Introduction
Gastric cancer is the third leading cause of
cancer-associated mortality worldwide, accounting for ~1,000,000
new cases and 738,000 mortalities each year, with a fatality rate
of 0.75 (1–3). Despite advancements in diagnostic
methods, gastric cancer is still frequently diagnosed at a
relatively advanced stage, where patients have a median survival of
<1 year (4). The incidence of
gastric cancer is affected by ethnic and geographical factors; the
incidence of gastric cancer is higher in Eastern Europe, Eastern
Asia and South America compared with North America and Africa
(5). Chemotherapy is the primary
treatment for patients with gastric cancer; however, the majority
of the patients will suffer from recurrence (6,7).
Therefore, it is important to identify novel biomarkers for the
early diagnosis of gastric cancer, in addition to novel therapeutic
candidates to improve the efficacy of gastric cancer treatment.
Long non-coding (Lnc)RNAs are a class of RNAs that
are >200 nucleotides in length and do not encode proteins due to
a lack of open reading frames (8,9).
According to the GENCODE project analysis (version 23), 27,817
transcripts from 15,931 genes were identified as LncRNAs (10). Accumulating evidence has demonstrated
the significant roles served by LncRNAs in various homeostatic
processes, including cellular differentiation (11), gene imprinting (12) and tumorigenesis (13). In addition, the aberrant expression
of LncRNAs in multiple tissues is frequently associated with
tumorigenesis. Recently, the roles of certain LncRNAs in the
tumorigenesis of gastric cancer were elucidated. For example, the
LncRNA ZNFX1 antisense RNA 1 was reported to markedly promote
gastric cancer cell proliferation and metastasis by epigenetically
repressing the expression of Kruppel like factor 2 and naked
cuticle homolog 2 (14). The LncRNA
RNA component of mitochondrial RNA processing endoribonuclease was
demonstrated to increase gastric carcinogenesis by acting as a
microRNA-206 regulator and is therefore a novel therapeutic target
for the treatment of gastric cancer (15). However, the underlying molecular
mechanisms of how these LncRNAs regulate tumorigenesis remains
unknown, whilst the effects of other LncRNAs remain unclear.
Mediator of DNA damage checkpoint protein 1 (MDC1)
serves a key role in the repair of DNA double-strand breaks and
acts as a tumor suppressor in bladder cancer (16). The antisense transcript of MDC1,
MDC1-antisense RNA (MDC1-AS), was recently identified as a novel
LncRNA in bladder cancer (16).
Another study demonstrated that the relative transcript level of
MDC1-AS was decreased in bladder cancer and glioma (17). In addition, overexpression of MDC1-AS
promoted human glioma cell proliferation and shifted the cell cycle
in an MDC1-dependent manner (17).
However, the molecular mechanism underlying this effect and the
role of MDC1-AS in gastric cancer remains unclear.
The present study examined the expression of MDC1-AS
in gastric cancer in vivo and in vitro. The
overexpression of MDC1-AS in poorly differentiated MKN28 cells
inhibited, whereas knockdown of MDC1-AS in well-differentiated
MKN45 cells increased the cellular proliferation rate and
metastatic potential. Alteration of the expression of MDC1 relieved
this inhibitory effect of MDC1-AS in gastric cancer cells. The
results of the present study revealed the oncogenic potential of
MDC1-AS in human gastric cancer, indicating that MDC1-AS may serve
as a novel therapeutic target for the diagnosis and treatment of
gastric cancer in the clinic.
Materials and methods
Human samples
A total of 80 patients (mean age, 50±5, male:female,
51:29) with gastric cancer were recruited from Shenzhen Second
People's Hospital (Shenzhen, China) between May 2014 to May 2015.
No patients had received radiotherapy or chemotherapy prior to
surgery. Written consent was obtained from each patient. Tumor
tissues and adjacent non-cancerous tissues were collected during
the gastric tumor resection, immediately frozen in liquid nitrogen
and stored until required for analysis. None of the patients
received radiotherapy or chemotherapy prior to surgery. The present
study was approved by the Ethics Committee of Shenzhen Second
People's Hospital.
Cell culture and transfection
The rat normal gastric epithelial cell RGM-1, and
the gastric cancer cell lines KATO III, SGC-7901 and AGS were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The gastric cancer cell lines MN45 and MKN28 were purchased
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cell line MKN28 is known to be an MKN74
derivative, which is also a gastric adenocarcinoma cell line
(17). All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C in a humidified incubator with 5%
CO2. Plasmid, short hairpin (sh)RNA and small
interfering (si)RNA transfection was conducted using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. pcDNA 3.1 vector with
MDC1-AS and MDC1 overexpression were established by our lab using
the polymerase chain reaction (PCR). shRNAs and siRNAs were
designed and synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). Sequences were not released by the company.
Plasmids and sh/siRNAs were dissolved in distilled water to produce
a stock solution.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR analysis
RNAs from the tissue samples and cultured cells were
extracted using TRIzol reagent (Takara Biotechnology Co., Ltd.,
Dalian, China) in an RNase free atmosphere according to the
manufacturer's protocol. cDNA was then reverse transcribed using a
Takara Biotechnology Co., Ltd. kit (Transcriptor First Strand cDNA
Synthesis kit) according to the manufacturers's instructions (37°C
for 15 min and 85°C for 5 sec). qPCR analysis with SybrGreen
reagent (Takara Bio, Inc., Otsu, Japan) was performed with the
following primers: MDC1-AS forward, 5′-TCCCAGATGTGCCAAAGTCAG-3′ and
reverse, 5′-AGCAACCCCAGTTGTCATTC-3′; MDC1 forward,
5′-GCAGCTTCCAGACAACAG-3′ and reverse, 5′-TACCCATGACTTTATCCACA-3′;
and GAPDH forward, 5′-AAGGTGAAGGTCGGAGTCAAC-3′ and reverse,
5′-GGGGTCATTGATGGCAACAATA-3′. GAPDH was used as an internal
control. The thermocycling conditions were as follows: 95°C for 5
min, followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec.
The expression of MDC1-AS and MDC1 was quantified using the
2−∆∆Cq method (18).
Cell proliferation assay
Cell proliferation was determined by
bromodeoxyuridine (BrdU) incorporation (EMD Millipore, Billerica,
MA, USA). Briefly, a total of 1×103 MKN28 cells were
transfected with the MDC1-AS overexpressing plasmid with or without
siRNA targeting MDC1 (siMDC1), and MKN45 cells were transfected
with shRNA directed against MDC1-AS in the presence or absence of
an MDC1 expression plasmid in a 6-well plate. The cells were then
cultured for 48 h at 37°C. Then, the cells were washed and the DMEM
was replaced with serum-free media for 24 h. Subsequently, the
cells were washed with PBS, trypsinized for ~3 sec at 37°C and
collected by centrifugation at 850 g for 5 min. Equal numbers of
MKN28 or MKN45 cells (5,000) from each group were then seeded into
96-well plates and incubated in DMEM supplemented with 10% FBS and
10 µM BrdU for 30 min. BrdU incorporation was detected with
additional peroxidase substrates according to the manufacturer's
protocols. The absorbance of the wells at a wavelength of 450 nm
was measured using a microplate reader.
Transwell migration and invasions
assays
Cell migration and invasion were explored using
Transwell® chambers (pore size, 8 µm; Corning
Incorporated, Corning, NY, USA). For the migration assay, MKN28 and
MKN45 cells were transfected and collected as described above.
Afterwards, 5×104 cells in serum-free media were seeded
into the upper chamber and 600 µl of DMEM supplemented with 10% FBS
was added into the lower chamber. After incubation for 24 h at
37°C, the cells were fixed with ice-cold methanol for 5 min and
stained with crystal violet (0.1%) at room temperature for 5 min.
The membrane was washed in water three times and cells on the upper
surface were removed using cotton swabs. Cells on the lower surface
were imaged at a magnification, ×100 and the number of cells in
five random fields of view were counted using a light microscope.
For the invasion assay, the membranes of the chambers were
pre-coated with Matrigel (Corning Incorporated) at 37°C for 6
h.
Wound healing assay
Wound-healing assays were performed by creating
identical ‘wounds’ using 10 µl sterile pipette tips. Briefly, a
total of 1×104 cells/well were seeded into 6-well plates
and co-incubated with the plasmids and/or sh/siRNAs described above
for 48 h. Afterwards, the cells were washed with PBS and a ‘wound’
was created across the center of each well. Then plates were then
washed again and fresh serum-free medium was immediately added.
After 24 h incubation at 37°C, cells were observed and imaged under
a light microscope at a magnification of ×200. Five random fields
of view were selected and the percentage of wound closure was
calculated (ImageJ Software; version 2; National Institutes of
Health; Bethesda, MD, USA.
Statistical analysis
All results are presented as the mean ± standard
derivation, unless otherwise stated. The student's t-test was used
to analyze the statistical significance of differences between
variables. All statistical analyses were performed using SPSS
software (version 18.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
MDC1-AS expression is decreased in
human gastric cancer in vivo and in vitro
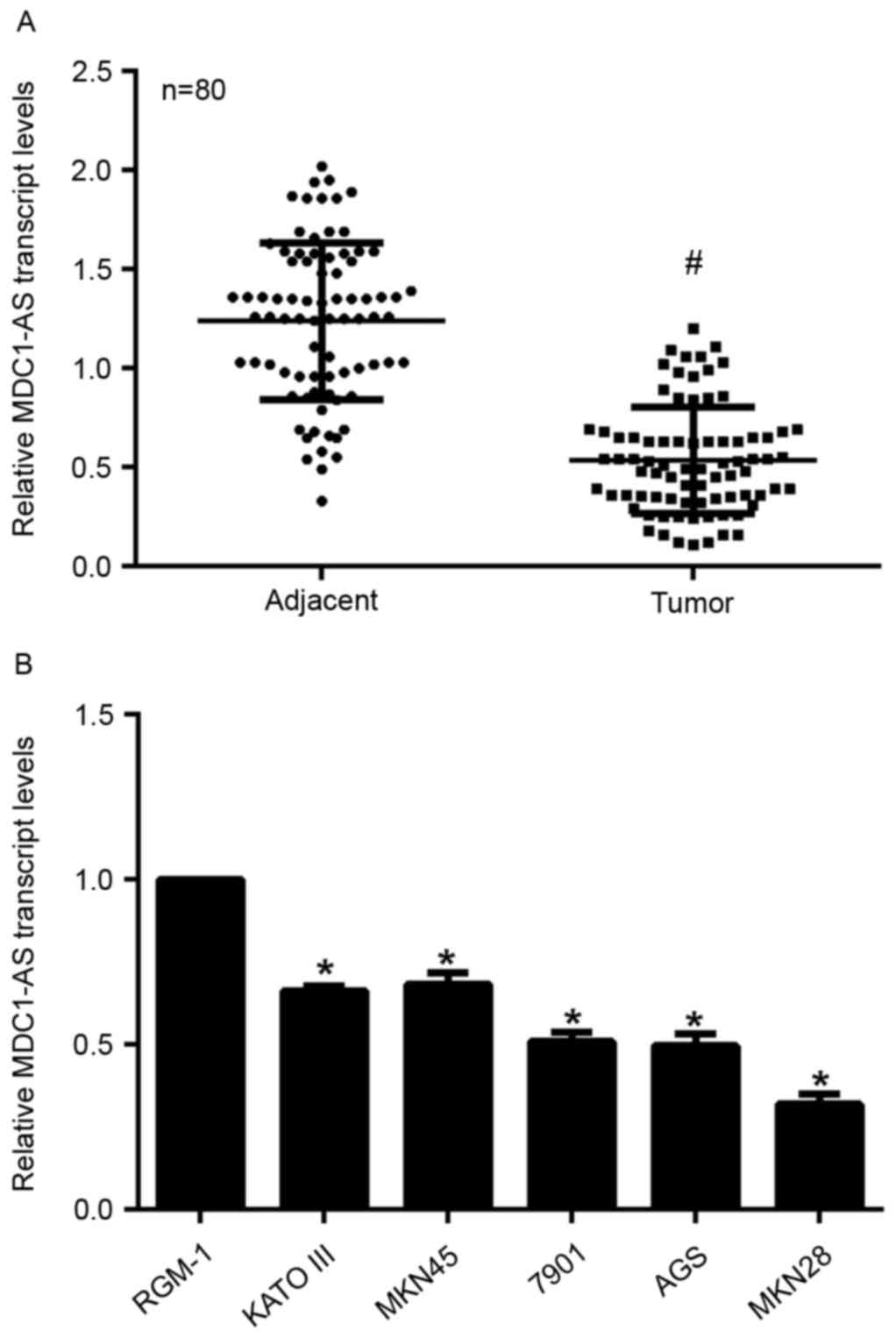
Samples from a total of 80 gastric cancer patients
were collected in the present study and subjected to RT-qPCR
analysis. As shown in Fig. 1A, the
relative transcript level of MDC1-AS in tumor tissues was
significantly decreased compared with that in their adjacent
non-cancerous tissues. RGM-1 cells are derived from normal rat
gastric epithelial tissue, while KATO III, MKN45, SGC-7901, AGS and
MKN28 cells are human gastric cancer cells with increased migration
abilities. KATO III and MKN45 cells are well differentiated,
SGC-7901 and AGS cells are moderately differentiated and MKN28
cells are poorly differentiated. The expression of MDC1-AS was
measured in these cell lines and it was identified that MDC1-AS
expression was significantly suppressed in all five of the gastric
cancer cell lines compared with the RGM-1 cells (Fig. 1B). Notably, the relative MDC1-AS
transcript level was decreased as the level of differentiation of
the cancer cells decreased. Among the five cancer cell lines, MKN28
and MKN45 cells exhibited the lowest and highest expression of
MDC1-AS, respectively (Fig. 1B).
Thus, MKN28 and MKN45 cells were selected for the subsequent
experiments. These data indicate that the expression of MDC1-AS is
suppressed in human gastric cancer cells in vivo and in
vitro.
MDC1-AS inhibits the proliferation of
human gastric cancer cells
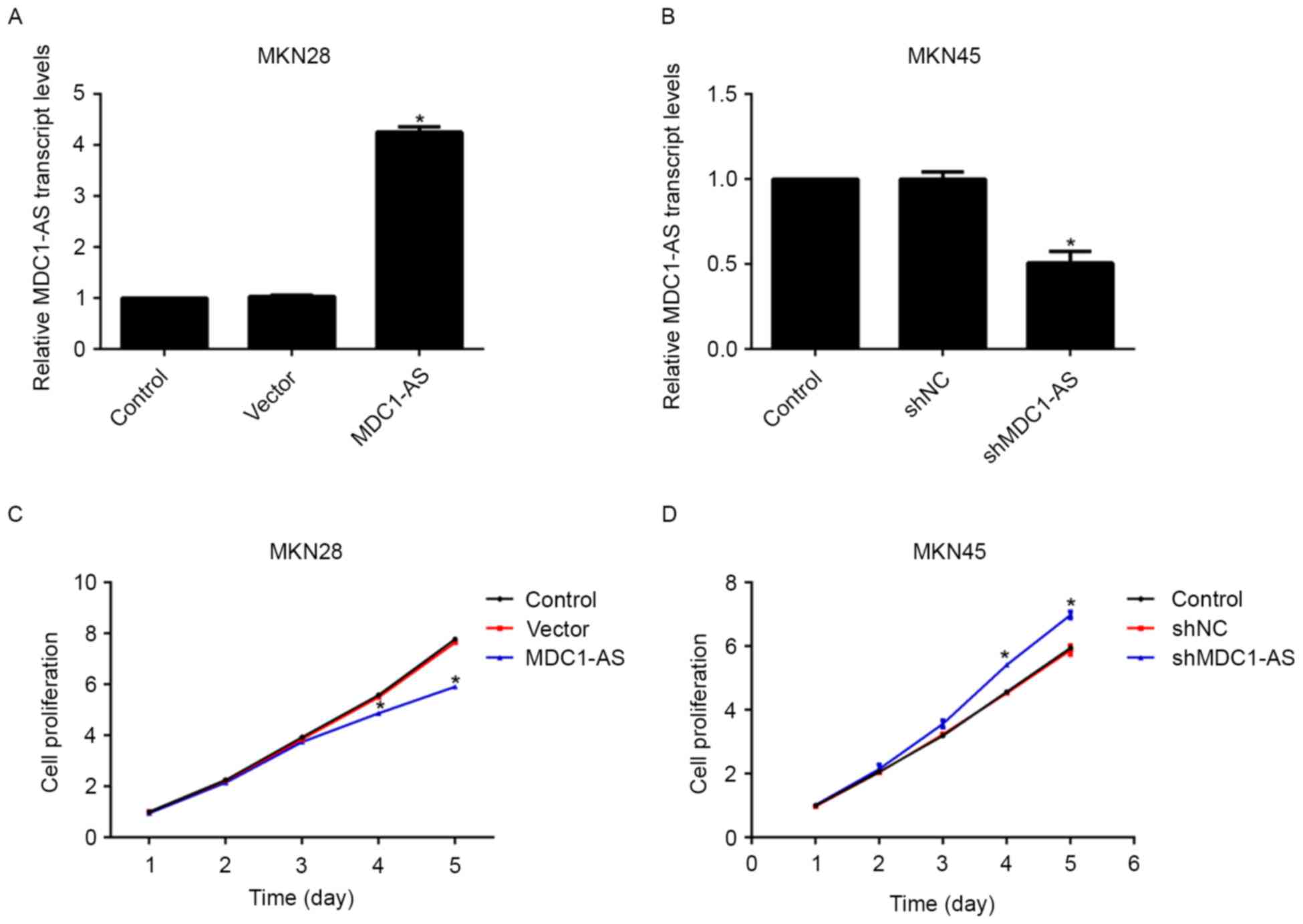
The effect of MDC1-AS on the proliferation of
gastric cancer cells was investigated in the present study. An
MDC1-AS-expressing plasmid and shRNA targeting MDC1-AS were
transfected into MKN28 and MKN45 cells, respectively. Upon plasmid
treatment, the relative transcript level of MDC1-AS in the MKN28
cells was significantly increased by 4-fold compared with the
control group (Fig. 2A). The
expression of MDC1-AS in MKN45 cells was suppressed to 50% of the
preliminary baseline after shRNA treatment (Fig. 2B). Afterwards, the effect of MDC1-AS
on cell proliferation was examined using cell viability assays. No
significant difference in cell proliferation was observed between
the three groups of MKN28 and MKN45 cell lines in the 3 days
following plasmid or shRNA transfection (Fig. 2C and D). However, the proliferative
rate of MKN28 cells was significantly inhibited by 13% on day 4 and
24% on the day 5 after transfection with the MDC1-AS-expressing
plasmid compared with the control cells (Fig. 2C). Conversely, MKN45 cell
proliferation was increased significantly by 16 and 15% on the days
4 and 5, respectively, after shMDC1-AS transfection (Fig. 2D). These results suggest that MDC1-AS
suppresses the proliferation of human gastric cancer cells.
MDC1-AS inhibits the metastasis of
human gastric cancer cells
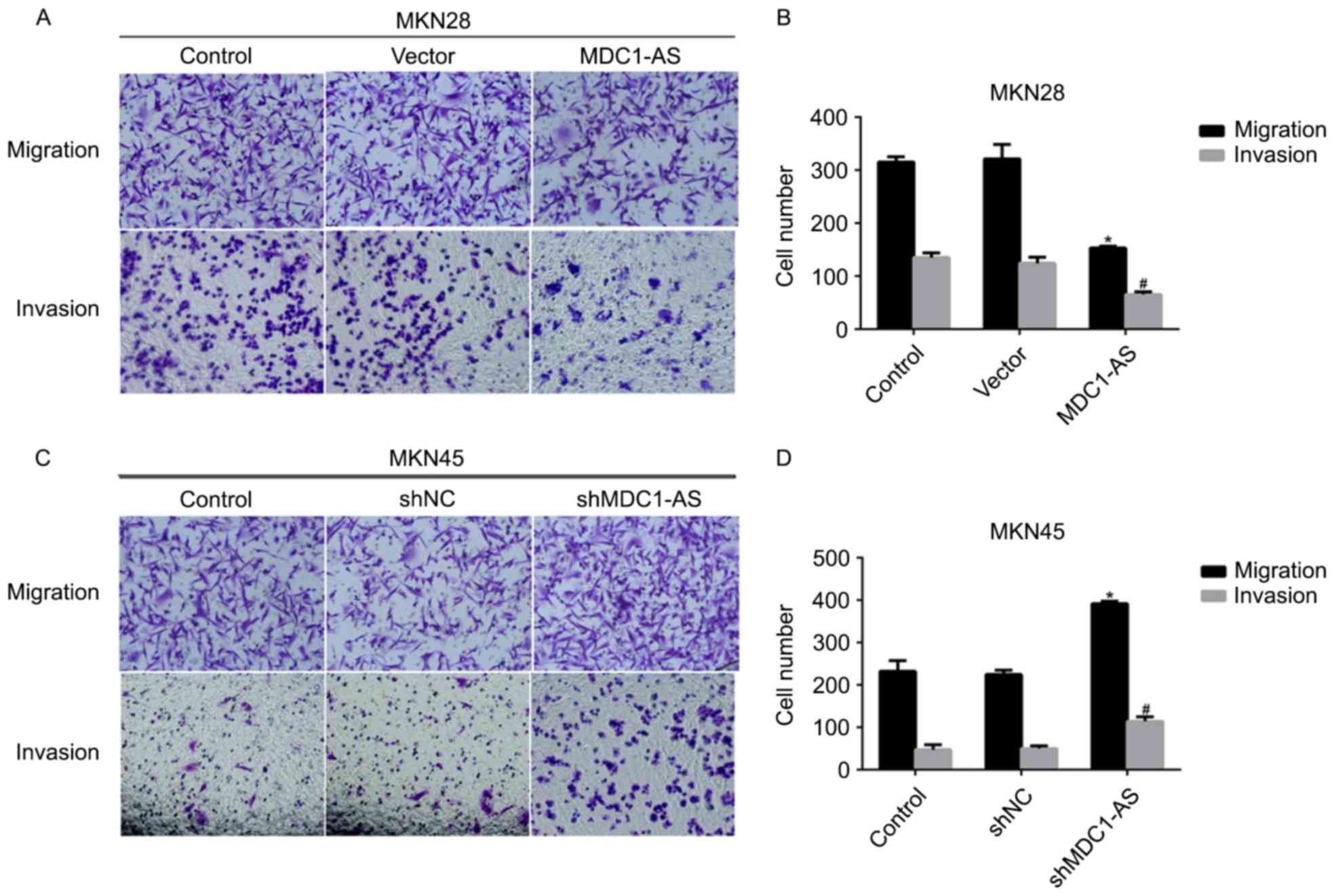
Cell proliferation and metastasis are hallmarks of
cancer, including gastric cancer. Therefore, the role of MDC1-AS in
gastric cancer cell metastasis was investigated with Transwell
assays. MKN28 cells were transfected with an MDC1-AS-expressing
plasmid and MKN45 cells were treated with shRNA directed against
MDC1-AS. As shown in Fig. 3A and B,
after transfection MKN28 cell migration and invasion was
significantly decreased compared with the control. Specifically,
~300 MKN28 cells were observed on the lower surface in the cell
migration assays in the control group; however, only ~150 cells
successfully migrated through the membrane in the group transfected
with the MDC1-AS-expressing plasmid (Fig. 3B). Likewise, invasive potential was
inhibited by >50% in the MDC1-AS plasmid treatment group
compared with the control group in MKN28 cells (Fig. 3B). A similar phenomenon was observed
in MKN45 cells (Fig. 3C and D).
Transfection of shMDC1-AS significantly increased cell migration by
43% and cell invasion by 60% in MKN45 cells (Fig. 3D). These results indicate that
MDC1-AS inhibits the metastasis of human gastric cancer cells.
Knockdown of MDC1 relieves the
inhibitory effect of MDC1-AS on human gastric cancer cell
proliferation
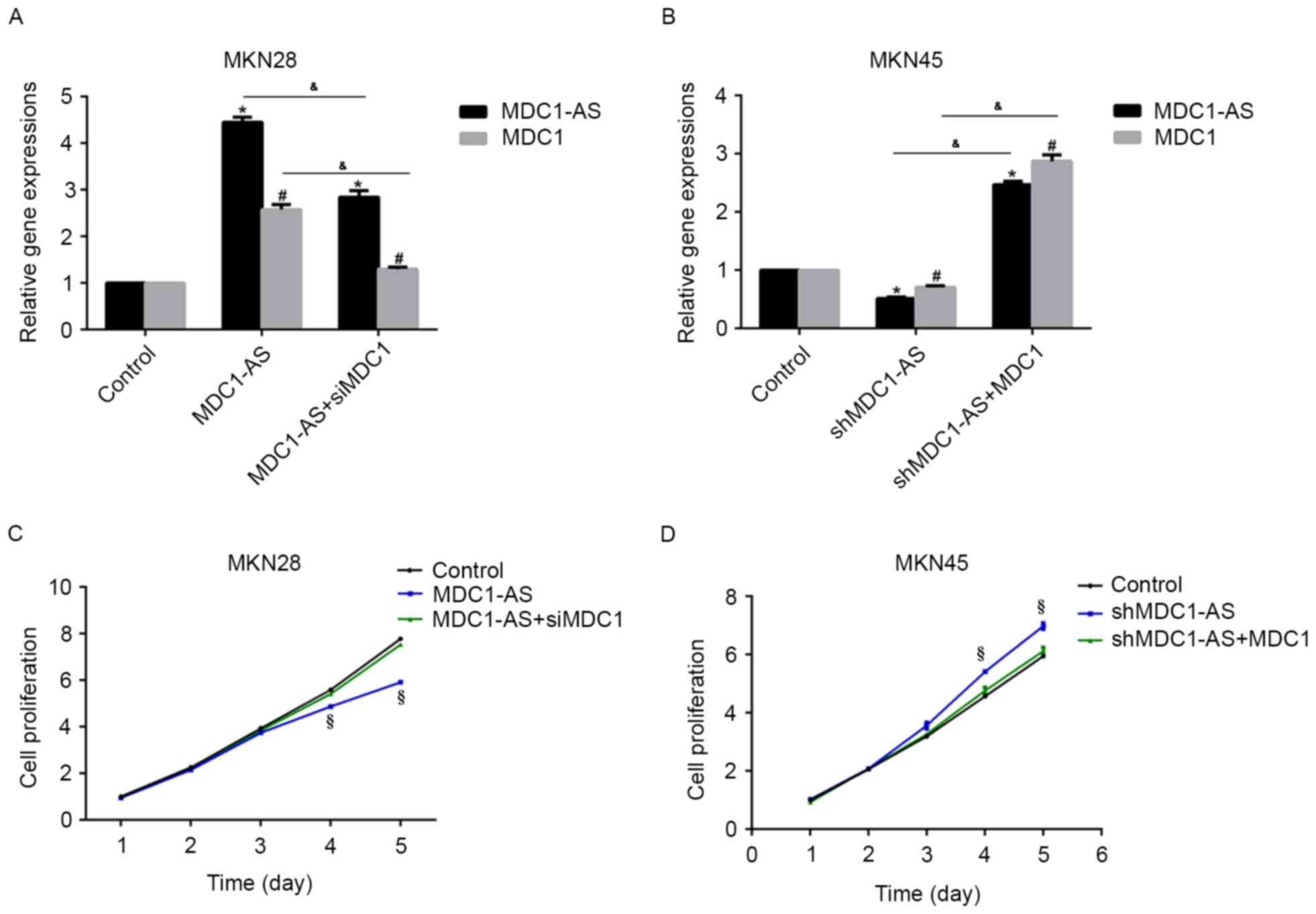
The expression of MDC1 has been reported to involved
in the regulation of MDC1-AS in human bladder cancer (16); therefore, the present study examined
the effect of MDC1 on the expression of MDC1-AS in human gastric
cancer cells. Firstly, MKN28 cells treated with the
MDC1-AS-expressing plasmid were transfected with siMDC1, and
shMDC1-AS-treated MKN45 cells were transfected with an
MDC1-expressing plasmid, after which RT-qPCR analysis was
performed. As shown in Fig. 4A,
MDC1-AS plasmid transfection significantly increased MDC1-AS and
MDC1 mRNA levels compared with the control group, whereas
additional siMDC1 treatment significantly suppressed MDC1-AS and
MDC1 mRNA expression in MKN28 cells. Likewise, the mRNA expression
of MDC1-AS and MDC1 in MN45 cells was significantly suppressed by
shMDC1-AS transfection compared with the control group, whereas
additional treatment with an MDC1-expressing plasmid significantly
upregulated MDC1-AS and MDC1 mRNA expression compared with the
control (Fig. 4B). These results
indicate a potential interaction between MDC1-AS and MDC1.
Subsequently, the cell proliferation rate was
investigated in these cells. The inhibitory effect of the
MDC1-AS-expressing plasmid on MKN28 cell proliferation was
attenuated by siMDC1 treatment (Fig.
4C). Similarly, the stimulating effect of shMCD1-AS on the
proliferation of MKN45 cells was inhibited by co-transfection with
the MDC1-expressing plasmid (Fig.
4D). These data suggest that MDC1-AS inhibits human gastric
cancer cell proliferation through an interaction with MDC1.
Knockdown of MDC1 abolishes the
suppressive effect of MDC1-AS on cell metastasis in human gastric
cancer cells
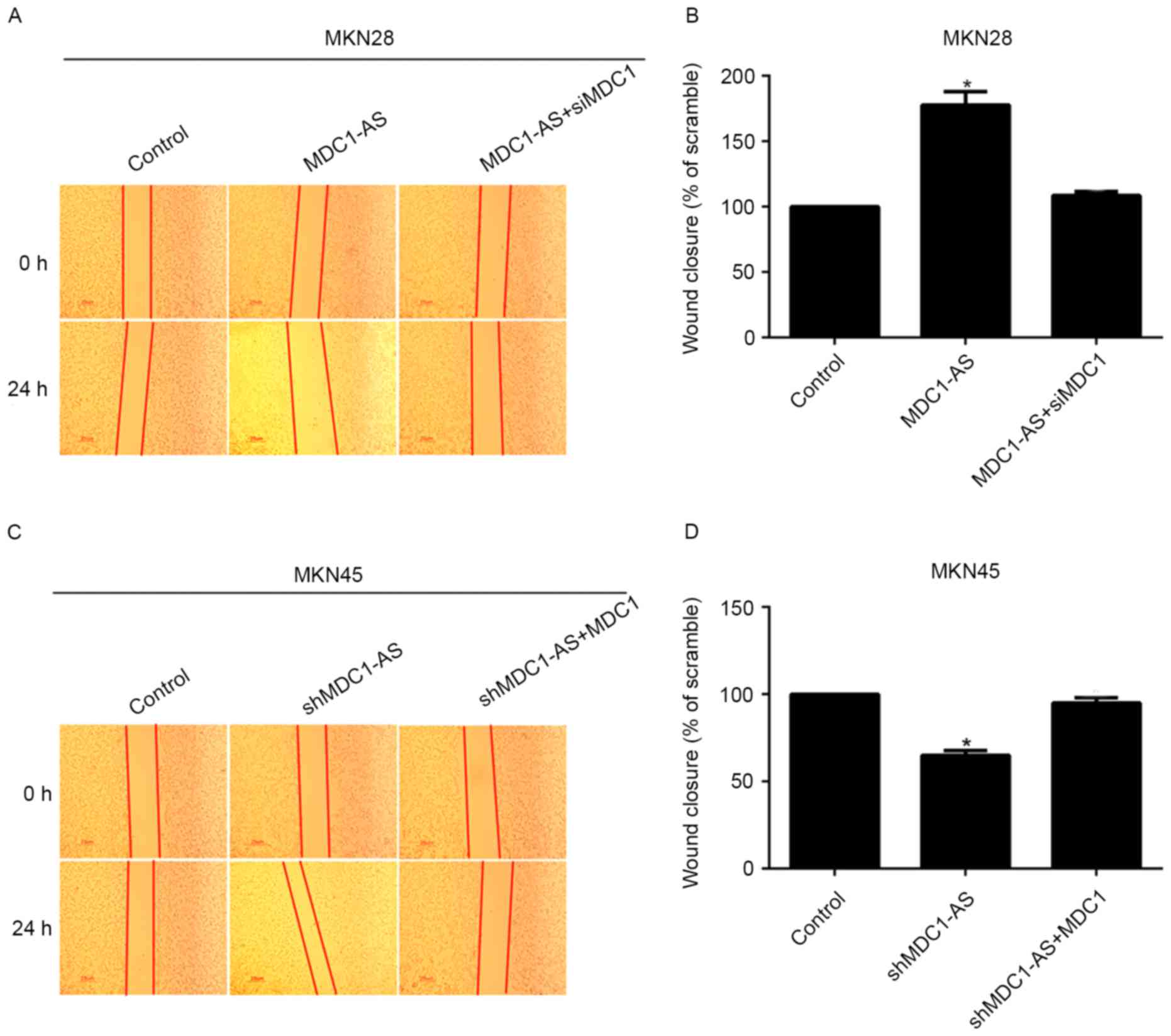
The effect of MDC1 and MDC1-AS on human gastric
cancer cell metastasis was investigated using wound healing assays.
This revealed that wound closure was significantly increased by 70%
when MKN28 cells were treated with the MDC1-AS-expressing plasmid
compared with the control, whereas the percentage of wound closure
was decreased to control levels after co-transfection with siMDC1
(Fig. 5A and B). Likewise, the
knockdown of MDC1-AS significantly decreased wound closure in MKN45
cells; however, additional treatment with an MDC1-expressing
plasmid increased the percentage of wound closure to a similar
level as the control (Fig. 5C and
D). These data suggest that the inhibitory effect of MDC1-AS on
cell metastasis is dependent upon the expression of MDC1 in human
gastric cancer cells.
Discussion
Gastric cancer is primarily caused by infection with
the bacterium Helicobacter pylori, which accounts for ~60%
of gastric cancer cases (19);
however, the risk of developing gastric cancer is also affected by
genetic and geographic factors (19). Gastric cancer can metastasize to
other parts of the body, including the liver, bones and lymph nodes
(20), which makes it difficult to
cure since the majority of patients are diagnosed at an advanced
stage (21). Therefore, novel
strategies to identify gastric cancer at an early stage are
required.
MDC1 is a key component of the DNA damage response
machinery that is involved in the early cellular response to DNA
damage in order to protect genome integrity (22). MDC1 serves an important role in the
process of cell death or survival after DNA damage through
regulating cellular tumor antigen p53 (23). The role of MDC1 in carcinogenesis is
thus an area of interest and has been widely studied recently
(16). LncRNAs can be classified by
their location as follows: Intergenic, intronic, antisense and
enhancer LncRNAs (24). Antisense
LncRNAs are transcripts encoded on the antisense strand of DNA
(25). MDC1-AS is the antisense
LncRNA of MDC1, and has been demonstrated to regulate the role of
MDC1 in bladder cancer (16) and
glioma (17).
The present study revealed that MDC1-AS had an
inhibitory effect on gastric tumorigenesis, which was dependent
upon the expression of MDC1. In poorly differentiated MKN28 cells,
the transfection of an MDC1-AS expression plasmid suppressed cell
proliferation and metastasis; however, when the cells were
co-transfected with siMDC1 this effect was inhibited, indicating
that the effect of MDC1-AS is dependent upon MDC1. This hypothesis
was also verified in the well-differentiated gastric cancer cell
line MKN45. Knockdown of MDC1-AS in MKN45 cells increased
proliferation and metastasis, while co-transfection with an
MDC-1-expressing plasmid inhibited this effect. However, the exact
molecular mechanisms by which MDC1 affects the function of MDC1-AS
requires further study.
In conclusion, the present study demonstrated that
the expression of the LncRNA MDC1-AS was suppressed in human
gastric cancer cells in vitro and in vivo. In
addition, the overexpression of MDC1-AS in MKN28 cells inhibited
proliferation, migration and invasion, while the knockdown of
MDC1-AS in MKN45 cells promoted these hallmarks of cancer. This
inhibitory effect of MDC1-AS on tumorigenesis was identified to be
MDC1-dependent. These findings indicate that MDC1-AS is a tumor
suppressor, which may provide new directions for the diagnosis and
treatment of gastric cancer in the clinic.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Okines AF and Ashley S:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 362:858–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li R, Zhu H and Luo Y: Understanding the
functions of long non-coding RNAs through their higher-order
structures. Int J Mol Sci. 17:pii: E7022016. View Article : Google Scholar
|
|
9
|
Qi P, Zhou XY and Du X: Circulating long
non-coding RNAs in cancer: Current status and future perspectives.
Mol Cancer. 15:392016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harrow J, Frankish A, Gonzalez JM,
Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa
A, Searle S, et al: GENCODE: The reference human genome annotation
for the ENCODE project. Genome Res. 22:1760–1774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pauli A, Rinn JL and Schier AF: Non-coding
RNAs as regulators of embryogenesis. Nat Rev Genet. 12:136–149.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi P, Zhou XY and Du X: Circulating long
non-coding RNAs in cancer: Current status and future perspectives.
Mol Cancer. 15:392016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie F, Yu X, Huang M, Wang Y, Xie M, Ma H,
Wang Z, De W and Sun M: Long noncoding RNA ZFAS1 promotes gastric
cancer cells proliferation by epigenetically repressing KLF2 and
NKD2 expression. Oncotarget. 8:38227–38238. 2017.PubMed/NCBI
|
|
15
|
Shao Y, Ye M, Li Q, Sun W, Ye G, Zhang X,
Yang Y, Xiao B and Guo J: LncRNA-RMRP promotes carcinogenesis by
acting as a miR-206 sponge and is used as a novel biomarker for
gastric cancer. Oncotarget. 7:37812–37824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue Y, Ma G, Zhang Z, Hua Q, Chu H, Tong
N, Yuan L, Qin C, Yin C, Zhang Z and Wang M: A novel antisense long
noncoding RNA regulates the expression of MDC1 in bladder cancer.
Oncotarget. 6:484–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yue H, Zhu J, Xie S, Li F and Xu Q:
MDC1-AS, an antisense long noncoding RNA, regulates cell
proliferation of glioma. Biomed Pharmacother. 81:203–209. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang AH and Parsonnet J: Role of bacteria
in oncogenesis. Clin Microbiol Rev. 23:837–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruddon RW: Introduction to the molecular
biology of cancer: Translation to the clinic. Prog Mol Biol Transl
Sci. 95:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montenegro RC, Clark PG, Howarth A, Wan X,
Ceroni A, Siejka P, Nunez-Alonso GA, Monteiro O, Roger C, Gamble V,
et al: BET inhibition as a new strategy for the treatment of
gastric cancer. Oncotarget. 7:43997–44012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart GS, Wang B, Bignell CR, Taylor AM
and Elledge SJ: MDC1 is a mediator of the mammalian DNA damage
checkpoint. Nature. 421:961–966. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakanishi M, Ozaki T, Yamamoto H, Hanamoto
T, Kikuchi H, Furuya K, Asaka M, Delia D and Nakagawara A:
NFBD1/MDC1 associates with p53 and regulates its function at the
crossroad between cell survival and death in response to DNA
damage. J Biol Chem. 282:22993–23004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morris KV and Vogt PK: Long antisense
non-coding RNAs and their role in transcription and oncogenesis.
Cell Cycle. 9:2544–2547. 2010. View Article : Google Scholar : PubMed/NCBI
|