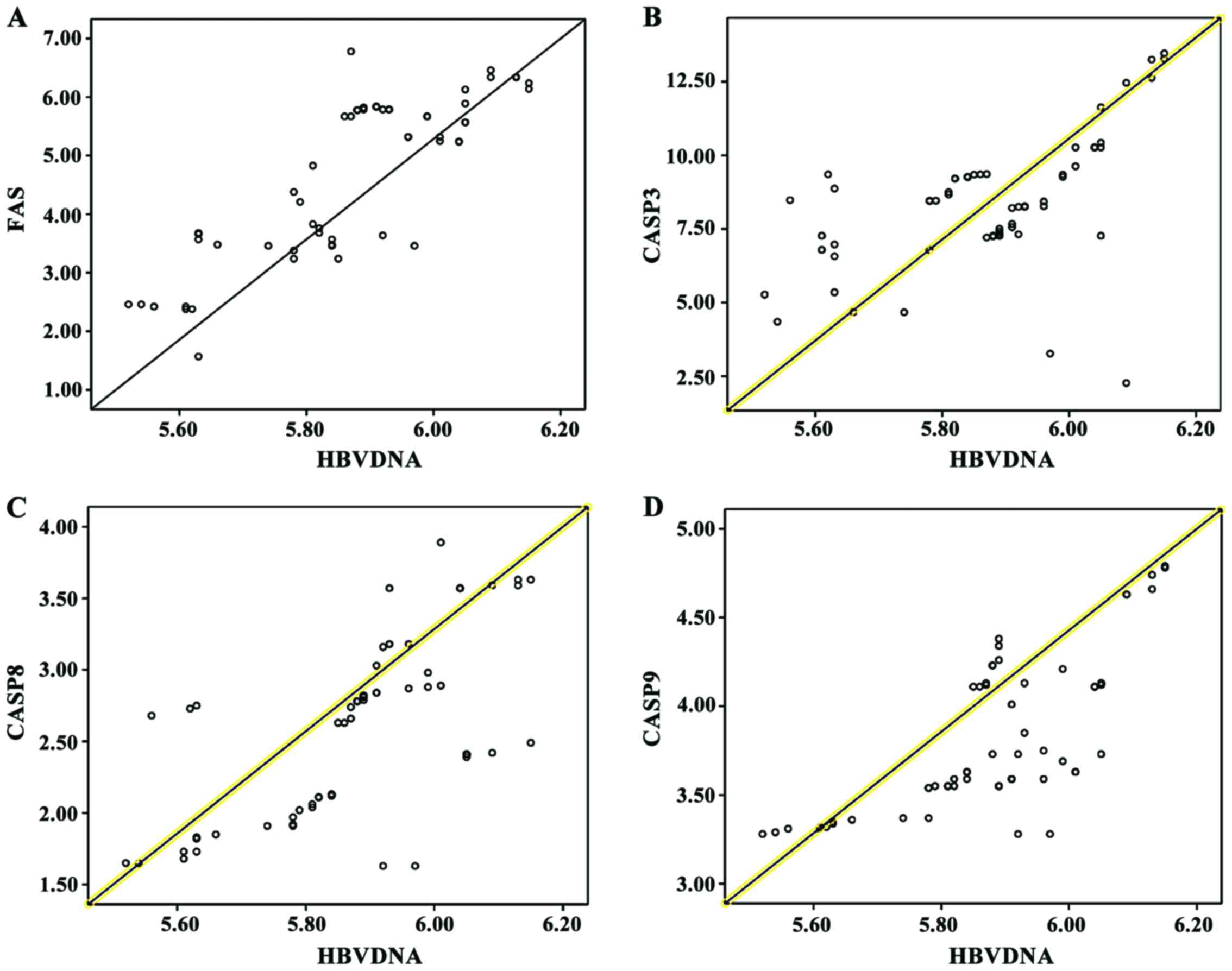
Correlation analysis of FAS, CASP3,
CASP8 and CASP9 protein expression levels and HBV DNA
quantification
The Pearson correlation coefficient analysis shows
that FAS, CASP3, CASP8 and CASP9 were positively correlated with
HBV DNA (P<0.05) (Table VI and
Fig. 1).
 | Table VI.Correlation analysis of apoptotic
molecules in PBMC and HBV DNA quantitative log value. |
Table VI.
Correlation analysis of apoptotic
molecules in PBMC and HBV DNA quantitative log value.
| Item | r | P-value |
|---|
| FAS | 0.508 | 0.003 |
| CASP3 | 0.531 | 0.017 |
| CASP8 | 0.506 | 0.004 |
| CASP9 | 0.475 | 0.014 |
Protein expression levels of
apoptosis-related factors in PBMC of patients with positive and
negative HBV-DNA
The mRNA expression levels of FAS, CASP3, CASP8 and
CASP9 in patients with positive HBV-DNA were significantly higher
than those with negative HBV-DNA (P<0.05) (Table VII).
 | Table VII.Comparisons of protein expression
levels of apoptosis-related factors in PBMC between the two groups
(ng/ml). |
Table VII.
Comparisons of protein expression
levels of apoptosis-related factors in PBMC between the two groups
(ng/ml).
| Group | Cases | FAS | CASP3 | CASP8 | CASP9 |
|---|
| Positive HBV-DNA | 32 | 4.53±0.64 | 7.38±0.74 | 2.86±0.53 | 3.35±0.53 |
| Negative HBV-DNA | 30 | 2.34±0.35 | 3.98±0.63 | 1.37±0.42 | 1.98±0.47 |
| t-value |
| 23.340 | 27.284 | 17.174 | 15.088 |
| P-value |
| <0.05 | <0.05 | <0.05 | <0.05 |
Discussion
As one of the most important organs that regulates
body metabolism, liver is responsible for a variety of biological
transformation and storage, such as storage and metabolism of
protein and fat, bile secretion, synthesis of coagulation factors
and detoxification function (7). The
various types of hepatitis generally occur after pathogen
infection, and among them, CHB accounts for the majority of cases
of hepatitis, which has the highest incidence rate of infectious
diseases in China (8). CHB is
usually caused by hepatitis B virus infection, and approximately
5–10% of cases transform to CHB, and 10–20% of CHB will progress to
cirrhosis, thereby increasing the risk of primary liver cancer,
eventually leading to the death of patients (9,10). As
one of the most basic life phenomena, cell apoptosis is an active
process with steps that are regulated by the combined action of
various apoptosis-related factors. Normal cell apoptosis is an
important mechanism which regulates the regeneration of tissue and
cells as well as the growth and development of organisms in order
to maintain the stability of the internal environment. Viral
infection, heart failure, neurodegenerative diseases, myocardial
ischemia and other diseases can be caused by excessive apoptosis,
and insufficient apoptosis will lead to tumors, atherosclerosis,
autoimmune diseases and so forth. Therefore, cell apoptosis has
become a hot topic in clinical research (11). Severe liver dysfunction and a large
number of apoptosis in hepatocytes are the pathological features of
CHB, which are manifested by the abnormal biochemical indicators in
liver function.
In the process of HBV infection, lymphatic monocytes
will migrate and infiltrate to the infected liver tissues, which is
infected by the virus, thus leading to proliferation and
activation. Apoptosis will be induced and removed by the apoptotic
mechanism after being activated (12,13).
PBMC-mediated antiviral immune response is an important mechanism
for HBV clearance from the body, however, the continuous
replication of virus and increasing viral load in CHB patients are
mainly caused by the reduction of antiviral immune response
function (14).
Caspase family members can play catalytic roles,
which only show relatively low activity levels in the normal
condition without any damage to normal cells and tissue, and
protein substrates can be decomposed when they are largely
activated, thus inducing cell apoptosis (15). FAS is a member of the tumor necrosis
factor receptor superfamily, which is widely expressed in PBMCs,
and initiates a caspase cascade activation process after binding to
FAS ligand (FASL) (16). Apoptosis
is usually carried out by the exogenous and endogenous pathway. The
exogenous pathway is triggered by the binding of FAS and FASL,
which leads to the activation of CASP8, and subsequently, CASP9 and
CASP3 are activated. The endogenous pathway is induced by the viral
protein response, DNA damage and oxidative stress, which results in
the activation of CASP9 (17,18).
CASP3 is a member of the CASP family, which plays an important role
in regulating activated T-cell apoptosis and maintaining B-cell
homeostasis (19). The results of
this study indicate that the mRNA and protein expression levels of
FAS, CASP3, CASP8 and CASP9 in the observation group were
significantly higher than those in the control group (P<0.05),
which suggests that PBMC apoptosis can be induced by HBV infection,
and FAS, CASP8 and CASP9 are activated by an exogenous and
endogenous pathway and is highly expressed, resulting in the
activation of the common effector, CASP3, which will combine these
two pathways in order to execute the process of cell apoptosis.
The replication of HBV in the body can be reflected
by the HBV-DNA content level in blood, and evidence-based medicine
has shown that the hepatitis activity can be reduced by the
inhibition of HBV replication, which ameliorates the prognosis of
patients (20). The results of this
study demonstrated that the positive rate of HBV DNA and the log
value of copy amount of HBV DNA in the observation group were
significantly higher than those in the control group, and FAS,
CASP3, CASP8 and CASP9 were positively correlated with HBV DNA
(P<0.05). The mechanism at play is that the bodys antiviral
immune response can be inhibited and reduced by apoptosis-related
factors in PBMCs through the exogenous and endogenous pathway,
leading to a continuous infection and replication of HBV, which
will increase the copy amount of HBV DNA and activate inflammatory
reaction, thereby aggravating liver tissue injury through various
inflammatory factors, such as TNF-α, IL-6 and IL-8 h, which will
participate in the development process of CHB. The excessive
activation of the PBMC apoptosis is closely associated with the
content of HBV-DNA. The reduction of lymphocyte activity and number
that is caused by excessive PBMC apoptosis will lead to an
imbalance of immune function, thereby weakening anti-HBV immune
response. Therefore, CHB will be aggravated due to an ineffective
clearance of HBV. This study also reveals that the mRNA expression
levels of FAS, CASP3, CASP8 and CASP9 in patients with negative
HBV-DNA were significantly lower than those with positive HBV-DNA
(P<0.05), suggesting that PBMC apoptosis-related factors are
inhibited via treatment and other interventions. Therefore, the
illness of patients with CHB can be controlled and HBV-DNA is
transformed to negative, which is conducive to prognosis.
In conclusion, apoptosis-related factors in PBMCs
play important roles in the occurrence and development of CHB,
which affect the bodys immune response and prognosis of patients.
Therefore, intervention for their expressions can be considered to
achieve the goal of complete clearance of HBV.
References
|
1
|
Zeisel MB, Lucifora J, Mason WS, Sureau C,
Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, et
al: Towards an HBV cure: State-of-the-art and unresolved questions
- report of the ANRS workshop on HBV cure. Gut. 64:1314–1326. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papastergiou V, Lombardi R, Macdonald D
and Tsochatzis EA: Global epidemiology of hepatitis B virus (HBV)
infection. Curr Hepat Rep. 14:171–178. 2015. View Article : Google Scholar
|
|
3
|
Roberts H, Kruszon-Moran D, Ly KN, Hughes
E, Iqbal K, Jiles RB and Holmberg SD: Prevalence of chronic
hepatitis B virus (HBV) infection in U.S. households: National
Health and Nutrition Examination Survey (NHANES), 1988–2012.
Hepatology. 63:388–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo G, Feng X, Huang Y, Yi T, Wang D, Guo
X, Yan H and Zhang G: Effects of antiviral therapy on the cellular
immune response in patients with chronic hepatitis B. Mol Med Rep.
11:1284–1291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamb C and Arbuthnot P: Activating the
innate immune response to counter chronic hepatitis B virus
infection. Expert Opin Biol Ther. 16:1517–1527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Filippelli M, Garozzo MT, Capizzi A, Spina
M, Manti S, Tardino L, Salpietro C and Leonardi S: Immune response
to hepatitis B virus vaccine in celiac subjects at diagnosis. World
J Hepatol. 8:1105–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Himaja N and Shama SN: Herbal wealth for
hepatotoxicity: A review. Asian J Pharm Clin Res. 8:3–9. 2015.
|
|
8
|
Gonzalez SA and Keeffe EB: Chronic
hepatitis B and C: Update on therapy. Future Virol. 4:437–452.
2015. View Article : Google Scholar
|
|
9
|
World Health Organization (WHO), . WHO
Guidelines Approved by the Guidelines Review Committee: WHO
Guidelines for the Prevention, Care and Treatment of Persons with
Chronic Hepatitis B Infection. World Health Organization; Geneva:
2015
|
|
10
|
The Korean Association for the Study of
the Liver (KASL): KASL Clinical Practice Guidelines: Management of
chronic hepatitis B. Clin Mol Hepatol. 18:109–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv J, Lin S, Peng P, Cai C, Deng J, Wang
M, Li X, Lin R, Lin Y, Fang A, et al: Arenobufagin activates p53 to
trigger esophageal squamous cell carcinoma cell apoptosis in vitro
and in vivo. Onco Targets Ther. 10:1261–1267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong CA and Nam YS: Functional
nanostructures for effective delivery of small interfering RNA
therapeutics. Theranostics. 4:1211–1232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao BB, Zheng SJ, Gong LL, Wang Y, Chen
CF, Jin WJ, Zhang D, Yuan XH, Guo J, Duan ZP, et al: T lymphocytes
from chronic HCV-infected patients are primed for
activation-induced apoptosis and express unique pro-apoptotic gene
signature. PLoS One. 8:e770082013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Somal A, Aggarwal A and Upadhyay RC:
Effect of thermal stress on expression profile of apoptosis related
genes in peripheral blood mononuclear cells of transition Sahiwal
cow. Iran J Vet Res. 16:137–143. 2015.PubMed/NCBI
|
|
15
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 7:a0086562015. View Article : Google Scholar
|
|
16
|
Karev VE: Fas, FasL, and bcl-2 expression
on hepatic intralobar lymphocytes in different variants of the
natural course of chronic HBV and HCV infection and in its
outcomes. Arkh Patol. 76:16–21. 2014.(In Russian). PubMed/NCBI
|
|
17
|
Martins D'Oliveira F, Gomes BC, Rodrigues
AS and Rueff J: Genetic susceptibility in acute pancreatitis:
Genotyping of GSTM1, GSTT1, GSTP1, CASP7, CASP8, CASP9, CASP10,
LTA, TNFRSF1B, and TP53 gene variants. Pancreas. 46:71–76. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng P, Yoshida H, Tanji K, Matsumiya T,
Xing F, Hayakari R, Wang L, Tsuruga K, Tanaka H, Mimura J, et al:
Carnosic acid attenuates apoptosis induced by amyloid-β 1–42 or
1–43 in SH-SY5Y human neuroblastoma cells. Neurosci Res. 94:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo X, Dong Z, Yamada S, Li Y, Guo Y, Shen
S, Liang J, Tanimoto A and Guo W: Association of Casp3 microRNA
target site (1049216) SNP with the risk and progress of cervical
squamous cell carcinoma. Int J Gynecol Cancer. 27:206–213. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng CY, Hsieh TC, Hsieh TY, Tseng KC, Lin
CL, Su TH, Tseng TC, Lin HH, Wang CC and Kao JH: HBV-DNA level at 6
months of entecavir treatment predicts HBeAg loss in HBeAg-positive
chronic hepatitis B patients. J Formos Med Assoc. 114:308–313.
2015. View Article : Google Scholar : PubMed/NCBI
|