Introduction
Osteoporosis (OP) is considered as one of the most
common chronic skeletal diseases in the aged population of modern
society. OP is a disorder of the skeletal system that leads to
decreased bone density and increased fracture risk due to an
imbalance in bone matrix turnover. According to the World Health
Organization, patients diagnosed with OP demonstrate significantly
decreased bone mineral density (BMD) compared with that of
sex-matched adults (1). Osteoporotic
fractures occur so frequently as to affect 1 in 3 women and 1 in 5
men over 50 years of age (2). The
increasing number of osteoporotic fractures not only places a heavy
economic burden on the medical care system but also leads to
chronic pain, deformity, depression, disability and even death of
patients.
Cannabinoid receptors (CNRs) are members of the
G-protein coupled receptors superfamily and have a major role in
the pathophysiology of a wide range of chronic diseases. CNR2 (also
abbreviated as CB2 receptor), is considered to be a
promising drug target for the treatment of a number of diseases,
including osteoporosis (3). Due to
the lack of CNR2 in the central neural system (CNS), CNR2
receptor-targeting drugs usually show only few adverse effects on
the CNS (4). Great progress has been
made in the past few decades in the identification of novel ligands
and optimization of the binding efficiency to the CNR2 (5). However, not until recently, endogenous
cannabinoids and corresponding receptors were found to be involved
in the regulation of osteoblast differentiation and bone formation
(6,7). For instance, CB1/2 receptor
agonist CP 55,940 and CNR2-selective agonist HU 308 have been shown
to stimulate the early differentiation of bone marrow-derived
osteoblast precursors and enhance bone nodule formation in
osteoblast cultures in vitro (8). Consistent with these discoveries, a
previous study by our group found that activation of CNR2 by
chemical ligand UR-144 enhanced osteogenic differentiation of bone
marrow-derived mesenchymal stem cells (BMSCs) (9), whereas treatment with the CNR1
antagonist AM 251 suppressed osteoblast number and function
(10). Therefore, it appears that
cannabinoid signaling functions differently in terms of different
cannabinoid receptors, but its role in osteoporosis has remained
elusive.
Previous studies have revealed the loss of CNR2
expression in the bone marrow of osteoporotic patients (9). The present study hypothesized that
lentiviral vector-mediated overexpression of CNR2 reverses the
osteogenic differentiation and mineralization ability of BMSCs
isolated from osteoporotic patients. To test this hypothesis,
patient-derived BMSCs were transfected with CNR2-expressing
lentiviral vector, and their osteogenic differentiation ability and
associated cell signaling were tested. Regulation of CNR2 in
osteoporosis patient-derived BMSCs may provide a promising
therapeutic strategy, which transforms local stem cells from a
disease state to a reparative state.
Materials and methods
Cell culture and expansion
Bone marrow biopsies were obtained from patients who
underwent bone marrow examinations in October 2015. Patients
provided informed consent before bone marrow collection. The
protocol for human tissue preparation was approved by the Medical
Ethical Committee of Xiangyang Central Hospital (Xiangyang, China).
MSCs were isolated from bone marrows of osteoporotic patients as
well as healthy donors as described previously (11). In brief, total bone marrow was plated
at a density of 50,000 cells/cm2 in culture flasks in
MSC proliferation medium [α-modified Eagle's medium (α-MEM)
supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, 0.2
mM ascorbic acid, 100 U/ml penicillin, 10 µg/ml streptomycin and 1
ng/ml basic fibroblast growth factor], plus 1% heparin. The medium
was changed every 3–4 days until confluence. BMSCs from four
healthy and four osteoporotic patients were used in this study. All
reagents used for cell culture were purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and chemicals
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany),
unless otherwise specified.
Osteogenic differentiation
Osteogenic differentiation was induced by treating
MSCs with osteogenic medium containing Dulbecco's modified Eagle's
medium plus 10% FBS, 0.1 nM dexamethasone, 10 mM
β-glycerophosphate, 0.01 µM 1,25-dihydroxy vitamin D3 and 50 µM
ascorbic acid in α-MEM (12).
Alizarin red staining
After 3 weeks of culture in osteogenic medium
supplemented with 10 nM CNR2 receptor agonist UR-144, MSCs were
fixed with 10% formalin. After rinsing in PBS, cells were incubated
with 40 mM alizarin red S (pH 4.2) for 10 min with agitation. Cells
were then rinsed 5 times with water, followed by washing with PBS
for 15 min to reduce non-specific staining. The stained nodules
were observed and recorded by using a phase contract
microscope.
Alkaline phosphatase (ALP) activity
staining
Cytochemical analysis with
5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro blue
tetrazolium chloride (NBT) was used for the staining for ALP. MSCs
were first fixed in 10% formalin and incubated with 300 µl BCIP/NBT
premixed solution (Sigma-Aldrich; Merck KGaA) for 8–10 min at room
temperature. Cells were then rinsed with water, dried and examined
by phase contract microscopy.
RNA isolation and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was isolated from BMSCs with the QIAamp
DNA Mini kit (Qiagen, Hilden, Germany). One microgram of total RNA
was reverse-transcribed into complementary (c)DNA using the iScript
cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The cDNA samples were amplified with a Pfu PCR kit (Tiangen,
Beijing, China) with specific primers listed in Table I. All PCR products then underwent
electrophoresis on a 2% agarose gel. Quantitative PCR was performed
on cDNA samples by using the iQ SYBR Green Supermix (Bio-Rad,
Laboratories, Inc.). PCR was performed on a MyiQ2 Two-Color
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) under
the following conditions: cDNA was pre-heated for 15 min at 95°C
and denatured for 5 min at 95°C, followed by 45 cycles consisting
of 15 sec at 95°C, 15 sec at 60°C and 30 sec at 72°C. For each
reaction, a melting curve was generated to confirm the binding
specificity of the primers. Calculation of relative expression was
performed using iQ5 optical system software (version 2.0; Bio-Rad
Laboratories, Inc.) using the ΔΔCq method (13). GAPDH was used as housekeeping gene
for normalization.
 | Table I.Sequences of primers used for
polymerase chain reaction. |
Table I.
Sequences of primers used for
polymerase chain reaction.
| Gene name | NCBI Gene ID | Sequence (5′-3′) | Length of amplicon
(bp) |
|---|
| Cannabinoid receptor
2 | 1,269 | Forward,
AGCCCTCATACCTGTTCATTGG; | 154 |
|
|
| Reverse,
GTGAAGGTCATAGTCACGCTG |
|
| Runt-related
transcription factor 2 | 860 | Forward,
TGGTTACTGTCATGGCGGGTA; | 101 |
|
|
| Reverse,
TCTCAGATCGTTGAACCTTGCTA |
|
| Osterix |
| Forward,
CCTCTGCGGGACTCAACAAC; | 128 |
|
|
| Reverse,
AGCCCATTAGTGCTTGTAAAGG |
|
| Integrin-binding
sialoprotein | 3,381 | Forward,
CACTGGAGCCAATGCAGAAGA; | 106 |
|
|
| Reverse,
TGGTGGGGTTGTAGGTTCAAA |
|
| Osteocalcin | 632 | Forward,
CACTCCTCGCCCTATTGGC; | 112 |
|
|
| Reverse,
CCCTCCTGCTTGGACACAAAG |
|
| Secreted
phosphoprotein 1 | 6,696 | Forward,
GAAGTTTCGCAGACCTGACAT; | 91 |
|
|
| Reverse,
GTATGCACCATTCAACTCCTCG |
|
|
GAPDH | 2,597 | Forward,
CTGGGCTACACTGAGCACC; | 101 |
|
|
| Reverse,
AAGTGGTCGTTGAGGGCAATG |
|
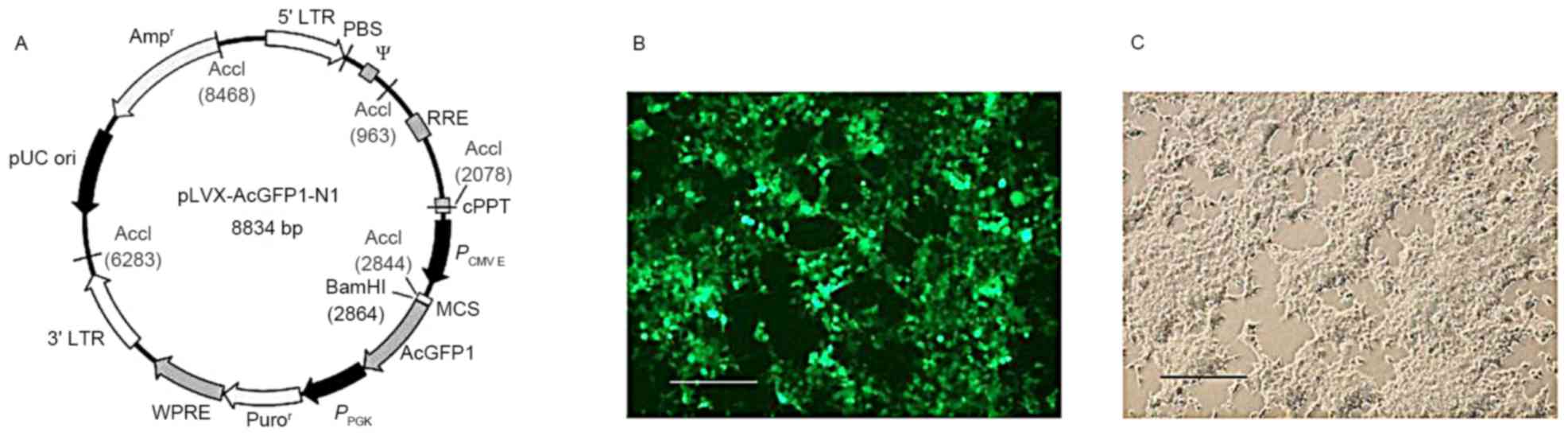
Overexpression of CNR2 by lentiviral
vector in MSCs
Cloning of CNR2 and lentiviral vectors were prepared
by Hanbio Biotechnology (Shanghai, China) to overexpress CNR2 in
target cells. In brief, the cDNA of CNR2 was amplified with
specific primers from a cDNA library and sub-cloned into a T
vector. The coding sequence of CNR2 was then cloned into lentiviral
vectors (pLVX-IRES-ZsGreen1) that expressed GFP and
puromycin-resistant proteins for screening. Lentiviral supernatants
(Lenti-CNR2) were packaged by triple transfection of HEK293T cells
(Fig. 1). MSCs were infected by
adding virus-containing supernatants to the culture medium.
Transduced cells were selected by addition of puromycin (1 µg/ml)
at 2 days after infection for 1 week. Transduction was verified by
visual examination for cells expressing green fluorescent protein
(GFP) under a fluorescent microscope. Cells infected with
lentivirus containing empty vectors (Lenti-GFP) were used as
controls.
Immunofluorescent staining
MSCs with or without viral infection were seeded on
glass cover slips in six-well plates 24 h prior to staining. Cells
were washed with PBS, fixed in 4% paraformaldehyde for 30 min at
room temperature and then permeabilized with 1% Triton-X 100 prior
to being blocked in 1% bovine serum albumin for 15 min at room
temperature. Slips were subsequently incubated overnight at 4°C
with rabbit polyclonal primary antibodies against CNR2 receptor
with a dilution of 1:100 (cat. no. ab3561, Abcam, Cambridge, MA).
Sequentially, slides were incubated with secondary antibodies
conjugated to Alexa 594 with a dilution of 1:500 for 1 h at room
temperature (cat. no. A-11037, Invitrogen; Thermo Fisher
Scientific, Inc.) and cell nuclei were counterstained with
4,6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR, USA).
After rinsing with PBS, cells were examined and imaged using a DMi
6000 B fluorescent microscope (Leica Microsystems, Wetzlar,
Germany).
Total protein isolation and western
blot analysis
Prior to lysing cells, cell layers were washed three
times with PBS. Total protein was extracted by RIPA Lysis and
Extraction buffer (Thermo Fisher Scientific, Inc.), followed by
centrifugation at 13,000 × g for 15 min to remove cell debris. The
protein concentration was measured and normalized using a
bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.).
Twenty-five micrograms of total protein from each sample was loaded
and separated by 15% SDS-PAGE gel, then bands were transferred to
PVDF membranes. Then, membranes were incubated with polyclonal
rabbit anti-CNR2 antibody (cat. no. ab3561, Abcam) or
anti-phospho-p38 mitogen-activated protein kinase (MAPK)
(Thr180/Tyr182) antibody (cat. no. 4511S, Cell Signaling
Technology, Inc., Danvers, MA, USA) with dilution of 1:1,000 at 4°C
overnight. After washing, membranes were incubated with
HRP-conjugated secondary antibodies (cat. no. 31460, Thermo Fisher
Scientific, Inc.) with dilution of 1:1,000 for 1 h at room
temperature. Immunocomplexes were visualized using enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.). Band
intensities were analyzed by Image J software (NIH, Bethesda, MD,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-tests for paired samples were performed to determine
statistical significance between two groups, with GraphPad Prism
7.0 (GraphPad Software, Inc. La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
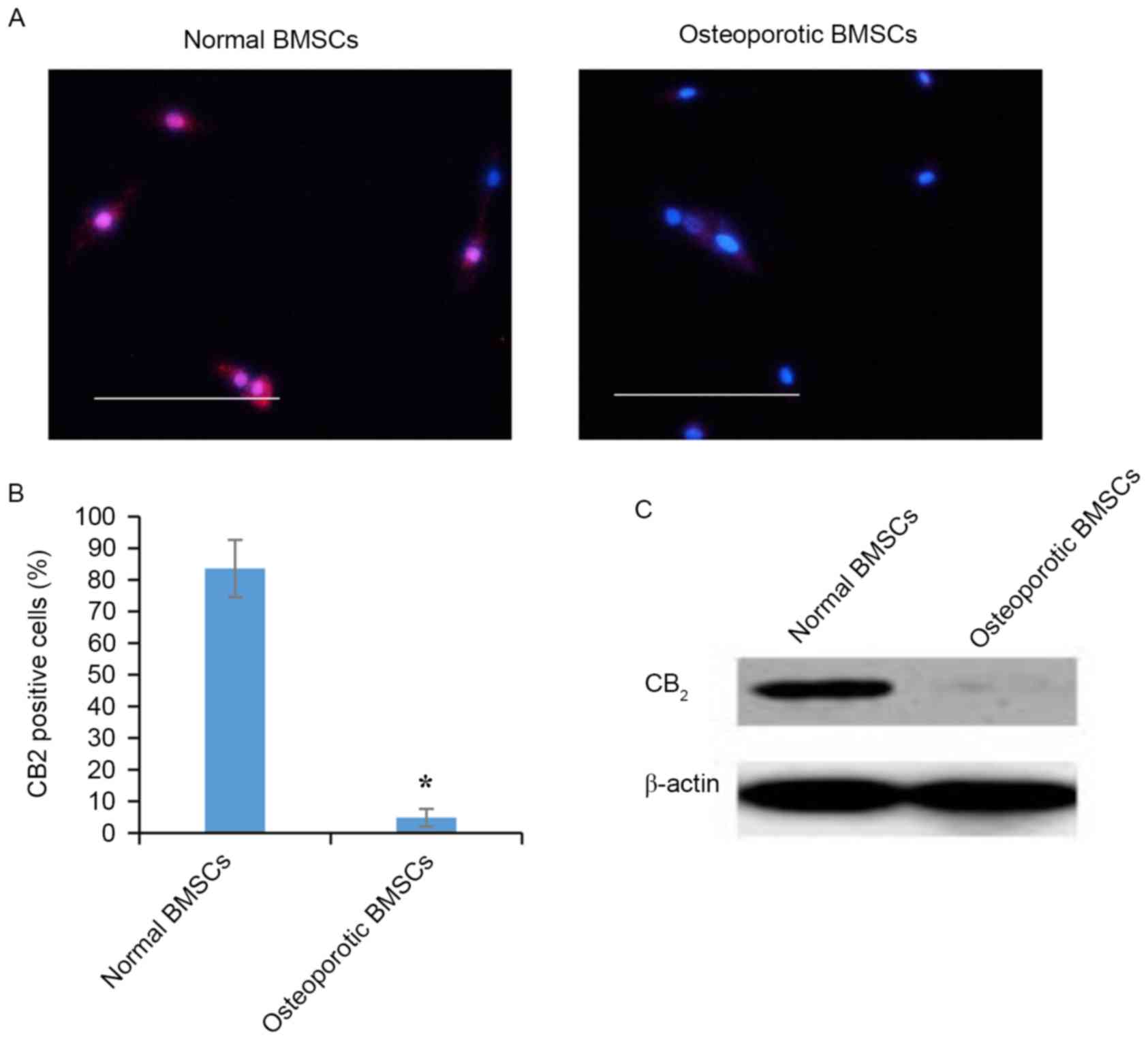
CNR2 is expressed in normal BMSCs,
while absent in osteoporotic BMSCs
Previous studies have revealed the loss of CNR2s
expression in the bone marrow of osteoporotic patients. However,
MSC-specific expression patterns of CNR2 in bone marrow has
remained to be fully elucidated. The present study assessed the
expression of CNR2 in cultured BMSCs isolated from healthy
individuals and osteoporotic patients. Immunofluorescent staining
showed that CNR2 was only expressed in normal BMSCs but absent in
osteoporotic BMSCs (Fig. 2A).
Quantification of immunofluorescent images indicated that
CNR2-expressing cells accounted for >80% of the overall BMSC
population in healthy individuals but <5% in osteoporotic BMSCs
(Fig. 2B). Western blot analysis
also demonstrated significantly lower protein levels of CNR2 in
BMSCs from osteoporotic patients compared to in BMSCs from healthy
individuals (Fig. 2C).
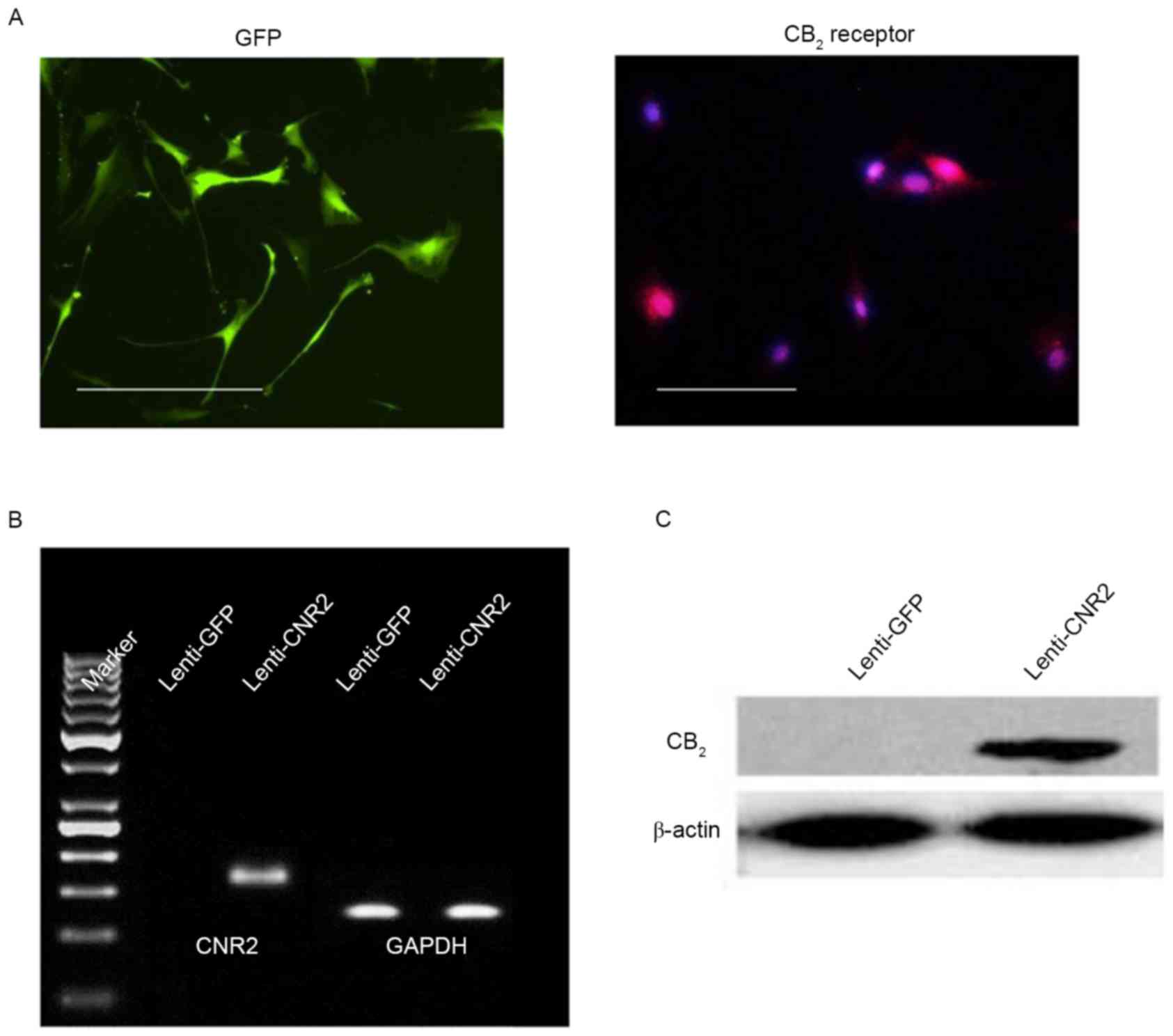
CNR2 expression in osteoporotic BMSCs
may be restored by lentiviral infection
To overexpress CNR2 in osteoporotic BMSCs, the
coding sequence of CNR2 was cloned into lentiviral vectors
(Fig. 1), which were transfected
into BMSCs. Successful transduction was identified by GFP
expression after screening with antibiotics. After puromycin
treatment, all live cells expressed GFP, indicating a homogenously
transfected cell population after selection (Fig. 3A). Immunofluorescent staining,
RT-qPCR and western blot analysis were performed to assess the
expression of CNR2. As shown in Fig.
3A, CNR2 was present in BMSCs transduced with Lenti-CNR2, but
absent in BMSCs transduced with control virus (Lenti-GFP). RT-qPCR
and western blot confirmed the expression of CNR2 at the mRNA and
protein level (Fig. 3B and C).
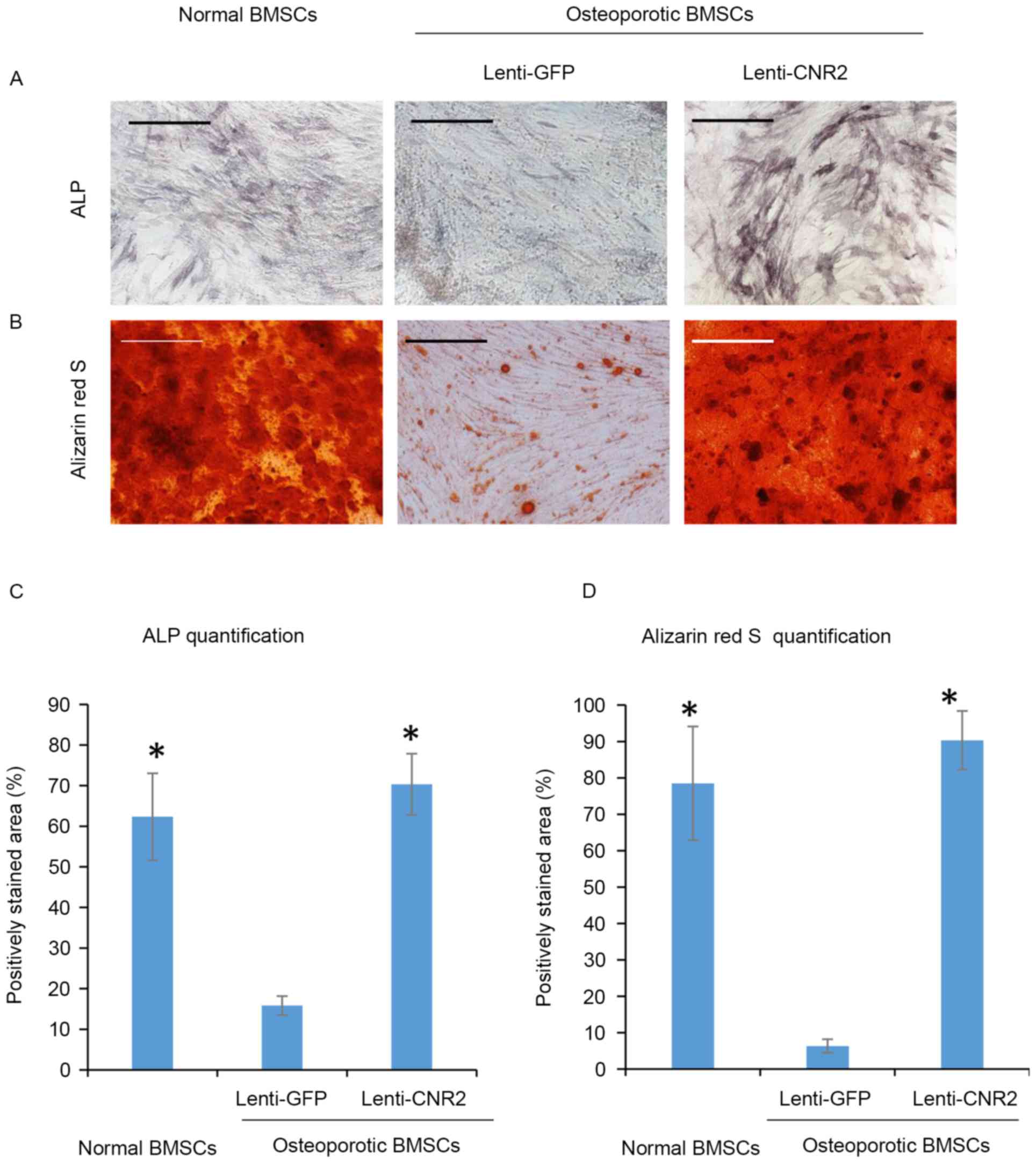
Overexpression of CNR2 promotes
osteogenic differentiation of BMSCs isolated from osteoporotic
patients
To test the possible influence of CNR2
overexpression on the mineralization of osteoporotic BMSCs, cells
transfected with Lenti-CNR2 were cultured in osteogenic medium to
induce osteogenic differentiation. BMSCs isolated from healthy
individuals were used as positive controls, while osteoporotic
BMSCs infected with Lenti-GFP were used as a negative control. All
groups of cells were cultured in osteogenic medium for 2 weeks for
ALP activity assay and 3 weeks for the mineralization assay. As
presented in Fig. 4A, osteoporotic
BMSCs infected with Lenti-CNR2 exhibited evidently increased ALP
activity compared to those infected with Lenti-GFP only, suggesting
the rescue of ALP activity by lentivirus-mediated CNR2
overexpression. Alizarin red S staining revealed a significantly
higher amount of mineral deposition in extracellular matrix
secreted by CNR2-overexpressing BMSCs (Fig. 4B). Quantification of staining images
(Fig. 4C and D) confirmed that ALP
activity and calcium accumulation were higher in osteoporotic BMSCs
infected with Lenti-CNR2 than in those transfected with Lenti-GFP.
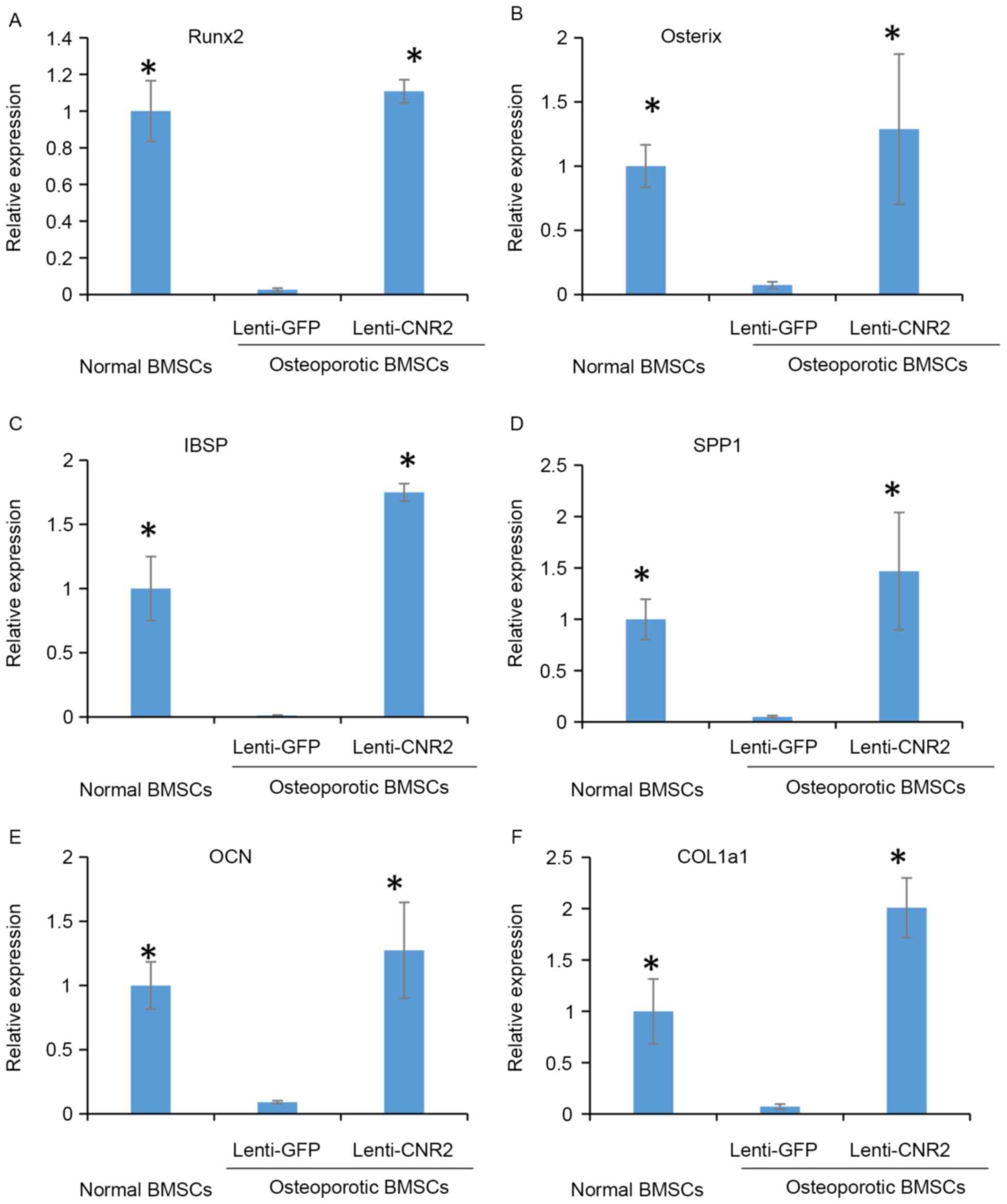
These findings were also consistent with the difference in
expression levels of genes associated with mineralization among
groups, including runt-related transcription factor 2,
integrin-binding sialoprotein, secreted phosphoprotein 1,
osteocalcin and collagen type I α 1 chain (Fig. 5).
Overexpression of CNR2 increases
phosphorylation of p38 MAPK
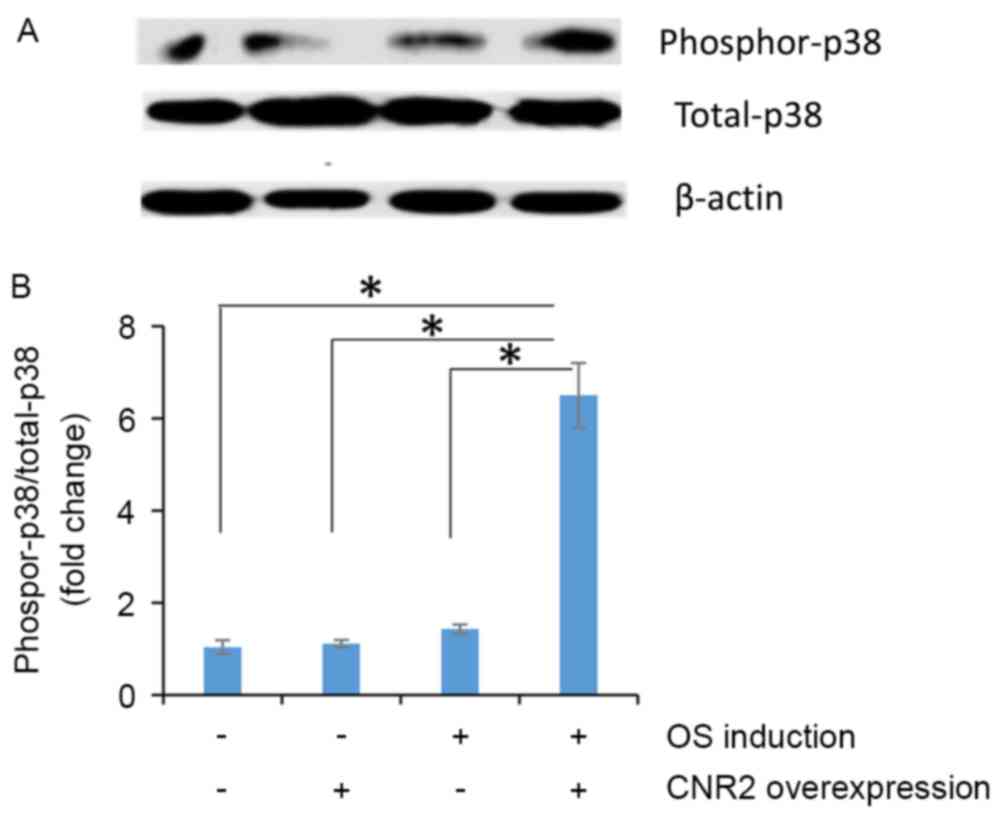
Since activation of p38 MAPK is known to be involved
in CNR2-mediated mineralization, the present study examined the
phosphorylation of p38 MAPK in the different groups by western blot
analysis. As displayed in Fig. 6A,
overexpression of CNR2 increased the phosphorylation of p38 MAPK in
osteoporotic BMSCs. Quantification of western blot images confirmed
significantly higher phosphorylation level of p38 MAPK in BMSCs
transfected with Lenti-CNR2 than that in BMSCs transfected with
Lenti-GFP (P<0.001; Fig. 6B).
Discussion
The current US Food and Drug Administration-approved
pharmacological therapies for osteoporosis prevention and/or
treatment are bisphosphonates, calcitonin and hormone therapy,
including estrogens, parathyroid hormone and estrogen
agonists/antagonists (14). The
mechanism of bisphosphonate-induced bone mineralization involves
the inhibition of osteoclasts by bisphosphonates (15). Orally administered bisphosphonates
reduced the vertebral and non-vertebral fracture risk in 20–50% of
subjects relative to the placebo group, while it was also
associated with gastrointestinal toxicity and adverse side effects
(16). Hormone therapy reduced the
fracture risk in 30–40% of subjects relative to the placebo group
(17); however, continuous use is
required to achieve efficacy, and hormone therapy may confer
beneficial effects on bone and also stimulation of other tissues
(18). Long-term therapy may
increase the risk of adverse health outcomes, such as stroke and
breast cancer in women (19). An
enhanced understanding of the pathogenesis of osteoporosis may
provide more precise cellular targets.
Stem cells have an important role in tissue
hemostasis and repair (20,21). However, aging and diseases may cause
a loss of function of stem cells (22,23). A
previous study by our group showed that bone marrow CNR2 levels in
osteoporotic patients were significantly reduced (9). In the present study, overexpression of
CNR2 was shown to restore the osteogenic differentiation and
mineralization of BMSCs isolated from osteoporotic patients. Thus,
it is possible that modification of stem cells from a disease state
into a reparative state has a therapeutic effect.
The present study found that osteoporotic BMSCs
transfected with Lenti-CNR2 exhibited higher ALP activity, higher
expression levels of osteogenic genes and increased mineral
deposition in the extracellular matrix than BMSCs transfected with
Lenti-GFP. It was further demonstrated that p38 MAPK
phosphorylation was increased by CNR2 overexpression. Human CNR1/2
may be activated by endogenous release of ‘endocannabinoids’ or
exogenous administration of drugs to ameliorate and cure diseases
induced by CNR1/2 inactivation. A number of medicines that activate
CNR1/2 are now used in clinical practice, including Cesamet
(nabilone), Marinol {dronabinol; Δ[9]-tetrahydrocannabinol
[Δ(9)-THC]} and Sativex [Δ(9)-THC with cannabidiol]. Particular
research interests have focused on the development of
CNR2-selective medicines with little activating effect on CNR1, due
to the fact that adverse effects induced by mixed CNR1/2 receptor
agonists primarily result from CNR1 rather than from CNR2
activation, and that CNR2-selective agonists have demonstrated
greater potential for therapeutic applications (24,25). The
present study provided a specific strategy for treating
osteoporosis, as bone marrow stem cells regained their osteogenesis
ability following restoration of CNR2. It is important for future
studies to assess whether restoration of CNR2 inhibits
osteoporosis-associated bone remodeling in vivo by using
animal models.
In conclusion, the present study supported that CNR2
in BMSCs is a therapeutic target of osteoporosis and overexpression
of CNR2 restores the osteogenic differentiation ability of BMSCs
from osteoporotic patients. The present study provided a cellular
therapeutic target for osteoporosis, which may lead to the
development of more effective drugs in the near future.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant no. 81272167).
References
|
1
|
Assessment of fracture risk and its
application to screening for postmenopausal osteoporosis. Report of
a WHO study group. World Health Organ Tech Rep Ser. 843:1–129.
1994.PubMed/NCBI
|
|
2
|
Bhutani G and Gupta MC: Emerging therapies
for the treatment of osteoporosis. J Midlife Health. 4:147–152.
2013.PubMed/NCBI
|
|
3
|
Han S, Chen JJ and Chen JZ: Latest
progress in the identification of novel synthetic ligands for the
cannabinoid CB2 receptor. Mini Rev Med Chem. 14:426–443. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasileiou I, Fotopoulou G, Matzourani M,
Patsouris E and Theocharis S: Evidence for the involvement of
cannabinoid receptors' polymorphisms in the pathophysiology of
human diseases. Expert Opin Ther Targets. 17:363–377. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang P, Wang L and Xie XQ: Latest advances
in novel cannabinoid CB (2) ligands for drug abuse and their
therapeutic potential. Future Med Chem. 4:187–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Idris AI, van't Hof RJ, Greig IR, Ridge
SA, Baker D, Ross RA and Ralston SH: Regulation of bone mass, bone
loss and osteoclast activity by cannabinoid receptors. Nat Med.
11:774–779. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ofek O, Karsak M, Leclerc N, Fogel M,
Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, et
al: Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc
Natl Acad Sci USA. 103:pp. 696–701. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sophocleous A, Landao-Bassonga E, Van't
Hof R, Ralston S and Idris A: The type 2 cannabinoid receptors
(CB2) protects against age-related osteoporosis by affecting bone
formation and CB2 agonists exhibit anabolic activity in vivo. Bone.
44:S2192009. View Article : Google Scholar
|
|
9
|
Sun YX, Xu AH, Yang Y, Zhang JX and Yu AW:
Activation of cannabinoid receptor 2 enhances osteogenic
differentiation of bone marrow derived mesenchymal stem cells.
Biomed Res Int. 2015:8749822015.PubMed/NCBI
|
|
10
|
Idris AI, Sophocleous A, Landao-Bassonga
E, Canals M, Milligan G, Baker D, van't Hof RJ and Ralston SH:
Cannabinoid receptor type 1 protects against age-related
osteoporosis by regulating osteoblast and adipocyte differentiation
in marrow stromal cells. Cell Metab. 10:139–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernandes H, Dechering K, Van Someren E,
Steeghs I, Apotheker M, Leusink A, Bank R, Janeczek K, Van
Blitterswijk C and de Boer J: The role of collagen crosslinking in
differentiation of human mesenchymal stem cells and MC3T3-E1 cells.
Tissue Eng Part A. 15:3857–3867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu L, Wu Y, Lin Y, Jing W, Nie X, Qiao J,
Liu L, Tang W and Tian W: Osteogenic differentiation of adipose
derived stem cells promoted by overexpression of osterix. Mol Cell
Biochem. 301:83–92. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giusti A and Bianchi G: Treatment of
primary osteoporosis in men. Clin Interv Aging. 10:105–115.
2014.PubMed/NCBI
|
|
15
|
Maruotti N, Corrado A, Neve A and
Cantatore FP: Bisphosphonates: Effects on osteoblast. Eur J Clin
Pharmacol. 68:1013–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russell RG: Bisphosphonates: The first 40
years. Bone. 49:2–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rudic JS, Poropat G, Krstic MN, Bjelakovic
G and Gluud C: Hormone replacement for osteoporosis in women with
primary biliary cirrhosis. Cochrane Database Syst Rev: CD009146.
2011. View Article : Google Scholar
|
|
18
|
Montagnani A and Gonnelli S: Antidiabetic
therapy effects on bone metabolism and fracture risk. Diabetes Obes
Metab. 15:784–791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewiecki EM: Current and emerging
pharmacologic therapies for the management of postmenopausal
osteoporosis. J Womens Health (Larchmt). 18:1615–1626. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dore-Duffy P: Pericytes: Pluripotent cells
of the blood brain barrier. Curr Pharm Des. 14:1581–1593. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sousa-Victor P, Garcia-Prat L, Serrano AL,
Perdiguero E and Muñoz-Cánoves P: Muscle stem cell aging:
Regulation and rejuvenation. Trends Endocrinol Metab. 26:287–296.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carrió E and Suelves M: DNA methylation
dynamics in muscle development and disease. Front Aging Neurosci.
7:192015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramirez SH, Haskó J, Skuba A, Fan S,
Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A,
Zhang M, et al: Activation of cannabinoid receptor 2 attenuates
leukocyte-endothelial cell interactions and blood-brain barrier
dysfunction under inflammatory conditions. J Neurosci.
32:4004–4016. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adhikary S, Li H, Heller J, Skarica M,
Zhang M, Ganea D and Tuma RF: Modulation of inflammatory responses
by a cannabinoid-2-selective agonist after spinal cord injury. J
Neurotrauma. 28:2417–2427. 2011. View Article : Google Scholar : PubMed/NCBI
|