Introduction
Herba Gelsemii elegantis (GE) is a toxic
plant that is well-known throughout the world. However, it has been
used as a Chinese folk medicine for the treatment of malignant
tumors, pain, rheumatic arthritis, psoriasis and immune function
(1–3). Despite the benefits of GE preparations
for human health, it is not broadly used due to its high toxicity.
Although the toxic effects of GE have been evaluated using animal
models (3), there has been no
scientific report on how to decrease its toxicity, to the best of
our knowledge.
In Chinese folk medicine, GE is commonly taken with
Ramulus et Folium Mussaendae pubescentis (MP) to decrease
its toxicity (4). MP belongs to the
Rubiaceae family and has been used as an antipyretic and
antidotal herb for thousands of years. Taking the preliminary
results on the toxicity and efficacy of the two Chinese herbs, GE
and MP, into account, a novel formula comprising the
dichloromethane extract of GE and the aqueous extract of MP at the
ratio of 1:40 (GM) was derived. However, the mechanisms of this
detoxification have remained elusive.
It is generally known that organisms have the
ability to detoxify exogenous toxicants. Proposed mechanisms to
explain this detoxification include inhibition of the bioactivation
of xenobiotics by phase-I metabolic enzymes, induction of phase-II
detoxification enzymes and scavenging of ultimate electrophilic
species (5). The phase-I
detoxification system, composed primarily of cytochrome P450
(CYP450) enzymes, is frequently the first line of enzymatic defense
against exogenous toxicants. CYP450 enzymes are important for the
function of the toxicant metabolism in the body. Chief among them
are CYP2E1 and CYP1A2. CYP2E1 is a significant enzyme that is
responsible for not only drug metabolism but also the catalysis of
numerous poisons and carcinogens (6,7). CYP1A2
is abundantly expressed in the human and rodent liver and may have
a vital role in activating numerous pro-poisons and pro-carcinogens
(8). Previous studies suggested that
GE dichloromethane extracts and active constituents were able to
selectively inhibit CYP2E1 and CYP1A2 activities in vitro
(9). Thereafter, the phase-II
detoxification enzymes are involved in the metabolism of a variety
of phase-I metabolic intermediates. Glutathione (GSH) S-transferase
(GST) is a crucial phase-II metabolic enzyme in the body. The major
GST enzymes associated with detoxification include GST mu 1 (GSTm1)
and GST-pi (10). They catalyze the
combination of GSH and toxic metabolic intermediates to detoxify
them, leading to the formation of water-soluble substances
(11).
In light of the importance of metabolic enzymes in
the detoxification-associated metabolism, the present study
postulated that detoxification mechanisms of MP may be caused in
inducing or inhibiting phase-I metabolic enzymes and phase-II
detoxification enzymes. However, few studies have assessed whether
MP detoxification mechanisms are associated with induction or
inhibition of metabolic enzymes (12). For this reason, the aim of the
present study was to assess the modulation effect of MP on the
toxicity of GM by inhibition of hepatic CYP450 and GST enzymes in
rats. For this purpose, reverse-transcription quantitative
polymerase chain reaction (RT-qPCR), western blot and
immunohistochemical (IHC) assays were separately performed to
examine the effects of MP on the mRNA and protein expression of
CYP450 enzymes in GE-induced rat livers. Furthermore, RT-qPCR,
western blot analysis and a colorimetry assay were separately
performed to detect the effects of MP on the mRNA, protein
expression and enzyme activity of GST enzymes in GE-induced rat
livers.
Materials and methods
Chemicals and reagents
Primary antibodies against CYP2E1 (cat. no.
ab28146), CYP1A2 (cat. no. ab22717), GSTm1 (cat. no. ab108084),
GST-Pi (cat. no. ab138491) and β-actin (cat. no. ab8226) were
obtained from Abcam (Cambridge, MA, USA). TRIzol reagent was
supplied by Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The
TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix was
purchased from TransGen Biotech (Beijing, China). Biotinylated
secondary antibodies (goat anti-rat; cat. no. Ab202-01; and goat
anti-rabbit; cat. no. Ab203-01), enhanced chemiluminescence (ECL)
plus (cat. no. E411-04) and bicinchoninic acid (BCA) protein
determination (cat. no. E112-02) were provided by Vazyme Biotech
Co., Ltd. (Nanjing, China). GST assay kits (cat. no. A004) were
provided by Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). All the other chemicals were at least of analytical grade
and were obtained from common commercial sources.
Preparation and chemical determination
of extracts
The extraction method of GE with dichloromethane was
identical to those described previously (9). The concentration of GE in the
dichloromethane fraction was 0.05 g/ml. The extraction of MP was
also identical to that described previously (13). After dispersion in 150 ml water, it
was extracted with 450 ml petroleum ether, 450 ml dichloromethane
and 450 ml ethyl acetate separately. Thereafter, the residual
aqueous part was collected. The concentration of MP in the aqueous
part was 2.0 g/ml. Two parts were filtered and evaporated under
reduced pressure to obtain GE dichloromethane and MP aqueous
extracts with typical yields of 0.67±0.071 and 5.78±0.20%,
respectively. The high-performance liquid chromatography
fingerprint of the chemical constituents of the dichloromethane
fraction of GE was determined as previously described (14). The dichloromethane extract mainly
contained indole alkaloids, including humantenmine, koumine and
humantenine (9,14). The major chemical constituents of the
aqueous extract of MP were triterpenoids. The triterpenoid content,
which was determined as previously described (15), was 13.26±1.46 mg/g.
Animals, treatments and tissue
preparation
A total of 50 healthy male Sprague Dawley rats (~200
g; 6–8 week old) were obtained from SLRC Laboratory Animal Co.,
Ltd. (Shanghai, China). The animals were had access to commercial
rat feed and distilled water ad libitum and were randomly
divided into five experimental groups containing 10 animals per
group. They were maintained in a controlled environment at a
temperature of 25±1°C under a 12 h light/dark cycle. After
acclimatizing for one week, they were treated with GE (0.36, 0.43
and 0.54 g/kg) alone and, at the highest dose, also in combination
with MP (21.6 g/kg) simultaneously by oral gavage once a day for
one week. The control group of animals received the same volume of
saline. All the procedures were according to international ethical
guidelines and the National Institutes of Health Guide for the Care
and Use of Laboratory Animals. The animal protocol was approved by
the Ethics Committee of Fujian University of Traditional Chinese
Medicine (Fuzhou, China).
A total of 2 rats succumbed in each of the high- and
medium-dose GE groups. Surviving rats were sacrificed 1 h after the
last lavage and the livers were quickly excised. The livers were
washed with cold saline and specimens of it were fixed with 4%
paraformaldehyde for IHC analysis. The remnants of the livers were
stored at −80°C for the other analyses.
Determination of CYP450 enzyme
expression
RT-qPCR analysis of CYP2E1 and CYP1A2 mRNA
levels
Total RNA isolation was performed using TRIzol and
the RNA concentration was determined using a NanoDrop 2000C
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA (1 µg) was
reverse-transcribed using SuperScript® III First-Strand
Synthesis System according to the supplier's instructions. The
obtained complementary DNA was used to determine the amount of
CYP2E1, CYP1A2 by mRNA PCR. β-actin was used as a housekeeping gene
for normalization. The sequences of the primers used for
amplification of CYP2E1, CYP1A2 and β-actin transcripts were as
follows: CYP2E1 forward, 5′-CATCAATCTTGTCCCTTCCAACCTA-3′ and
reverse, 5′-TTCTCTGGATCTGGAAACTCATGG-3′; CYP1A2 forward,
5′-CACGGCTTTCTGACAGACCC-3′ and reverse,
5′-GGTTGACCTGCCACTGGTTTAT-3′; β-actin forward,
5′-TGGGTATGGAATCCTGTGGCA-3′ and reverse,
5′-TGTTGGCATAGAGGTCTTTAGGG-3′. The thermal cycling conditions were
as follows: Initial denaturation at 95°C for 3 min, followed by 35
cycles of denaturation at 95°C for 30 sec, annealing at 53°C for 30
sec and extension at 72°C for 30 sec. PCR amplification products
were separated by 1.5% agarose gel electrophoresis and visualized
by ethidium bromide staining. The mRNA levels were expressed as the
ratio of the specified gene's signal in the selected amplification
cycle divided by the β-actin signal (16).
Western blot analysis of CYP2E1 and CYP1A2
protein expression
Liver tissue was homogenized in
radioimmunoprecipitation assay lysis buffer using a homogenizer and
centrifuged at 12,000 × g at 4°C for 10 min to obtain the
supernatants for further analysis. The protein concentration was
determined by the BCA method. Equal amounts of protein samples (40
µg/lane) were denatured in gel-loading buffer at 100°C for 5 min,
fractionated by 10% SDS-PAGE (standard gel) and transferred onto a
0.45 µm polyvinylidene fluoride membrane (EMD Millipore, Billerica,
MA, USA). The membranes were blocked for 2 h with 5% milk in PBS
containing 0.1% Tween-20 (PBST) and incubated with desired primary
antibody against CYP2E1, CYP1A2 and β-actin (at a dilution of
1:1,000, 1:2,500 and 1:7,000, respectively) overnight at 4°C. The
membranes were then incubated with desired secondary antibody (at a
dilution of 1:6,000, 1:7,000 and 1:5,000, respectively) for 1 h at
room temperature, followed by ECL detection. The protein expression
was expressed as the ratio to β-actin.
IHC analysis of CYP2E1 and CYP1A2 protein
expression
The fixed liver samples were embedded in paraffin
and cut into 5-µm-thick sections. The liver sections were then
deparaffinized and rehydrated by treatment with a series of xylenes
and graded alcohols. Thereafter, the sections were subjected to
antigen retrieval and the endogenous peroxidase activity was
quenched with hydrogen peroxide. After blocking non-specific
proteins with normal serum in PBST, the sections were incubated
with desired primary antibodies to CYP2E1 and CYP1A2 (at a dilution
of 1:200 and 1:400, respectively) overnight at 4°C. After washing
with PBS, the sections were incubated with 100 µl biotinylated
anti-rabbit immunoglobulin G antibody for 10 min at room
temperature and then treated with the avidin biotin complex (both
Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) for 10 min. They
were finally incubated with diaminobenzidine for 4 min. After
staining, twenty fields of view (magnification, ×100) were randomly
selected in each slide, and the density of stained cells in each
field was calculated using the true color multi-functional cell
image analysis management system (Image-Pro Plus, version 6.0;
Media Cybernetics, Rockville, MD, USA). A quantification assay is
represented as the surface density and number density of positively
stained cells. Surface density = area of positively stained
cells/total statistical area. Number density = number of positively
stained cells/total statistical area.
Determination of GST enzyme
activities
RT-qPCR analysis for GSTm1 and GST-pi mRNA
levels
RT-qPCR was performed as described above with slight
adjustments as follows: The primer sequences for GSTm1 and GST-pi
were as follows: GSTm1 forward, 5′-CGACGCTCCCGACTATGACA-3′ and
reverse, 5′-CACGAATCCGCTCCTCCTCT-3′; GST-pi forward,
5′-GCACCTGGGTCGCTCTTTA-3′ and reverse,
5′-GGGCCTTCACATAGTCATCCTT-3′. The following thermal cycling
conditions were applied: Initial denaturation at 95°C for 3 min,
followed by 35 cycles of denaturation at 95°C for 30 sec, annealing
at 58°C for 30 sec and extension at 72°C for 30 sec.
Western blot analysis of GSTm1 and GST-pi protein
expression
Western blot analysis was performed according to the
abovementioned procedure with the conditions lightly adjusted as
follows: The dilution of the primary antibodies to GSTm1, GST-pi
and β-actin was 1:1,000, 1:2,000 and 1:7,000, respectively. The
dilution of the secondary antibodies to the GSTm1, GST-pi and
β-actin goat anti-rabbit antibodies was 1:6,000, 1:6,000 and
1:5,000, respectively.
Colorimetric analysis of GST enzyme activity
Liver tissue was homogenized in saline using a
homogenizer and centrifuged at 15,000 × g for 10 min to obtain the
supernatants for further analysis. GST activity was measured by the
method of Habig et al (17)
via reduced GSH. The concentration of GSH indicated the activity of
GST. One unit of enzyme activity was defined as the amount that
reduced 1.0 µmol/l GSH per min at 37°C.
Statistical analysis
Values are expressed as the mean ± standard
deviation. A one-way analysis of variance followed by least
significant difference post-hoc tests was used for the comparison
of multiple groups using SPSS software (version 12.0; SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of extracts on CYP450 enzymes
expression in rat liver
RT-qPCR, western blot analysis and IHC assays were
separately performed to examine the effects of MP on the mRNA and
protein expression of hepatic CYPs in GE-induced rats. Two types of
CYP that were associated with detoxification were selected, namely
CYP2E1 and CYP1A2.
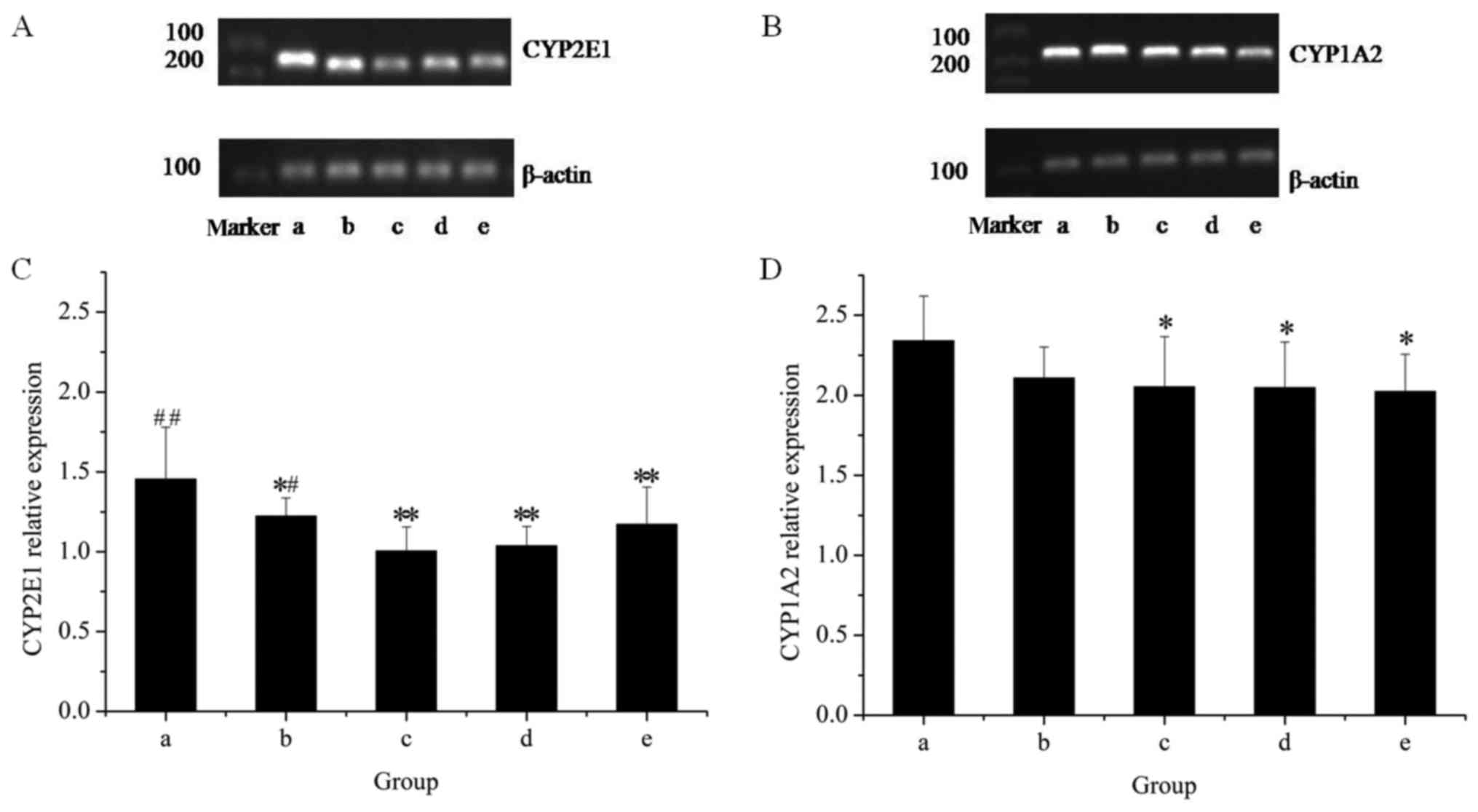
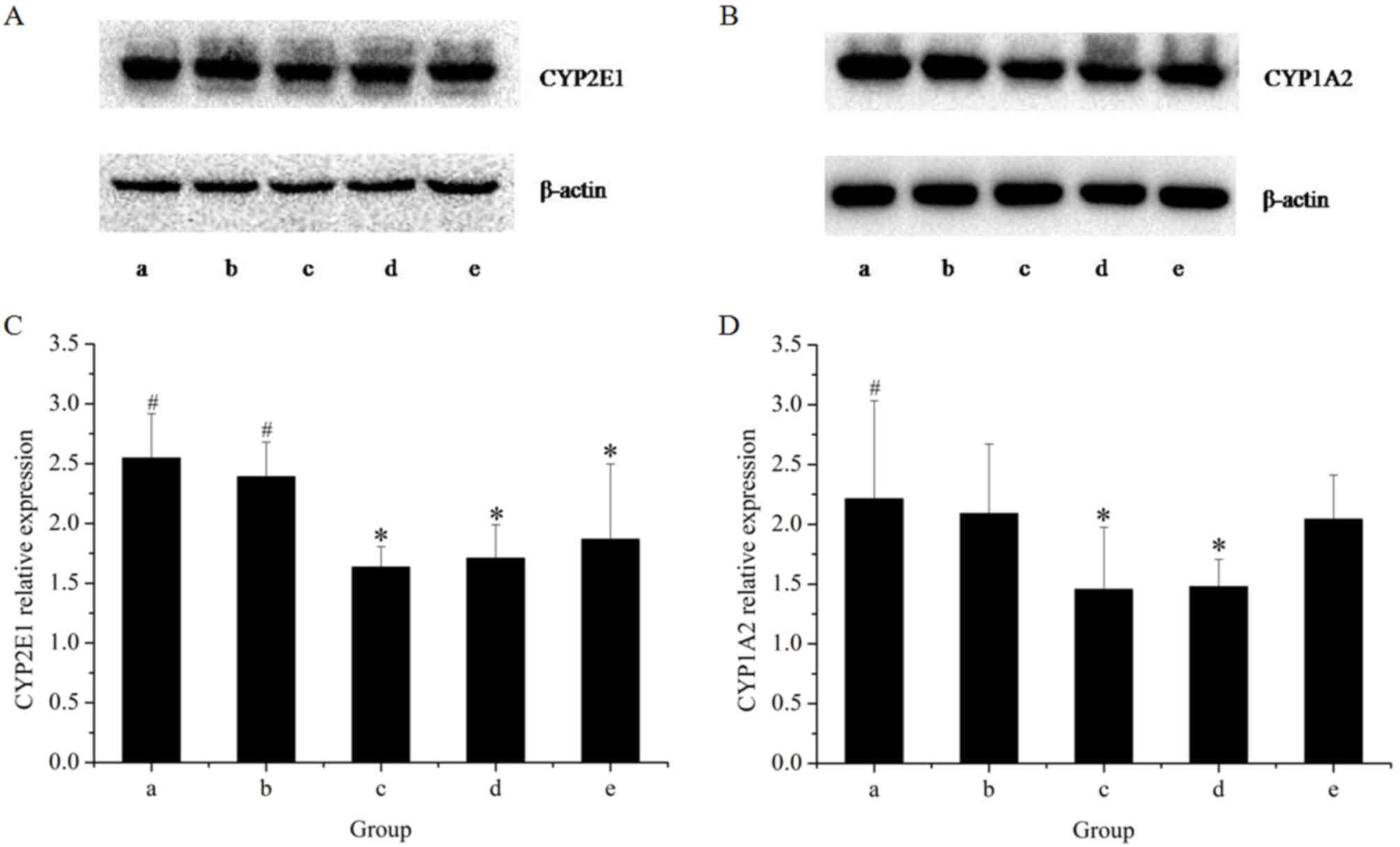
The effects of the extracts on the mRNA and protein
expression of CYP2E1 in rat livers are presented in Figs. 1 and 2, respectively. The results of the RT-qPCR
assay indicated that treatment with GE at all concentrations (0.36,
0.43 and 0.54 g/kg) significantly inhibited CYP2E1 mRNA expression
in a dose-dependent manner, as compared with the control. However,
co-administration of MP increased the mRNA expression of CYP2E1 as
compared with that in the high-dose GE group. Western blot analysis
revealed that the pattern of protein expression was similar to the
respective mRNA levels. These results suggested that CYP2E1
expression was decreased by GE, which was inhibited by
co-administration of MP.
To further reveal the modulation effects on hepatic
CYPs, the mRNA and protein expression of CYP1A2 was examined
(Figs. 1 and 2). The results of the RT-qPCR assay
revealed that GE treatments alone caused a significant decline in
CYP1A2 mRNA expression as compared with that in the control group.
Co-treatment with MP slightly increased the mRNA expression of
CYP1A2 as compared with that in the high-dose GE group, but the
difference was not statistically significant. Western blot analysis
indicated that treatments administered to the high- and medium-dose
GE groups significantly inhibited CYP1A2 protein expression as
compared with the control. The effect of co-treatment with MP was
similar to the results of mRNA levels.
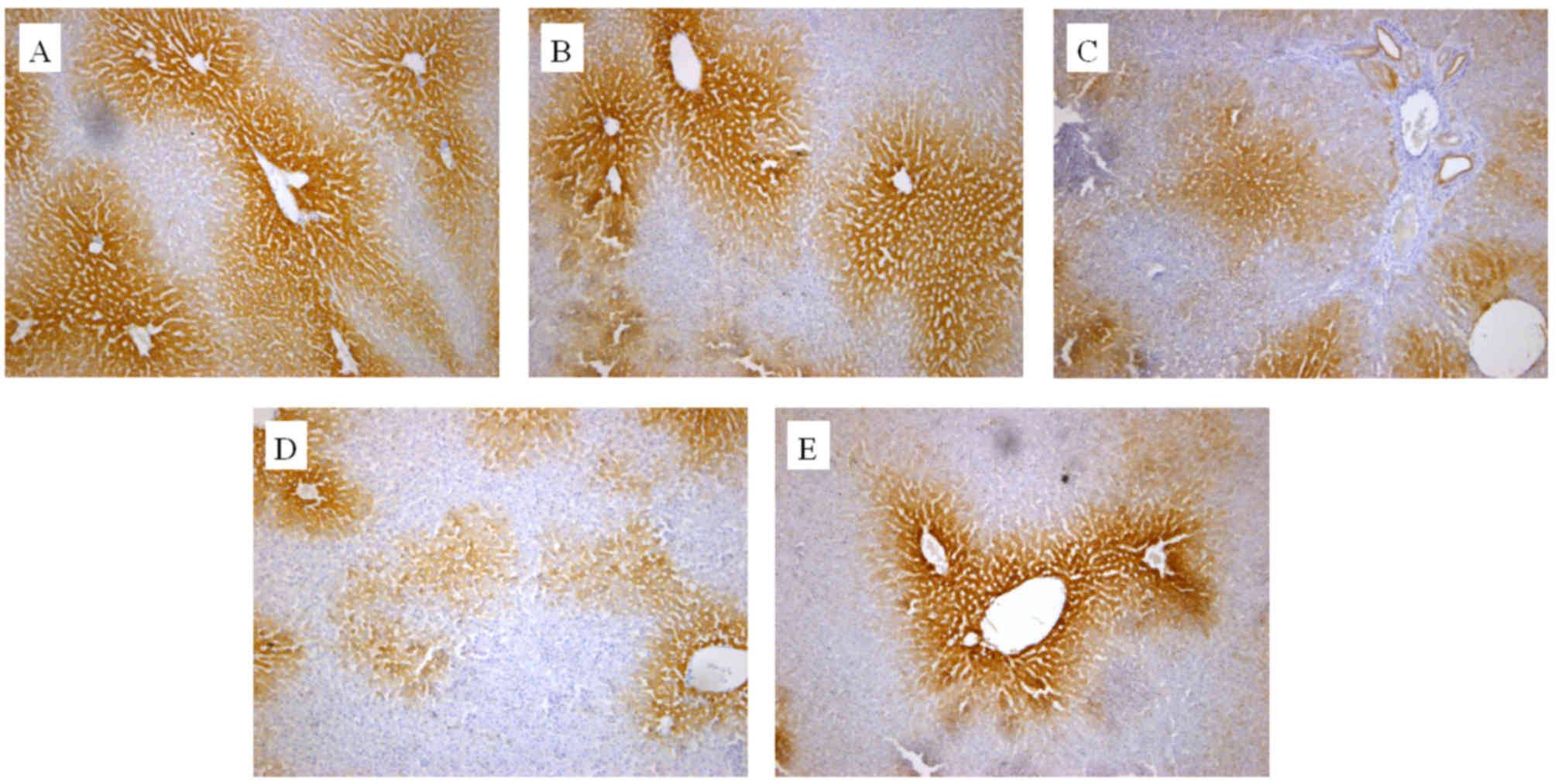
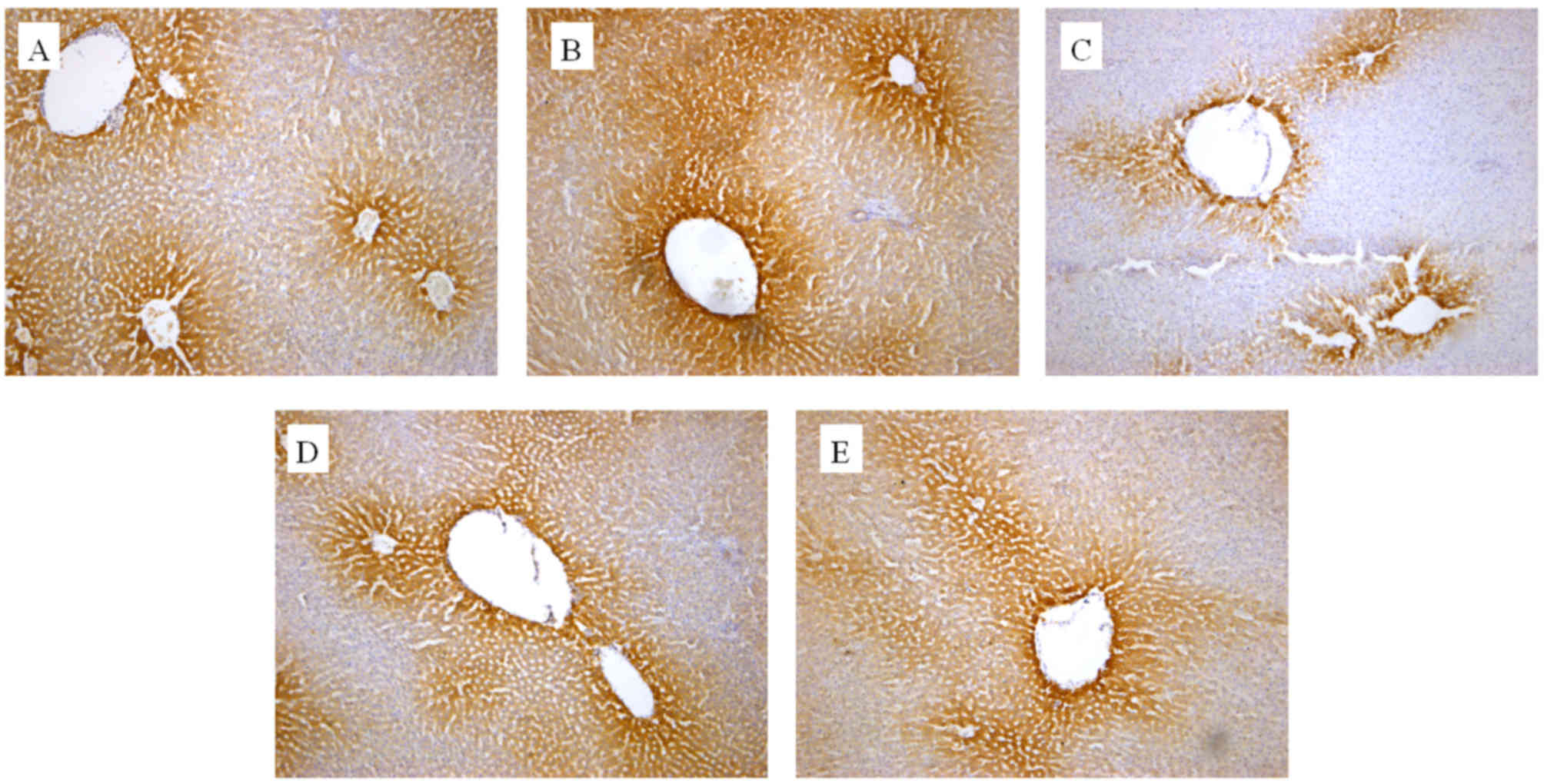
The in vivo effect of the extracts on CYP2E1
and CYP1A2 protein expression was also determined by an IHC assay.
As presented in Figs. 3 and 4, cells expressing the respective proteins
exhibited yellow brown or dark brown staining. These stained cells
were located around the hepatic vascular plexus with a clear
background. According to microscopic examination, CYP2E1 protein
expression was markedly reduced by administration of GE and mildly
increased by administration of GE + MP, which was in good agreement
with the results of RT-qPCR and western blot assays. Processing
with an image analysis system and statistical analysis revealed
that the surface density and number density of CYP2E1-expressing
cells in the control group was 54.29±6.53 and 0.71±0.065, while
that in high-dose GE group was 31.47±5.99 and 0.47±0.079 and that
in the GE and MP combination group was 43.77±8.01 and 0.70±0.11,
respectively (Table I). The results
for CYP1A2 protein expression were the similar to those for CYP2E1
protein expression (Fig. 4 and
Table II). Taken together, it was
demonstrated that MP upregulated CYP2E1 and CYP1A2 expression in
vivo.
 | Table I.Effect of herbal extracts on the
protein expression of cytochrome P450 2E1 in rat livers. |
Table I.
Effect of herbal extracts on the
protein expression of cytochrome P450 2E1 in rat livers.
| Group | n | GE dose (g/kg) | Surface density
(×10−2) | Number density (×10-2
cell/µm2) |
|---|
| Control | 10 | – |
54.29±6.53a |
0.71±0.065b |
| Combination of GE and
MP | 10 | 0.54 |
43.77±8.01ac |
0.70±0.11b |
| High-dose GE | 8 | 0.54 |
31.47±5.99c |
0.47±0.079d |
| Medium-dose GE | 8 | 0.43 |
34.28±5.32c |
0.53±0.10d |
| Low-dose GE | 10 | 0.36 |
35.33±6.72c | 0.69±0.13 |
 | Table II.Effect of herbal extracts on the
protein expression of cytochrome P450 1A2 in rat liver. |
Table II.
Effect of herbal extracts on the
protein expression of cytochrome P450 1A2 in rat liver.
| Group | n | GE dose (g/kg) | Surface density
(×10−2) | Number density (cell
×10−2/µm2) |
|---|
| Control | 10 | – |
48.66±6.32a |
0.69±0.089b |
| Combination of GE and
MP | 10 | 0.54 |
43.80±8.70b | 0.59±0.093 |
| High-dose GE | 8 | 0.54 |
37.18±9.99c | 0.53±0.1d |
| Medium-dose GE | 8 | 0.43 |
38.34±10.14c | 0.59±0.083 |
| Low-dose GE | 10 | 0.36 |
40.18±4.06c | 0.66±0.21 |
Effects of herbal extracts on GST
enzyme activities in rat liver
RT-qPCR, western blot and colorimetric assays were
performed to detect the effects of MP on the mRNA and protein
expression, as well as enzyme activity of hepatic GST in GE-induced
rats. Two types of GST associated with detoxification, namely GSTm1
and GST-pi, were selected for analysis.
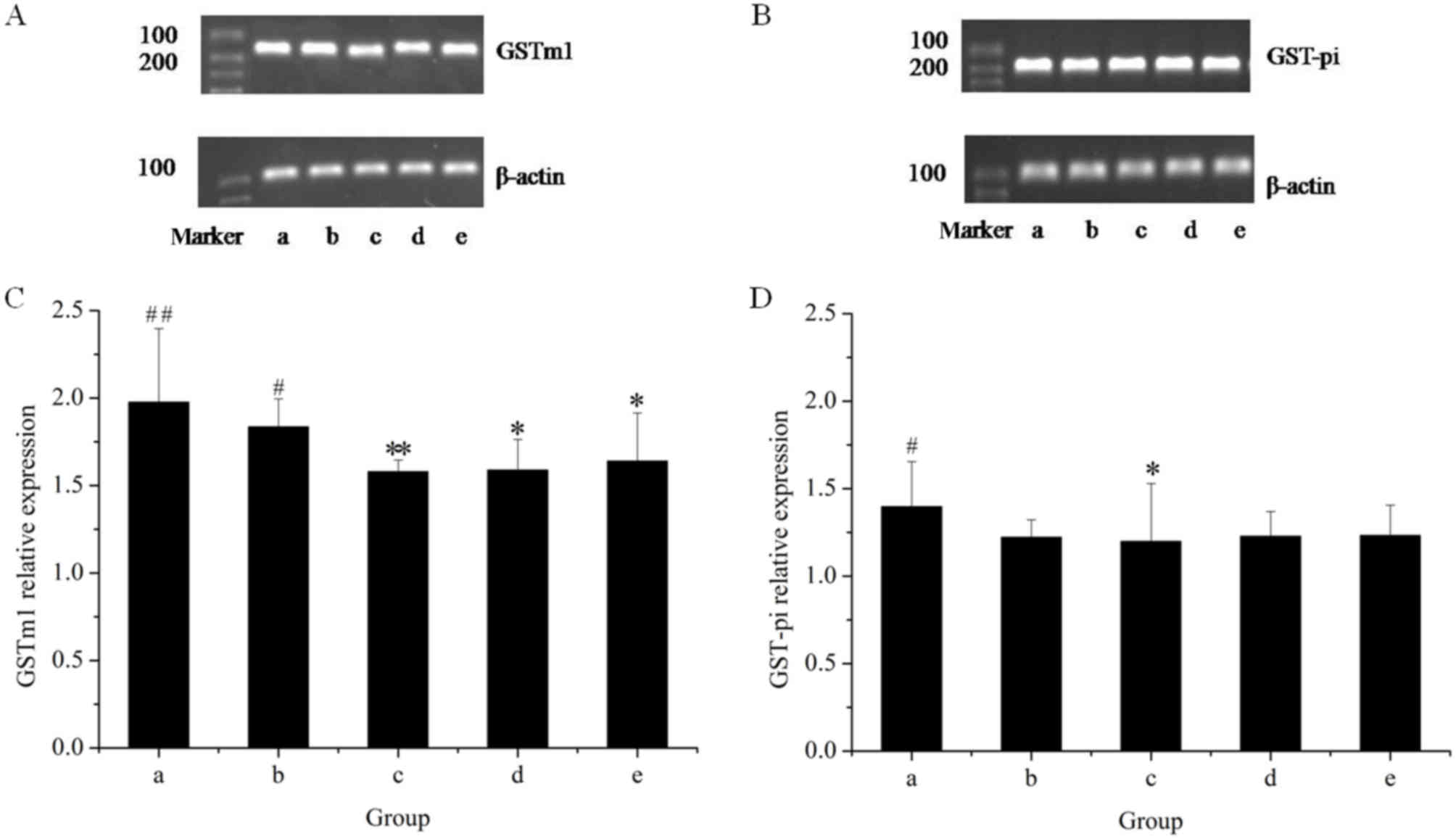
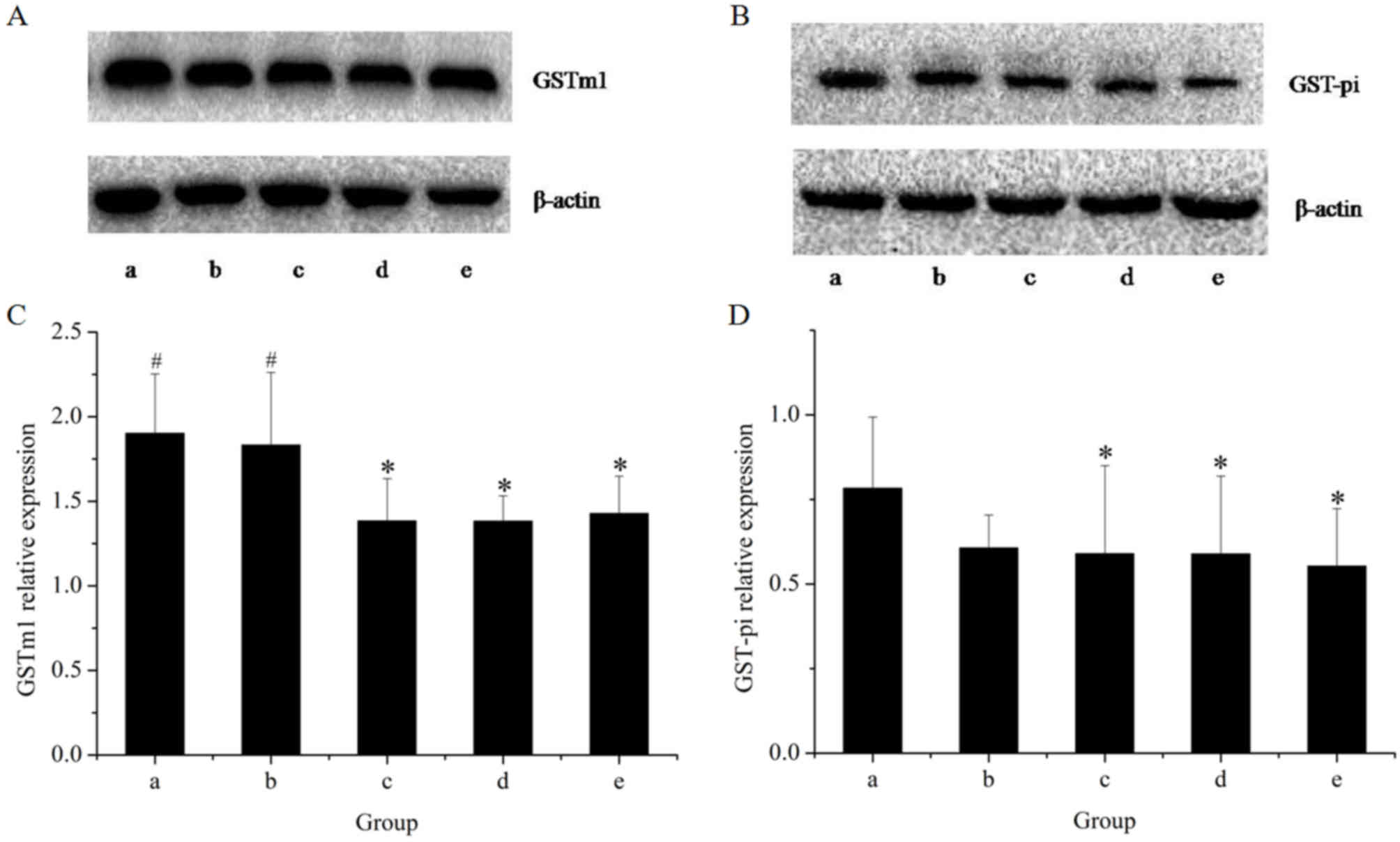
The effects of the herbal extracts on GSTm1 mRNA and
protein expression in rat livers are presented in Figs. 5 and 6, respectively. The results of the RT-qPCR
assay indicated that treatment with GE at all concentrations (0.36,
0.43 and 0.54 g/kg) significantly inhibited GSTm1 mRNA expression
in a dose-dependent manner. However, in the GE and MP combination
group, the mRNA expression of GSTm1 was increased as compared with
that in the high-dose GE group. Western blot analysis revealed the
same effects on GSTm1 protein expression as those on the respective
mRNA levels. The results indicated that downregulation of GSTm1
expression by GE was counteracted by MP.
To further explore the modulation effects on hepatic
GST, the present study examined the mRNA and protein expression of
GST-pi (Figs. 5 and 6, respectively). The results of the RT-qPCR
assay indicated that all treatments caused an evident decline in
GST-pi mRNA expression as compared with the control. The greatest
decline was observed in the high-dose GE group. Combined treatment
with MP slightly increased the mRNA expression of GST-pi as
compared with that in the high-dose GE group, but the difference
was not statistically significant. The results of the western blot
analysis illustrated that the pattern of protein expression was
similar to that for the respective mRNA levels.
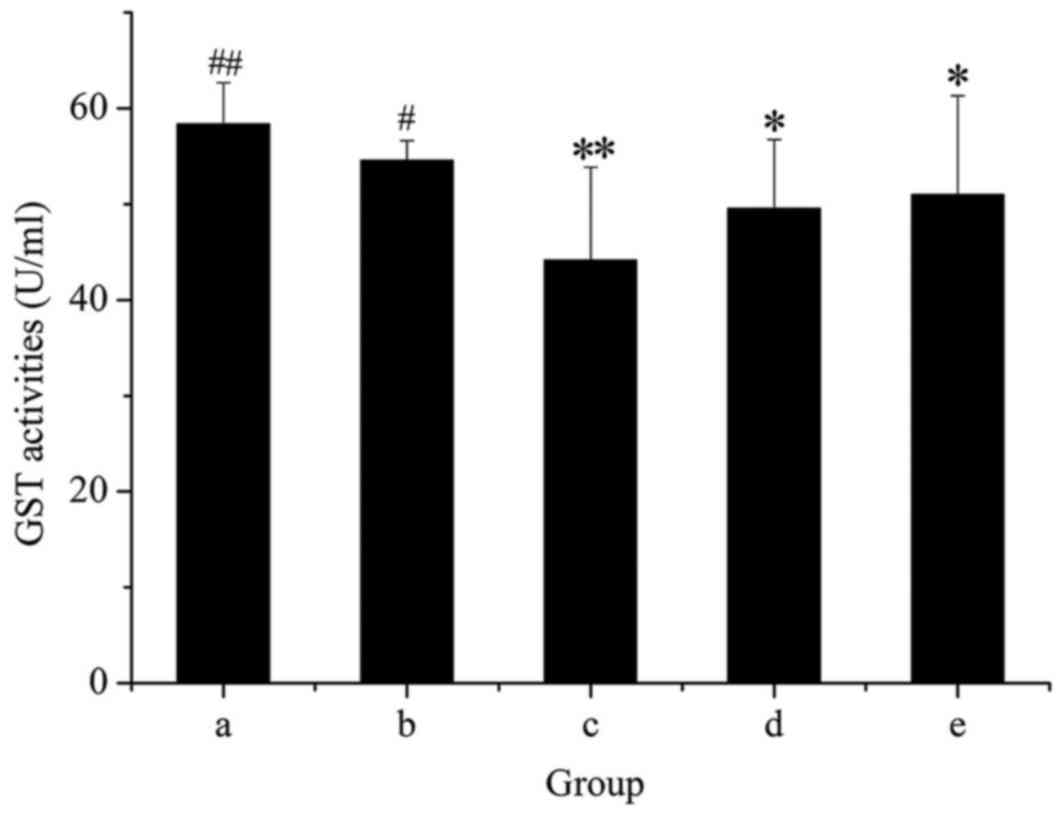
As presented in Fig.
7, administration of GE alone markedly decreased the activity
of GST, and this inhibitory effect was dose-dependent. However,
rats treated with GE + MP were significantly protected from the
decrease in GST activity produced by GE. This result suggested that
GST activity was enhanced after cotreatment with MP.
Discussion
The liver, as an important organ of metabolism and
excretion, is frequently endowed with the task of detoxification.
Detoxification mechanisms are generally considered to be associated
with induction or inhibition phase-I metabolic enzymes and phase-II
detoxification enzymes. CYP450 enzymes, which are significant
phase-I metabolic enzymes, have a vital role in the metabolism of
xenobiotics and endogenous substances (18). Of particular interest in an in
vitro toxicity study of GE was the involvement of CYP2E1 and
CYP1A2 in its metabolism (9). In the
present study, GE inhibited the mRNA and protein expression of
CYP2E1 and CYP1A2. However, administration of GE + MP increased the
mRNA and protein expression of CYP2E1 and CYP1A2 as compared with
that in the group treated with GE alone. This result suggested a
potential induction effect of MP on rat hepatic CYP450 inhibited by
GE. However, this result was not in accordance with the increased
expression of CYP2E1 in the case of liver injury (19). Several factors may explain for this
difference. First, GE may have been so poisonous that the enzyme's
expression was deactivated following continuous administration of
GE. Furthermore, the exogenous toxicant was transformed into its
metabolic intermediate by phase-I metabolic enzymes, followed by
later involvement of phase-II detoxification enzymes. Therefore,
the modulation effect of GST as a phase-II enzyme was explored in
the subsequent experiments.
GST, an important phase-II detoxification enzyme, is
a soluble protein located in the cytosol that functionally binds
GSH, as well as xenobiotics or endogenous substances (10). It has a significant role in the
detoxification and excretion of xenobiotics or metabolic
intermediates, since it increases the solubility of hydrophobic
substances (20). Following
assessment of the inhibition of the CYP450 pathway by GE and the
counteracting effect of MP, the detoxification pathway involving
GST was then investigated. The results indicated that GE inhibited
GST activity as well as the mRNA and protein expression of GSTm1
and GST-pi. However, the rats cotreated with MP were significantly
protected from the decrease in the activity, as well as mRNA and
protein expression of GSTm1 produced by GE. This result indicated a
potential induction effect of MP on GST to counteract the
inhibition by GE in rat livers. This modulation may explain for the
role of MP in reducing the acute intoxication effect of GE.
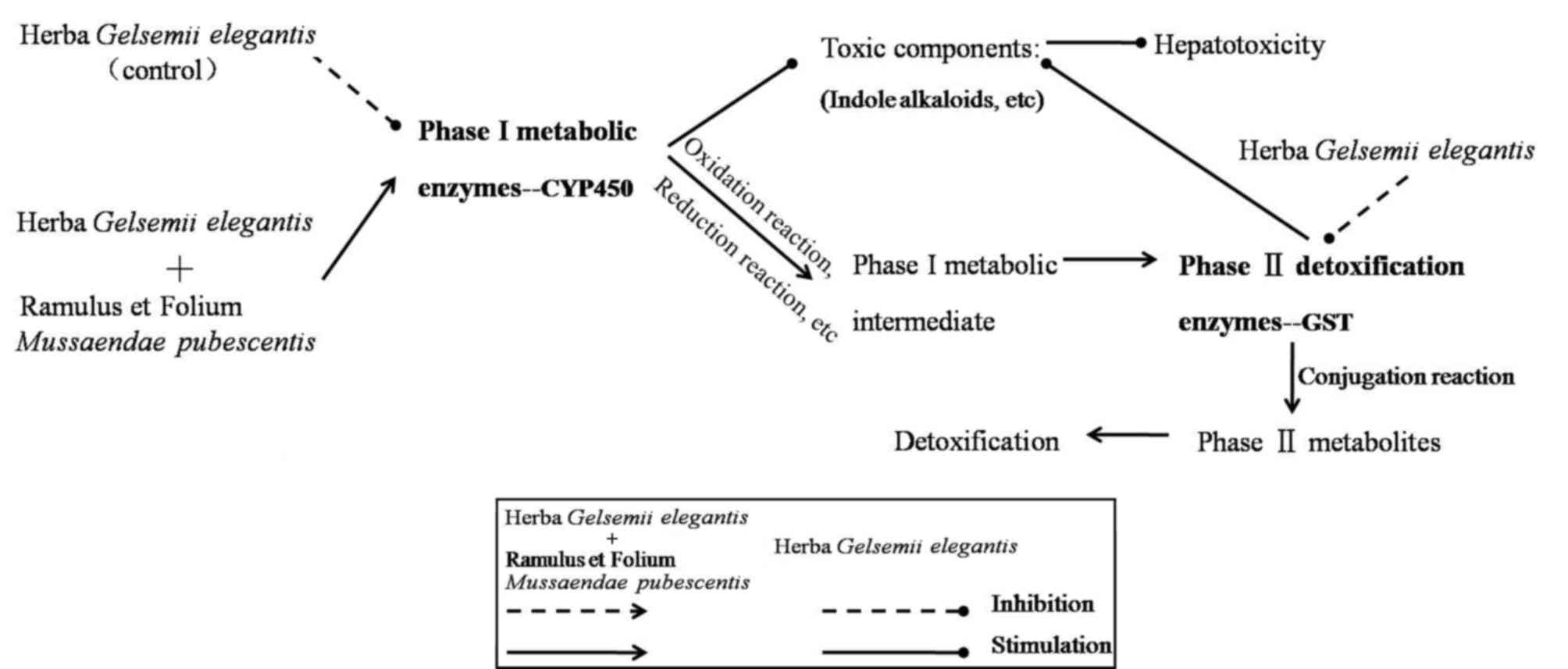
The scheme in Fig. 8
summarizes the mechanism deduced from the results obtained in the
present study. By inhibiting the expression of CYP450 and GST, GE
decreased phase I and II metabolic enzyme activity, which
metabolizes indole alkaloids and other toxic components (9). Following co-treatment with MP,
detoxification was achieved through upregulation of CYP450
expression in phase I metabolites [oxidation/reduction reaction of
toxic components (21)] and
enhancing GST enzyme activity in phase II metabolites [conjugation
reaction of phase I metabolites (10)]. It was demonstrated that MP had a
potent detoxification effect upon GE-induced acute intoxication in
rat livers. The detoxification mechanism may in part comprise the
modulation of CYP450 and GST enzymes. These results suggested that
MP may allow for the pharmaceutical application of GE due to
reducing its toxic side effects. Further study to characterize the
active components of MP and to elucidate the mechanisms of action
is in progress.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81773921 and
81303240) and the Specialized Research Fund for the Doctoral
Program of Higher Education (grant no. 20133519110005).
References
|
1
|
Xu Y, Qiu HQ, Liu H, Liu M, Huang ZY, Yang
J, Su YP and Yu CX: Effects of koumine, an alkaloid of Gelsemium
elegans Benth., on inflammatory and neuropathic pain models and
possible mechanism with allopregnanolone. Pharmacol Biochem Behav.
101:504–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Editorial Committee of Chinese Materia
Medica, . the Administration Bureau of Traditional Chinese
Medicine: Chinese Materia Medica (ZhonghuaBencao). Shanghai Science
and Technology: Shanghai. 6:1–215. 2000.
|
|
3
|
Rujjanawate C, Kanjanapothi D and Panthong
A: Pharmacological effect and toxicity of alkaloids from gelsemium
elegans benth. J Ethnopharmacol. 89:91–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang F, Lu Y, Li Y, Meng ZQ and Liang NS:
Experimental research on antineoplastic effect of extract from
Gelsemiu Elegans Benth. Guangxi Zhong Yi Yao. 1:51–53. 2004.(In
Chinese).
|
|
5
|
Yang CS, Chhabra SK, Hong JY and Smith TJ:
Mechanisms of inhibition of chemical toxicity and carcinogenesis by
diallyl sulfide (DAS) and related compounds from garlic. J Nutr.
131:1041S–1045S. 2001.PubMed/NCBI
|
|
6
|
Ronis MJJ, Lindros KO and Ingelman S: The
cytochrome P450 2E subfamilyIoannides C: Cytochromes P450,
metabolic and toxicological aspects. CRC Press; Boca Raton, FL: pp.
211–239. 1996
|
|
7
|
Bradford BU, Kono H, Isayama F, Kosyk O,
Wheeler MD, Akiyama TE, Bleye L, Krausz KW, Gonzalez FJ, Koop DR
and Rusyn I: Cytochrome P450 CYP2E1, but not nicotinamide adenine
dinucleotide phosphate oxidase, is required for ethanol-induced
oxidative DNA damage in rodent liver. Hepatology. 41:336–344. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gallagher EP, Kunze KL, Stapleton PL and
Eaton DL: The kinetics of aflatoxin B1 oxidation by human
cDNA-expressed and human liver microsomal cytochromes P450 1A2 and
3A4. Toxicol Appl Pharmacol. 141:595–606. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Wu S, Chen Z, Zhang H and Zhao W:
Inhibitory effects of cytochrome P450 enzymes CYP1A2, CYP2A6,
CYP2E1 and CYP3A4 by extracts and alkaloids of gelsemium elegans
roots. J Ethnopharmacol. 166:66–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dourado DF, Fernandes PA and Ramos MJ:
Glutathione transferase classes alpha, pi, and mu: GSH activation
mechanism. J Phys Chem B. 114:12972–12980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Townsend DM, Manevich Y, He L, Hutchens S,
Pazoles CJ and Tew KD: Novel role for glutathione S-transferase pi.
regulator of protein S-glutathionylation following oxidative and
nitrosative stress. J Biol Chem. 284:436–445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu TL, Su LL, Ji D, Gu W and Mao CQ:
Interaction between CYP450 enzymes and metabolism of traditional
chinese medicine as well as enzyme activity assay. Zhongguo Zhong
Yao Za Zhi. 40:3524–3529. 2015.(In Chinese). PubMed/NCBI
|
|
13
|
Wang Y, Wu S, Li D and Wang H: Uniform
design research on the compatibility toxicity of Gelsemium Elegans
Benth and Mussaenda Pubescens. Chin J Mod Appl Pharm. 2:150–154.
2016.
|
|
14
|
Lu XH, Wang YH, Wang HS, Li DS and Wu SS:
Study on the HPLC fingerprint of chemical constituents in
dichloromethane extraction of gelsemium elegans. Shizhen Guo Yi Guo
Yao. 5:1055–1057. 2016.(In Chinese).
|
|
15
|
Yu HM, Lu XH, Wang YH and Wu SS:
Establishment of determination method of total triterpenoids in
Mussaendapubescens. J Liaoning Univ TCM. 2:40–42. 2015.
|
|
16
|
Spencer WE and Christensen MJ: Multiplex
relative RT-PCR method for verification of differential gene
expression. Bio Techniques. 27:1044–1046. 1999.
|
|
17
|
Habig WH, Pabst MJ and Jacoby WB:
Glutathiene S-transferases. The first enzymatic step in the
mercapturic acid formation. J Biol Chem. 249:7130–7139.
1974.PubMed/NCBI
|
|
18
|
Autrup H: Genetic polymorphisms in human
xenobiotica metabolizing enzymes as susceptibility factors in toxic
response. Mutat Res. 464:65–76. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Li W, Kim YH and Lee YW: Chlorella
vulgaris extract ameliorates carbon tetrachloride-induced acute
hepatic injury in mice. Exp Toxicol Pathol. 65:73–80. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Truong Q, Bradley SA, Doss GA, Kumar S and
Reddy VB: Glutathione S-transferase catalyzed desulfonylation of a
sulfonylfuropyridine. Drug Metab Dispos. 38:108–114. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vancutsem PM and Babish JG: In vitro and
in vivo study the effects of enrofloxacin on hepatic cytochrome
P450. potential for drug interactions. Vet-Hum Toxicol. 38:254–256.
1996.PubMed/NCBI
|