Introduction
In an aging society, hip, knee and shoulder
replacement surgeries, as well as vertebrae fusion in spinal
surgeries, are increasing in prevalence. Bone graft is essential,
particularly for fusion in spinal surgery (1,2) and
augmentation in revision arthroplasty (3–5), Bone
grafting consists of filling the bone defect with a material to
support new bone formation, and enhance healing and successful
fusion of defects or nonunions (6,7).
Autograft, allograft and synthetic ceramics are used
as materials for bone grafting. Iliac crest bone autograft is
considered the gold standard for orthopedic surgery (6–8), as a
sufficient amount of cancellous bone obtained from the pelvis
exhibits all the desired properties of osteoconduction,
osteoinduction and osteogenicity. Furthermore, the use of
autologous bone has merits, including a lack of ethical issues, no
concerns for disease transmission and no risk of immunogenicity.
However, the autograft procedure requires an additional surgery to
collect the bone for grafting, which may lead to complications such
as paresthesia, long-lasting pain, hematoma and infection (9,10).
Allograft bone is harvested from tissue donors.
Compared with the bone graft substitutes (synthetic ceramics) in
use today, allograft bone is relatively inexpensive and readily
available. Furthermore, it retains substantial structural strength
and carries no associated donor site morbidity. However, the
incorporation of allograft into the host bone is slower and less
complete compared with autograft (11). In addition, there is a theoretical
risk of disease transmission and immune rejection (10).
Synthetic bone graft substitutes are osteoconductive
agents that consist of hydroxyapatite and β-tricalcium phosphate
(β-TCP), or a combination of these materials (12,13).
Hydroxyapatite is a naturally occurring mineral form of calcium
apatite with the formula
Ca5(PO4)3(OH), and possesses the
properties of biocompatibility and osteoconductivity (14,15).
However, it has various disadvantages, such as remaining in the
body for a long time and showing no progressive bone formation in
the course of bone tissue repair (16). By contrast, β-TCP is a synthetic
porous ceramic graft material composed of tricalcium phosphate
[TCP; Ca3(PO4)2,], which comprises
70% of human bone (17–21). During the bone remodeling process,
β-TCP is gradually degraded by osteoclastic resorption and finally
replaced with mature host bone (22,23).
Thus, β-TCP is a highly biocompatible material that provides a
resorbable interlocking network within a bone defect (24). However, β-TCP must be protected from
excessive loading forces until a solid fusion has taken place, due
to its brittle structure and low tensile strength (23,25–27).
Parathyroid hormone (PTH) is a peptide hormone
consisting of 84 amino acids that is involved in calcium
homeostasis; its secretion from the parathyroid gland is mainly
controlled by serum Ca2+ through negative feedback
(28). Teriparatide is a
biologically active fragment containing the N-terminal 34 amino
acids of human parathyroid hormone (hPTH), and daily and weekly
teriparatide administration has been approved for the treatment of
osteoporosis (29,30). When teriparatide is administered
intermittently, it has an anabolic effect on osteoblasts, thereby
stimulating bone formation and increasing bone mineral density
(31,32). In 1999, Andreassen et al
(33) reported the efficacy of
intermittent administration of teriparatide in a rat tibial
fracture model. Teriparatide was demonstrated to promote bone
formation by increasing the number and activity of osteoblasts,
enhancing the mean cortical thickness and trabecula volume, and
improving bone microarchitecture, thereby increasing fracture
strength and callus quantity. Similarly, intermittent
administration of teriparatide has previously been demonstrated to
increase the volume, stiffness, torsional strength and density of
fracture calluses in a rat femur fracture model (34,35).
These observations suggest that teriparatide enhances and
accelerates not only osteogenesis but also bone remodeling in the
process of bone repair (36,37).
The aim of the present study was to investigate
whether intermittent administration of teriparatide with low
frequency (three times per week) enhances the remodeling of bone
defects grafted with β-TCP, using a rabbit bone defect model, based
on radiographic and histological examinations, and mechanical
testing.
Materials and methods
Preparation of β-TCP
In the present study, two types of β-TCP granules
with a total porosity of 67% (KG-2) and 75% (KG-3) were supplied by
HOYA Corporation (Tokyo, Japan). β-TCP granules contain three types
of pores: Macropores (diameter, 100–300 µm), interconnected pores
(diameter, 50–100 µm) and micropores (diameter, 0.5–10 µm). β-TCP
slurry was produced by HOYA Corporation by mixing dibasic calcium
phosphate and calcium carbonate, and milled with water (quantities
not known); the slurry was subsequently sintered and dried to
produce β-TCP powder, which was burnt at 1,100°C with surfactant
and bubbled stabilizer (types not known) to produce β-TCP granules
containing three types of pores (macropore, interconnected pore and
micropore). The compressive strength is 15 MPa for 67% porosity and
1.5 MPa for 75% porosity.
Teriparatide
Teriparatide, also known as hPTH (1–34),
corresponds to the N-terminal part of hPTH, the full length of
which is 84 amino acids (38).
Teriparatide acetate was supplied by Asahi Kasei Pharma Corp.
(Tokyo, Japan), dissolved in 0.2% rabbit serum albumin solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and stored at
−30°C until use.
Bone defect model
The present study was approved by the Ethics
Committee of Juntendo University Institute of Casualty Center
(Juntendo University Shizuoka Hospital, Izunokuni, Japan). A total
of 60 male Japanese white rabbits (12 weeks old; 3.5 kg; Japan SLC,
Inc., Hamamatsu, Japan) were divided into 10 groups: Sham group for
4 weeks; 67% β-TCP without teriparatide for 4 weeks; 67% β-TCP with
teriparatide for 4 weeks; 75% β-TCP without teriparatide for 4
weeks; 75% β-TCP with teriparatide for 4 weeks; Sham group for 8
weeks; 67% β-TCP without teriparatide for 8 weeks; 67% β-TCP with
teriparatide for 8 weeks; 75% β-TCP without teriparatide for 8
weeks; and 75% β-TCP with teriparatide for 8 weeks. The rabbits
were housed separately in standard cages in a
temperature-controlled room (24±3°C and humidity of 55±15%) with a
12-h light/dark cycle, fed a commercial standard diet (NR-2;
Nisseiken Co. Ltd., Tokyo, Japan) and received drinking water ad
libitum.
Rabbits were anesthetized with an intravenous bolus
injection of 25 mg/kg sodium pentobarbital (Somnopentyl; Kyoritsu
Seiyaku Corporation, Tokyo, Japan) prior to surgery. The distal
metaphysis and lateral condyle of the femur were exposed through a
2-cm lateral longitudinal incision, and the thigh muscles were
divided under sterile conditions. The bone membrane was removed and
a dead-end defect (5 mm in diameter and 15 mm in depth) was created
in the lateral cortex just proximal to the epiphyseal plate using
an air drill with an intermittent drip of sterile saline to control
the temperature. The orientation of the bone defect was
perpendicular to the sagittal axis of the femur.
β-TCP granules (70 mg of those with 75% porosity or
80 mg of those with 67% porosity) respectively, were manually
grafted into the defected hole (5 mm in diameter and 15 mm in depth
corresponding to a volume of 294 mm3). The soft tissue
was closed in layers. All procedures were performed by the same
surgeon. Following the completion of surgery, teriparatide (40
µg/kg), or 0.2% rabbit serum albumin solution as a vehicle control,
was subcutaneously administered to rabbits in the PTH and Control
groups of animals, respectively, three times per week. In some
experiments, the bone defect was created in the distal metaphysis
and lateral condyle of the femur and no β-TCP granules were
grafted. These animals were subcutaneously injected with 0.2%
rabbit serum albumin solution three times per week and used as the
Sham group.
At 4 or 8 weeks post-surgery, rabbits were
anesthetized via the intraperitoneal injection of 50 mg/kg
pentobarbital sodium. Rabbits were sacrificed by exsanguination via
the femoral vein under deep anesthesia. Death of the animals was
confirmed by cardiac arrest and cessation of respiration. Blood
samples were harvested from the femoral vein, and the serum was
separated by centrifugation at 1,220 × g for 20 min at 4°C and
stored at −80°C. Following sacrifice, femurs was collected from
every rabbit.
In order to evaluate bone formation and remodeling
in vivo, all rabbits were subcutaneously injected twice with
calcein, a calcium-binding fluorescent dye (10 mg/kg; Wako Pure
Chemical Industries, Ltd., Osaka, Japan) on days 3 and 10 prior to
sacrifice.
Assay of Gla-osteocalcin
Serum levels of Gla-osteocalcin (Gla-OC) were
measured using an ELISA kit (Gla-OC EIA kit; Takara Bio, Inc.,
Shiga, Japan) as previously described (38,39).
Radiological analysis &
measurement of bone mineral density (BMD)
Distal femoral condyles containing graft sites were
dissected from the femurs, and X-ray images were captured using
micro computerized tomography (CT) with a SkyScan 1172 instrument
(Bruker microCT, Konitich, Belgium) to evaluate the absorption of
β-TCP and new bone formation. BMD values at the femoral condyle
graft sites were calculated from micro-CT images using Image Pro
(version 7; Media Cybernetics Inc., Rockville, MD, USA) with
reference to BMD phantom.
Histological analyses
Distal femoral condyles containing the graft sites
were fixed in 4% formaldehyde in 0.1 M phosphate buffer (pH 7.2) at
4°C for 20 h and subsequently dehydrated with 70% alcohol, embedded
in 2-hydroxyethyl methacrylate/methyl methacrylate/2-hydroxyethyl
acrylate mixed resin, and cut into 3-µm sections, as previously
reported (40–42). These sections were stained with
Giemsa. Alternatively, the sections were histochemically stained
for tartrate resistant acid phosphatase (TRAP) activity and
counterstained with hematoxylin and nuclear fast red (43). Histomorphometric analyses were
performed using BIOREVO (Keyence, Osaka, Japan), and Image Pro
(version 7), and the graft sites of femoral condyles were analyzed
in a region (0.55×2.2 mm), which was centrally aligned in a created
bone defect. The quantitative histomorphometric analysis of
trabecula remodeling was performed according to the following
stereologic calculations (44); i)
bone volume (BV)/tissue volume (TV; %); ii) mineralized surface
(MS)/bone surface[BS; %; (double labeled surface+single labeled
surface/2)/BS]; iii) mineral apposition rate (MAR; µm/day;
interlabel thickness/interlabel time); iv) bone formation rate
(BFR)/BS (µm3/µm2/year; MAR × MS/BS); v)
osteoclast number (N.Oc)/BS (N/mm); vi) osteoclast surface
(Oc.S)/BS (%); and vii) osteoid surface (OS)/BS (%). Furthermore,
the change in β-TCP volume in the graft was evaluated using the
following parameters; viii) β-TCP volume (TCPV)/TV (%); and ix)
newly formed bone volume (BV-TCPV)/TV (%).
MAR was measured by calculating calcein-labeling
under a fluorescence microscope with excitation and emission
wavelengths of 495 and 515 nm, respectively. BV/TV, MS/BS, MAR,
BFR/BS, TCPV/TV and (BV-TCPV)/TV were calculated using
Giemsa-stained sections. An osteoclast was defined as a
multinucleated giant cell in contact with the surface of a bone or
bone substitute, and N.Oc/BS and Oc.S/BS were calculated using
TRAP-stained sections.
In the Control group, histomorphometry was performed
using sections of distal femoral condyles without bone defect
obtained from unoperated femurs.
Mechanical testing
The grafts were evaluated with an axial push-out
load to failure test using MTS858 Mini Bionix2 (MTS Systems
Corporation, Eden Prairie, MN, USA) and the appropriate software
(MTS Test Star 790.00 version 4.00; MTS Systems Corporation). The
specimens were placed on a metal piston jig with a diameter of 4.0
mm, and continuous load-displacement data at a test speed of 0.5
mm/min were recorded until the graft surface was depressed to a
depth of 2.0 mm. The maximum shear stiffness (Mpa/mm) was obtained
from the slope of the linear section of the load-displacement
curve, and the total energy absorption (J/m2) was
calculated as the area under the load-displacement curve. Maximum
shear strength (MPa) was determined from the maximum force applied
until failure of the bone and artificial bone (45).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance followed by Bonferroni's multiple comparison
test (GraphPad Prism; GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
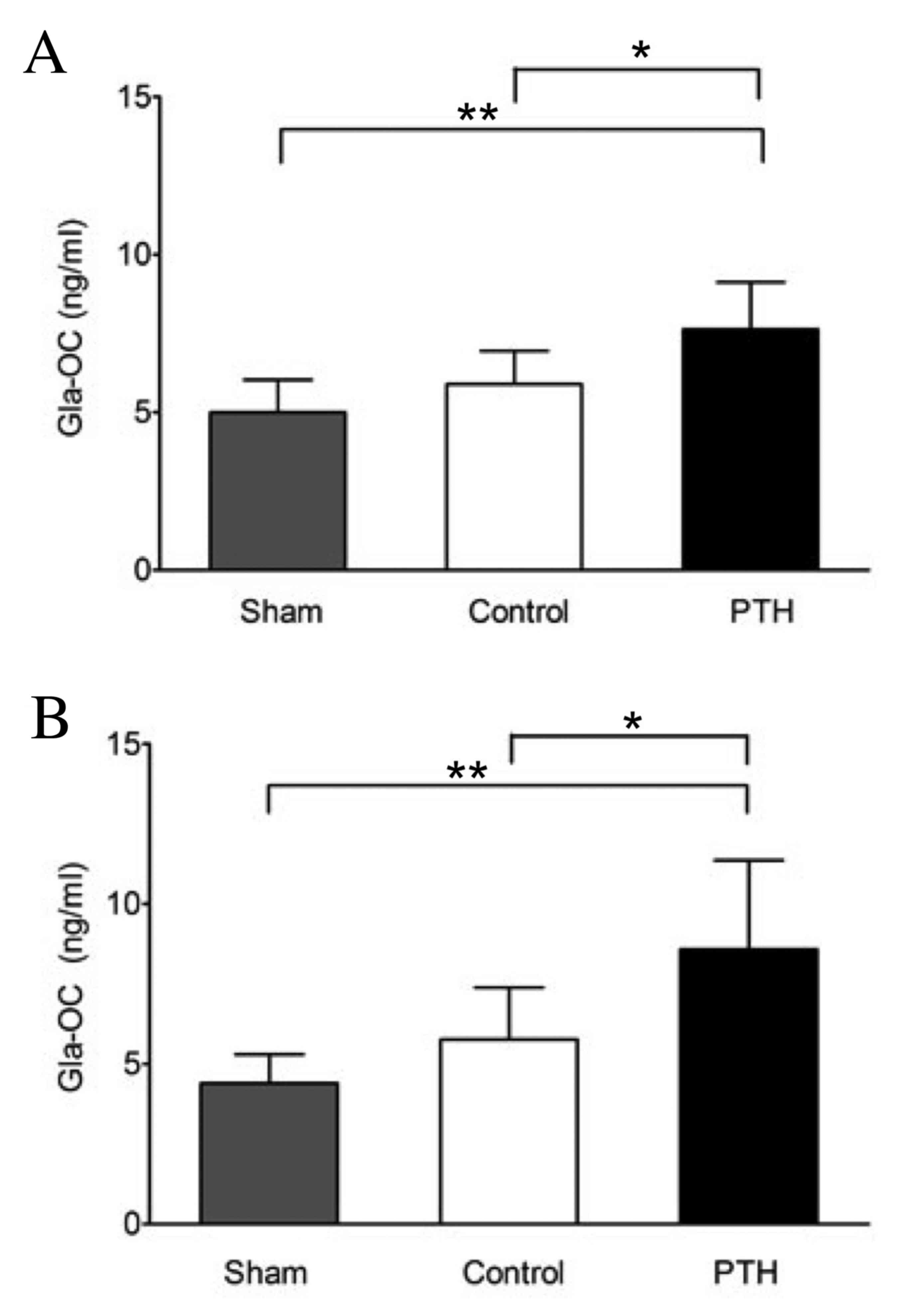
Evaluation of bone metabolism using
Gla-OC
Following grafting with β-TCP, teriparatide (40
µg/kg) or a vehicle was subcutaneously injected into rabbits for 4
or 8 weeks, and serum Gla-OC, which is a specific marker for bone
formation, was subsequently measured. The Gla-OC levels were
significantly higher in the PTH group than in the Sham (P<0.01)
and Control groups (P<0.05) at both 4 and 8 weeks (Fig. 1). By contrast, no significant
difference in Gla-OC levels was observed between the Control (with
β-TCP graft) and Sham (without β-TCP graft) groups at 4 or 8 weeks.
These observations suggest that increased levels of serum Gla-OC
may be due to teriparatide-induced bone formation but not the β-TCP
graft.
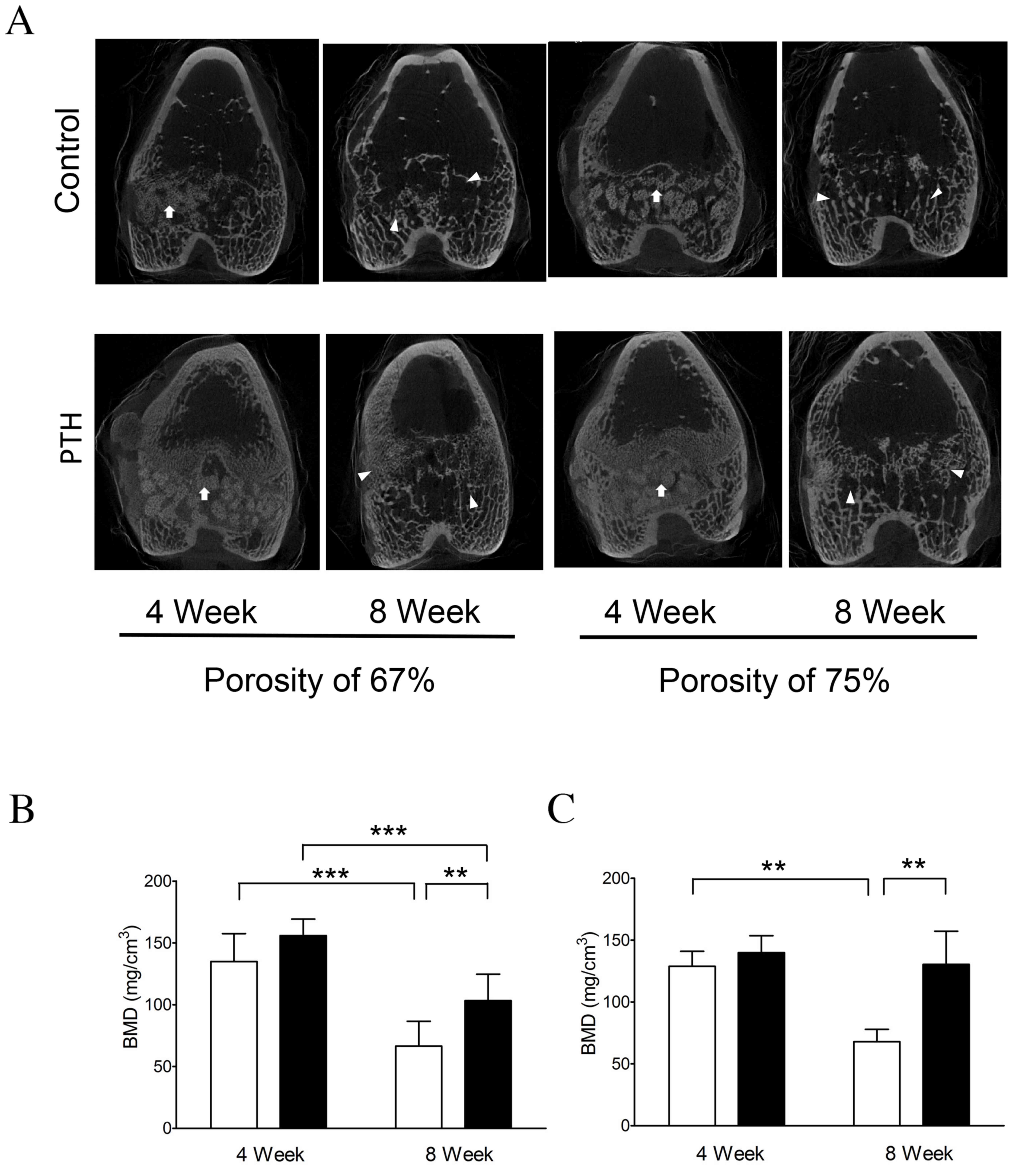
Radiological analysis &
measurement of BMD
Micro-CT analysis revealed that β-TCP was clearly
present in the grafted bone in the Control group at 4 weeks
following graft surgery; however, β-TCP was absorbed and the
granular structure was markedly increased in the grafted bone
around β-TCP granules in the PTH group for both porosities of β-TCP
(Fig. 2A).
Furthermore, micro-CT analysis indicated that, at 8
weeks, β-TCP was mostly absorbed and replaced with trabecular
structure, possibly cancellous bone, in the Control group (Fig. 2A), and the structure was notably
increased in the grafted bones with both the porosity of 67 and 75%
in the PTH group (Fig. 2A).
BMD was quantified based on the results of micro-CT
analysis. The results indicated that BMD was significantly
decreased (~50%) between 4 and 8 weeks following the graft with the
porosity of 67 (P<0.001) and 75% (P<0.01; Fig 2B) in the Control group. By contrast,
BMD was only decreased by ~30% in the PTH group following the graft
with 67% porosity, although this reduction was significant
(P<0.001; Fig. 2B). The reduction
in BMD was almost completely suppressed in the PTH group following
the graft with 75% porosity, with no significant differences in BMD
observed between weeks 4 and 8 (Fig.
2C). Notably, BMD was significantly increased in the PTH group
compared with the Control group at 8 weeks post-graft for both 67
and 75% porosity (P<0.01; Fig. 2B and
C). These observations suggest that teriparatide administration
enhances calcification in bone defects grafted with β-TCP of 67 and
75% porosity.
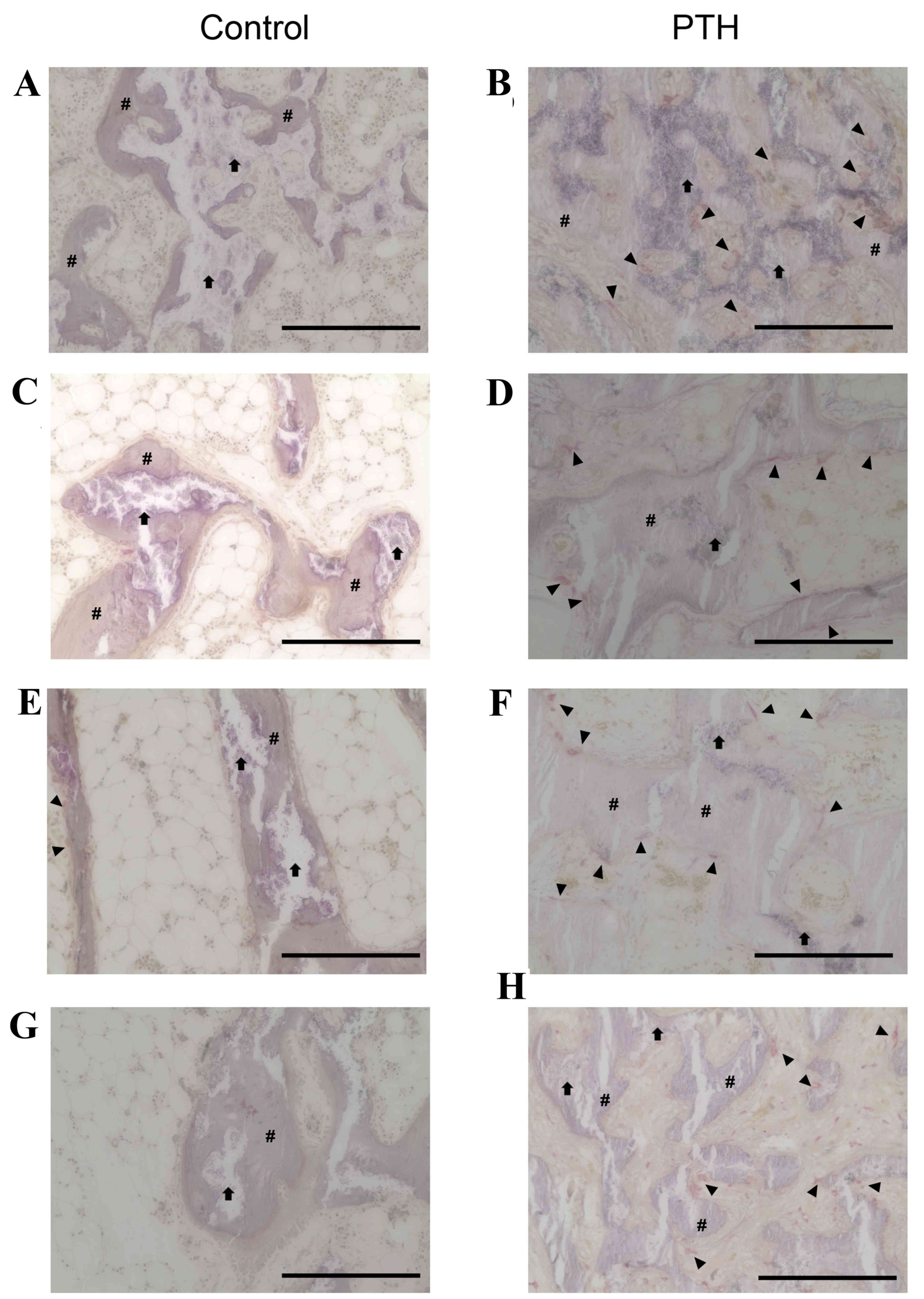
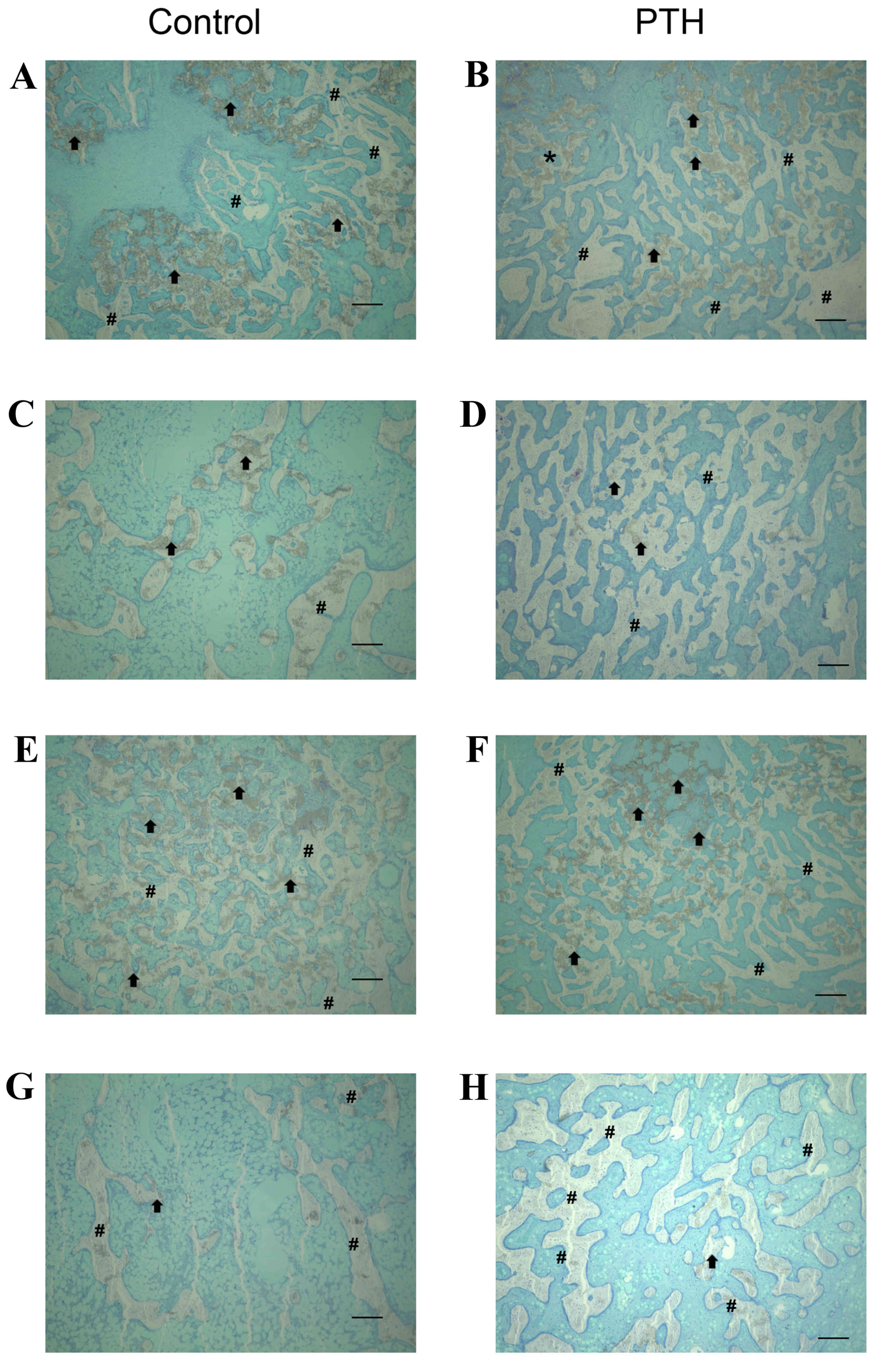
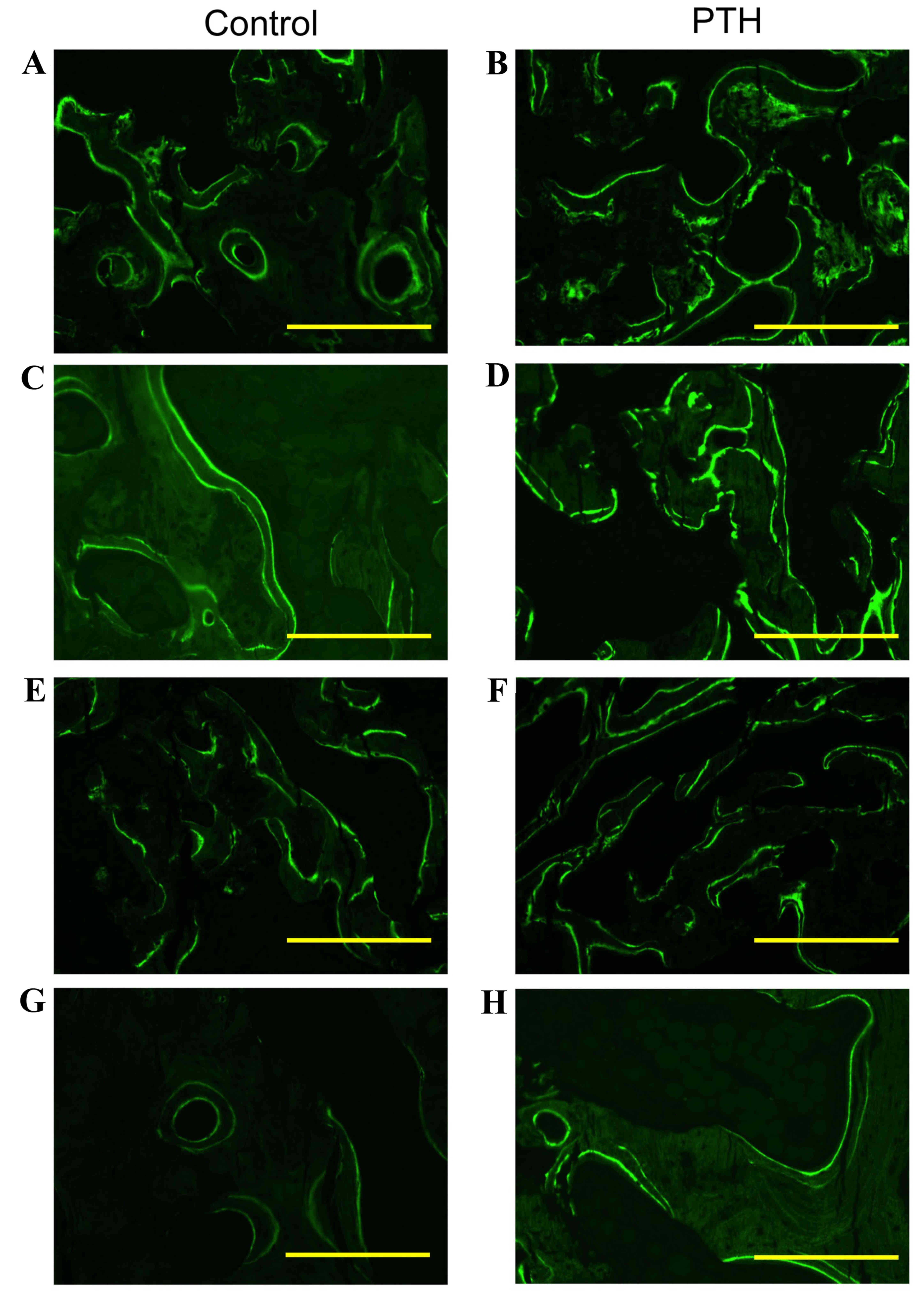
Histological analysis
Histological analysis revealed that the number of
TRAP-positive osteoclasts was markedly increased in the PTH group
compared with the Control group for bone grafted with β-TCP at
porosities of 67 and 75% (Fig. 3).
TRAP-positive osteoclasts were in direct contact with the surface
of newly formed bone as well as β-TCP, with a ragged appearance of
these structures. Furthermore, the amount of β-TCP at porosities of
67 and 75% was markedly decreased at 8 weeks compared with 4 weeks
in both the Control and PTH groups (Fig.
4). Notably, the amount of newly formed bone was increased in
the PTH group compared with the Control group at 4 and 8 weeks
following the graft for both porosities of β-TCP (Fig. 4).
Histomorphometry
Histomorphometric analysis was first performed using
sections of distal femoral condyles without bone defects, obtained
from unoperated femurs. The results demonstrated a significant
increase in BV/TV (P<0.05) and BFR/BS (P<0.01) at 4 weeks
(Fig. 5A and B), and significant
increases in BV/TV, MAR and BFR/BV at 8 weeks (P<0.01; Fig. 5A-C) in the PTH group compared with
the Control group (without teriparatide). These observations
suggest that teriparatide administration enhances the BV, MAR and
BFR in distal femoral condyles without bone defect. No significant
differences were observed in Oc.S/BS or OS/BS between the Control
and PTH groups at weeks 4 or 8 (Fig. 5D
and E).
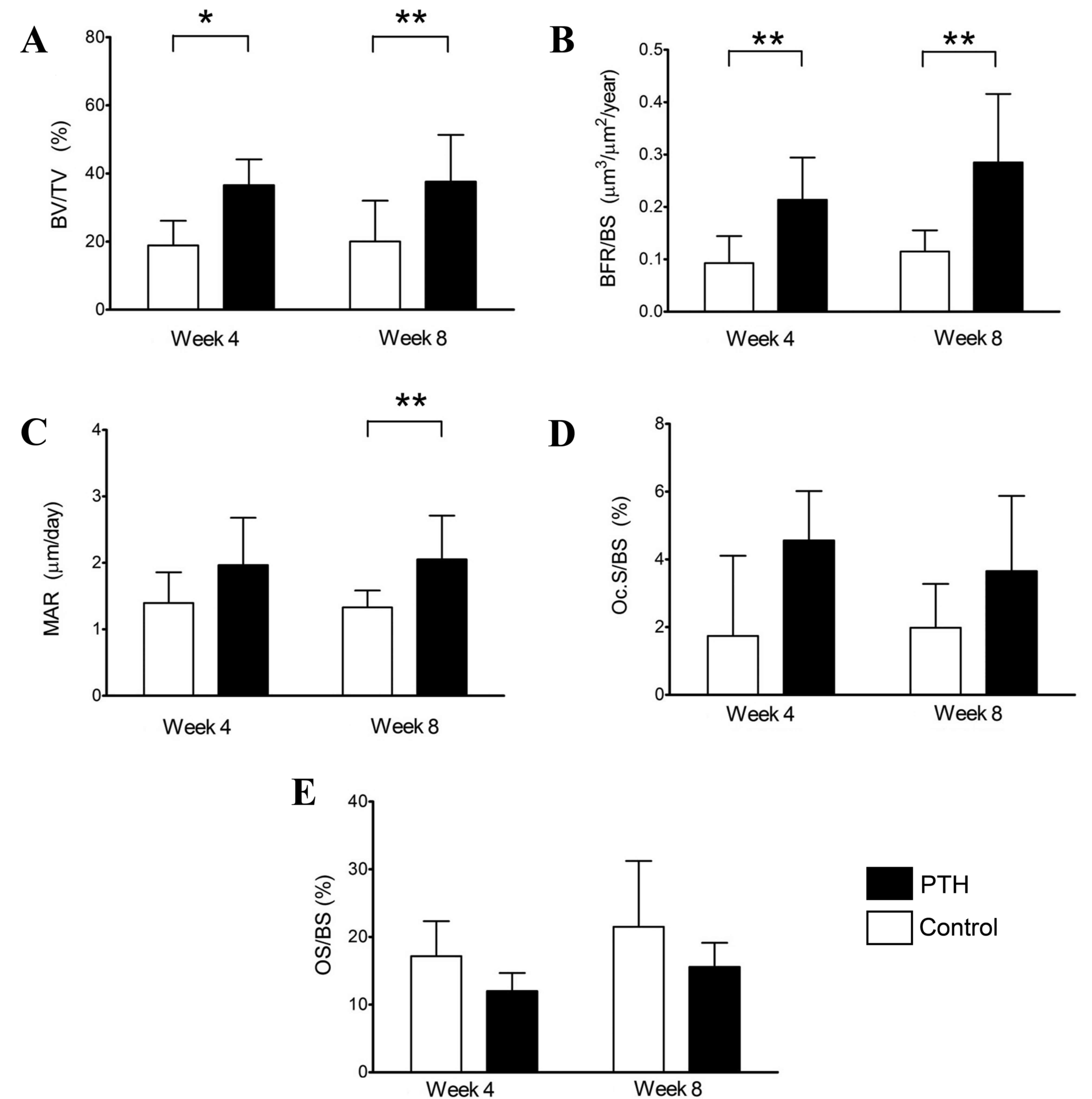 | Figure 5.Histomorphometry of the distal
femoral condyles without bone defects. Histomorphometric analyses
were performed using sections of the distal femoral condyles of the
Control and PTH groups, which were recovered at 4 and 8 weeks
post-surgery. (A) BV/TV, (B) BFR/BS, (C) MAR, (D) Oc.S/BS and (E)
OS/BS were determined. Data are expressed as mean ± standard
deviation (n=6). *P<0.05 and **P<0.01. PTH, parathyroid
hormone; BV bone volume; TV, tissue volume; BFR, bone formation
rate; BS, bone surface; MAR, mineral apposition rate/calcification;
Oc.S, osteoclast surface; OS, osteoid surface. |
Furthermore, histomorphometric analysis was
performed using sections of distal femoral condyles with bone
defects and β-TCP grafts (Figs. 6
and 7). The volume of β-TCP
(TCPV/TV) was decreased between weeks 4 and 8 in both the Control
and PTH groups following grafts with 67 and 75% porosity (Figs. 6A and 7A, respectively). By contrast, the volume
of newly formed bone, (BV- TCPV)/TV, was significantly increased
between weeks 4 to 8 in the PTH groups with grafts of both
porosities (P<0.05; Figs. 6B and
7B). Additionally, the volume of
newly formed bone was significantly increased in the PTH group
compared with the Control group grafted with 67% β-TCP at 4 weeks
(P<0.05; Fig. 6B).
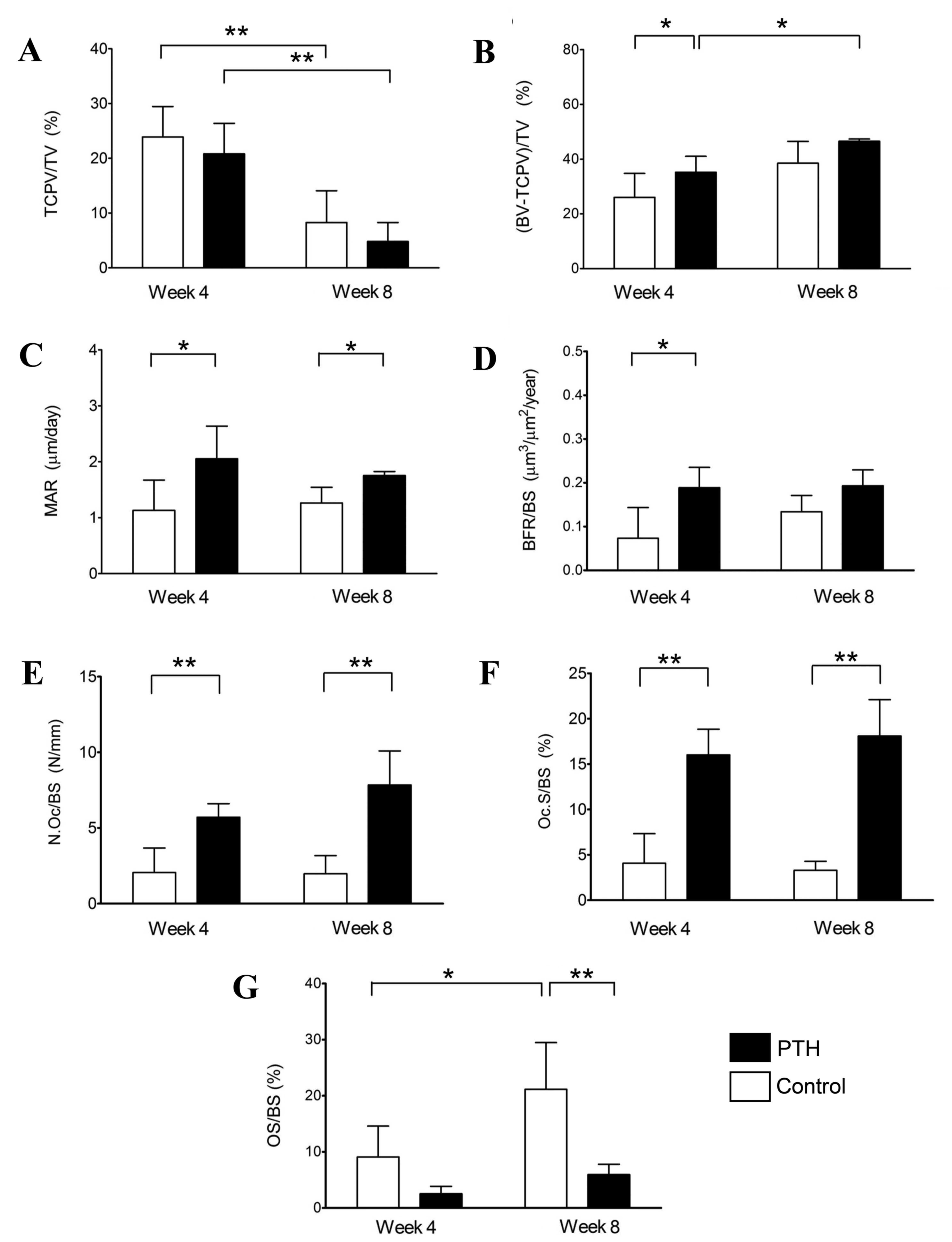 | Figure 6.Histomorphometry of bone defects
grafted with β-TCP of 67% porosity. Histomorphometric analyses were
performed using sections of distal femoral condyles of the Control
and PTH groups, which were recovered at 4 and 8 weeks post-surgery,
and (A) TCP/TV, (B) BV-TCPV/TV, (C) MAR, (D) BFR/BS, (E) N.Oc/BS,
(F) Oc.S/BS and (G) OS/BS were determined. Data are expressed as
mean ± standard deviation (n=6). *P<0.05 and **P<0.01. TCP,
tricalcium phosphate; PTH, parathyroid hormone; TV, tissue volume;
BV, bone volume; TCPV, TCP volume; BV-TCPV/TV, newly formed bone;
MAR, mineral apposition rate/calcification; BFR, bone formation
rate; BS, bone surface; N.Oc, osteoclast number; Oc.S, osteoclast
surface; OS, osteoid surface. |
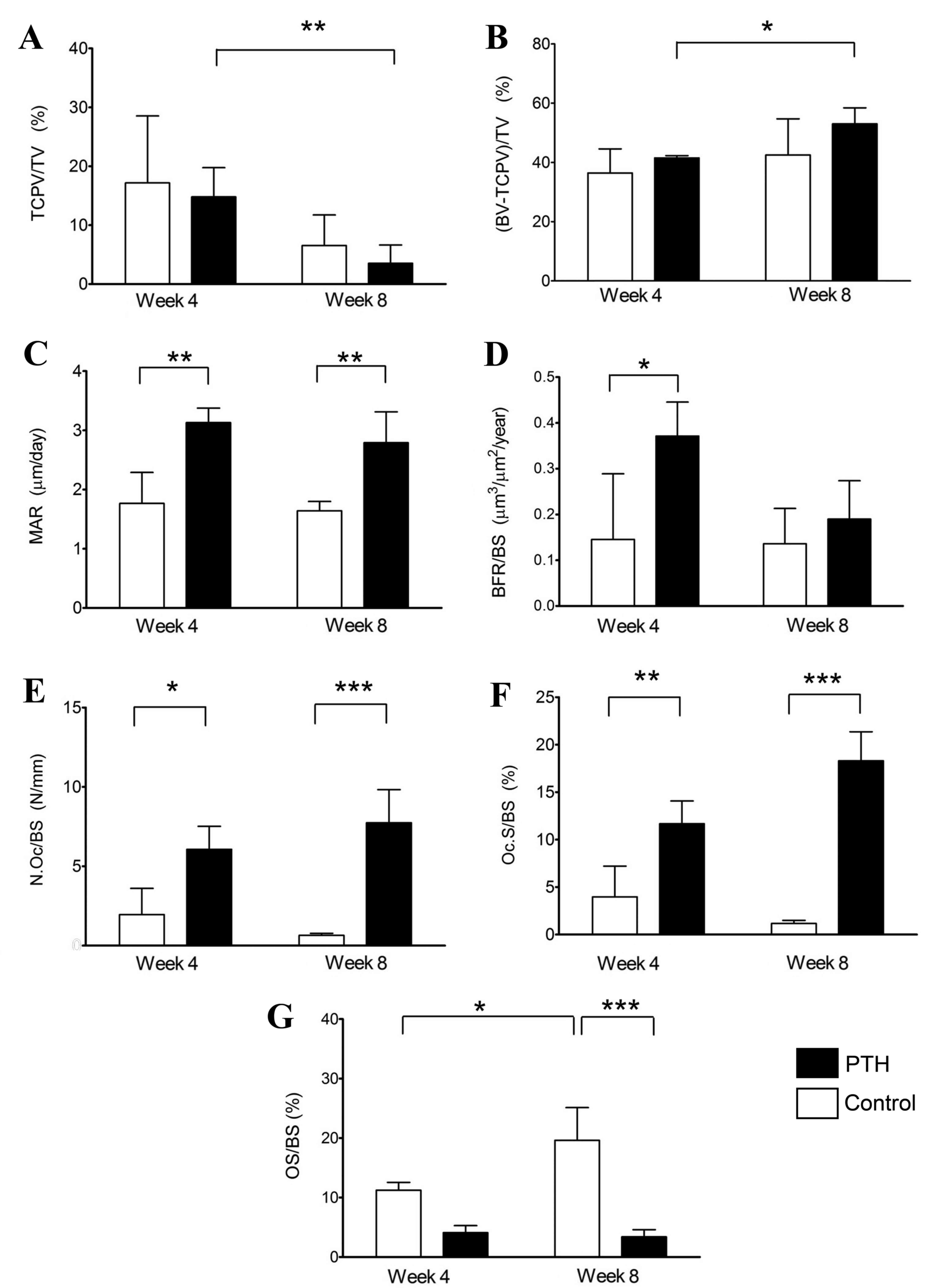 | Figure 7.Histomorphometry of the bone defect
grafted with β-TCP of 75% porosity. Histomorphometric analyses were
performed using sections of distal femoral condyles of the Control
and PTH groups, which were recovered at 4 and 8 weeks post-surgery,
and (A) TCP/TV, (B) BV-TCPV/TV, (C) MAR, (D) BFR/BS, (E) N.Oc/BS,
(F) Oc.S/BS and (G) OS/BS were determined. Data are expressed as
mean ± standard deviation (n=6). *P<0.05 and **P<0.01. TCP,
tricalcium phosphate; PTH, parathyroid hormone; TV, tissue volume;
BV, bone volume; TCPV, TCP volume; BV-TCPV/TV, newly formed bone;
MAR, mineral apposition rate/calcification; BFR, bone formation
rate; BS, bone surface; N.Oc, osteoclast number; Oc.S, osteoclast
surface; OS, osteoid surface. |
The calcification rate (MAR) was significantly
higher in the PTH group compared with the Control group at 4 and 8
weeks following grafting with both porosities of β-TCP (P<0.05
for 67%, P<0.01 for 75%; Figs. 6C
and 7C, respectively). Calcification
was indicated to be increased in the PTH group compared with the
Control group at 4 and 8 weeks following grafting with both
porosities of 67 and 75% β-TCP by labeling with a calcium-binding
fluorescent dye calcein (Fig. 8).
BFR/BS was significantly increased in the PTH group compared with
the Control group at 4 weeks for both porosities of β-TCP
(P<0.05); however, no significant differences were observed at 8
weeks after bone grafting (Figs. 6D
and 7D).
N.Oc/BS and Oc.S/BS were significantly increased in
the PTH group compared with the Control group for both porosities
of β-TCP at 4 weeks (P<0.05) and 8 weeks (P<0.01) following
the bone graft (Fig. 6E and F, and
Fig. 7E and F). Furthermore, OS/BS
significantly increased in the Control group from week 4 to week 8
for the two porosities of β-TCP (P<0.05); however, OS/BS was
significantly lower in the PTH group compared with the Control
group with β-TCP porosities of 67 (P<0.01) and 75% (P<0.001)
at 8 weeks following the bone graft (Figs. 6G and 7G). This reduction in OS in the PTH group
may be due to the action of teriparatide, which substantially
enhances the bone formation, thereby decreasing OS/BS.
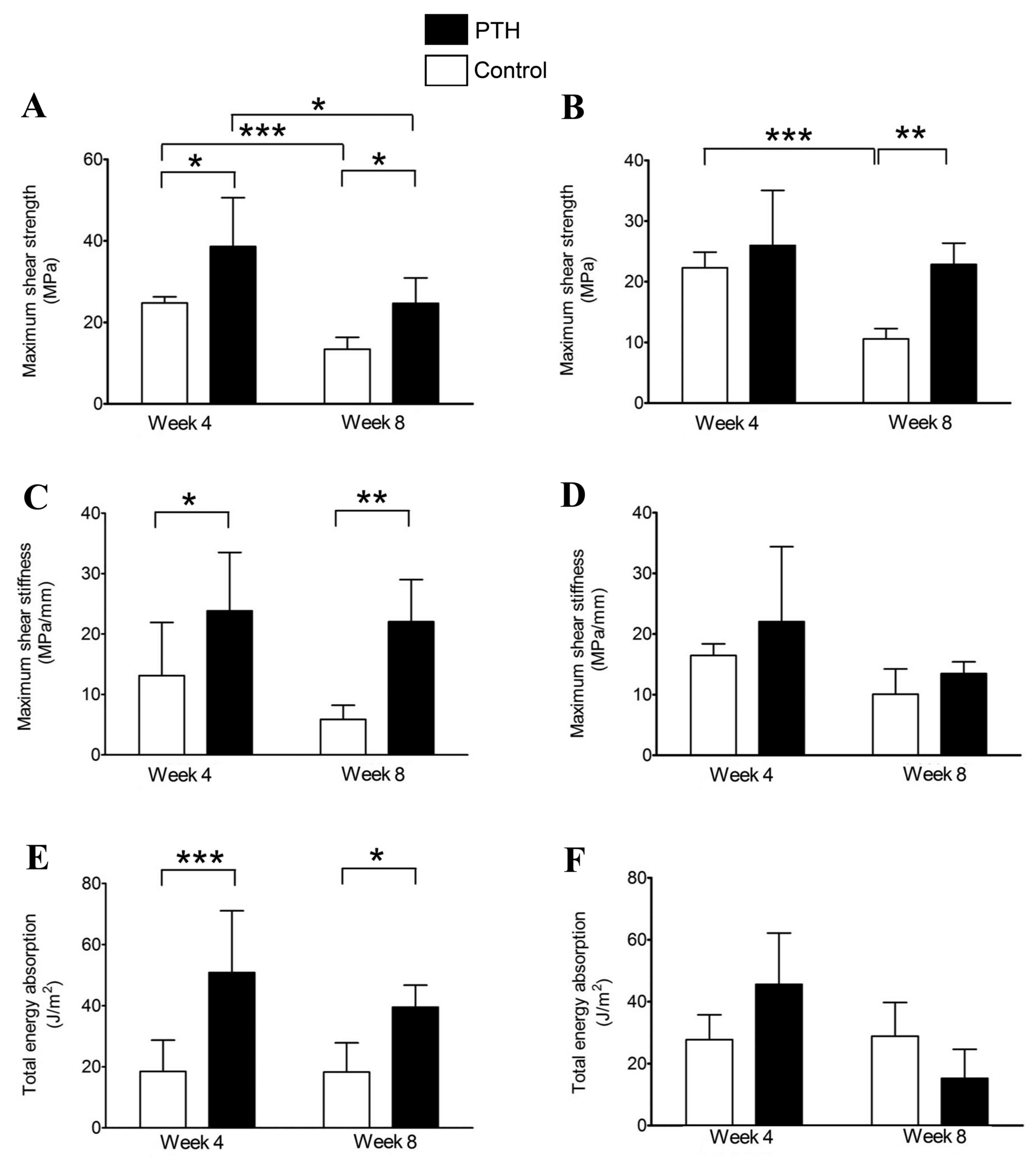
Mechanical strength analysis
Maximum shear strength of the bone grafts was
significantly increased in the PTH groups compared with the Control
groups at 4 weeks for β-TCP grafts of 67% porosity (P<0.05;
Fig. 9A) and at 8 weeks for β-TCP
grafts of both porosities (P<0.05 for 67%, P<0.01 for 75%;
Fig. 9A and B). Notably, the maximum
shear strength was significantly decreased between 4 and 8 weeks in
the Control groups at both porosities of β-TCP (P<0.001;
Fig. 9A and B); however, the maximum
shear strength was maintained by teriparatide administration to
almost the same levels at 8 weeks as those of the Control groups at
4 weeks for both porosities of β-TCP (Fig. 9A and B).
A significant increase in maximum shear stiffness
was observed in the PTH group compared with the control at 4
(P<0.05) and 8 (P<0.01) weeks following the application of
bone grafts with 67% porosity β-TCP (Fig. 9C); however, no significant difference
in this parameter was observed between groups for the 75% porosity
graft (Fig. 9D). Significant
increases in total energy absorption were also observed in the PTH
group compared with the control for the 67% porosity graft
(P<0.001 at week 4 and P<0.05 at week 8; Fig. 9E), but not for the graft using 75%
β-TCP (Fig. 9F).
Discussion
Grafting β-TCP is a well-established method for
restoring bone defects; however, there is a concern that the
mechanical stability of grafted β-TCP is not maintained during bone
translation (23). Notably, the
intermittent administration of teriparatide (hPTH 1–34) reduces the
risk of fracture in patients with osteoporosis and works as an
anabolic agent to stimulate bone formation (46). In the present study, therefore, the
effect of intermittent administration of teriparatide on new bone
formation and mechanical strength of bone defects grafted with
β-TCP was investigated. The results indicated that intermittent
teriparatide administration suppressed the reduction in mechanical
strength during the remodeling process in bone defects grafted with
β-TCP. Furthermore, the results of the present study indicate that
teriparatide increases the degradation of β-TCP by osteoclastic
resorption and promotes the formation of new bone following
grafting. These observations demonstrate that teriparatide is
effective at maintaining the mechanical stability of grafted β-TCP,
possibly by promoting new bone formation.
The experimental model used in the present study was
designed to evaluate the effect of intermittent administration of
teriparatide on the regeneration of cancellous bone defects grafted
with β-TCP. White Japanese rabbits were chosen as a testing model
due to the ease of handling and the anatomical characteristics of
their femurs (47,48), and bone metabolism closely resembling
that in humans (49–53). Previous studies have indicated that
the femoral condyle may be used as a site for evaluating the
mechanical strength and bone formation in defects grafted with bone
substitutes (47,48,54–56). In
the present study, the radiological, histological and mechanical
strength analyses of defected bone were performed at 4 and 8 weeks
following β-TCP graft; these intervals were selected because a
period of 6 weeks is required for bone formation in rabbits
(27,57–59), and
teriparatide accelerates the bone remodeling process 4 weeks
following intermittent administration (31,60,61).
The anabolic effect of teriparatide is dependent on
the dose, frequency and duration of administration (62–64). In
previous studies, 6–10 µg/kg teriparatide was subcutaneously
injected into rats and rabbits three times per week (62–64). In
the present study, to make the effect of teriparatide more evident,
40 µg/kg teriparatide was subcutaneously injected three times per
week, and it was confirmed that teriparatide significantly
increased the serum levels of Gla-OC at 4 and 8 weeks. Furthermore,
teriparatide significantly increased the calcification, bone
formation and newly formed bone following the graft compared with
that in the Control group. These results indicate that teriparatide
enhances bone formation in bone defects grafted with β-TCP.
Micro-CT analysis also revealed that the reduction in BMD during
the experimental period was suppressed, and BMD was maintained
during the experimental period in the teriparatide-treated group
following the graft.
In the Control group, TCPV was significantly
decreased between weeks 4 and 8, whereas the volume of newly formed
bone was increased. However, maximum shear strength significantly
decreased between weeks 4 and 8 in the Control group. These results
suggest that the bioresorption of β-TCP causes the mechanical loss
of bone grafted with β-TCP, although the volume of newly formed
bone increases, as previously reported (21,23,65).
Notably, teriparatide further decreased the volume of β-TCP and
increased the volume of newly formed bone between weeks 4 and 8
compared with the Control group at both porosities of β-TCP. These
results suggest that teriparatide increases both the biodegradation
of β-TCP and new bone formation in bone defects grafted with β-TCP.
In this context, it is interesting to note that based on the
activity of teriparatide (inducing osteoclast differentiation and
activation), bone resorption is transiently increased at an early
stage following teriparatide administration; however, bone
formation is significantly increased at a late phase following the
administration (66). Importantly,
teriparatide administration increased all mechanical parameters
(maximum shear strength, maximum shear stiffness and total energy
absorption) at 4 and 8 weeks compared with the Control group, when
β-TCP with 67% porosity was used. By contrast, only maximum shear
strength was increased by teriparatide at 8 weeks when using the
porosity of 75%. These results suggest that β-TCP of 67 but not 75%
porosity may be useful as a bone substitute, which enhances the
remodeling and mechanical strength of bone defects, potentially by
promoting the resorption of β-TCP and new bone formation.
It has been reported that during the bone remodeling
process in β-TCP grafted sites, osteoclasts continuously adhere to
the surface of β-TCP and resorb the material, and their
biodegradation stimulates bone formation (67–70).
Furthermore, a previous study indicated that the number of
osteoclasts peaks early, whereas the rate of new bone formation
peaks later in bone defects grafted with β-TCP (27). Based on these observations, it may be
speculated that the bioresorption of β-TCP by osteoclasts is
responsible for the reduction in mechanical strength of bone
grafted with β-TCP until new bone formation is completed.
Consistent with this, the maximum shear strength was reduced
between weeks 4 and 8 in the Control group. Notably, the maximum
shear strength was maintained by teriparatide administration,
possibly via the increase in calcification or new bone
formation.
In conclusion, the results of the present study
revealed that intermittent administration of teriparatide enhances
the remodeling of bone defects grafted with β-TCP. Teriparatide
likely increases the degradation of β-TCP by osteoclastic
resorption and promotes the formation of new bone following
grafting, thereby suppressing the reduction in mechanical strength
during the remodeling process of bone defects grafted with β-TCP.
Thus, the combination of an anabolic agent (e.g., teriparatide) and
a bone graft substitute (e.g., β-TCP) may have useful clinical
applications in spinal fusion surgery and augmentation in revision
arthroplasty.
Acknowledgements
The authors would like to thank Associate Professor
Kiyohito Naito (Department of Medicine for Motor Organs, Juntendo
University Graduate School of Medicine, Tokyo, Japan) for helpful
discussion, Masahiro Miyazaki (Department of Bio-Engineering,
Juntendo University Institute of Casualty Center, Shizuoka, Japan)
and the members of the Laboratory of Division of Molecular &
Biochemical Research, Research Support Center, Juntendo University
Graduate Faculty of Medicine (Tokyo, Japan), for technical
assistance. The authors would also like to thank Asahi Kasei Pharma
Corp. and HOYA Corporation for their supplies of teriparatide and
β-TCP, respectively. This study was supported in part by a grant
from the Strategic Research Foundation Grant-aided Project for
Private Universities from Ministry of Education, Culture, Sport,
Science, and Technology, Japan, 2014–2018 (grant no. S1411007).
References
|
1
|
Weinstein JN, Lurie JD, Olson PR, Bronner
KK and Fisher ES: United States trends and regional variations in
lumbar spine surgery: 1992–2003. Spine (Phila Pa 1976).
31:2707–2714. 2007. View Article : Google Scholar
|
|
2
|
Chau AM and Mobbs RJ: Bone graft
substitutes in anterior cervical discectomy and fusion. Eur Spine
J. 18:449–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bicanic G, Barbaric K, Bohacek I,
Aljinovic A and Delimar D: Current concept in dysplastic hip
arthroplasty: Techniques for acetabular and femoral reconstruction.
World J Orthop. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JM and Nam HT: Acetabular revision
total hip arthroplasty using an impacted morselized allograft and a
cementless cup: Minimum 10-year follow-up. J Arthroplasty.
26:1057–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dewei Z and Xiaobing Y: A retrospective
analysis of the use of cannulated compression screws and a
vascularised iliac bone graft in the treatment of displaced
fracture of the femoral neck in patients aged <50 years. Bone
Joint J. 96-B:1–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herkowitz HN and Kurz LT: Degenerative
lumbar spondylolisthesis with spinal stenosis. A prospective study
comparing decompression with decompression and intertransverse
process arthrodesis. J Bone Joint Surg Am. 73:802–808. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zdeblick TA: A prospective, randomized
study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976).
18:983–991. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwasaki K, Ikedo T, Hashikata H and Toda
H: Autologous clavicle bone graft for anterior cervical discectomy
and fusion with titanium interbody cage. J Neurosurg Spine.
21:761–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Summers BN and Eisenstein SM: Donor site
pain from the ilium. A complication of lumbar spine fusion. J Bone
Joint Surg Br. 71:677–680. 1989.PubMed/NCBI
|
|
10
|
Aurori BF, Weierman RJ, Lowell HA, Nadel
CI and Parsons JR: Pseudoarthrosis after spinal fusion for
scoliosis. A comparison of autogenic and allogenic bone grafts.
Clin Orthop Relat Res. 153–158. 1985.PubMed/NCBI
|
|
11
|
Park JJ, Hershman SH and Kim YH: Updates
in the use of bone grafts in the lumbar spine. Bull Hosp Jt Dis.
71:39–48. 2013.
|
|
12
|
Giannoudis PV, Dinopoulos H and Tsiridis
E: Bone substitutes: An update. Injury. 36 Suppl 3:S20–S27. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myoui A and Yoshikawa H: Regenerative
medicine in bone tumor surgery. Clin Calcium. 18:1767–1773.
2008.(In Japanese). PubMed/NCBI
|
|
14
|
Tamai N, Myoui A, Tomita T, Nakase T,
Tanaka J, Ochi T and Yoshikawa H: Novel hydroxyapatite ceramics
with an interconnective porous structure exhibit superior
osteoconduction in vivo. J Biomed Mater Res. 59:110–117. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumine A, Myoui A, Kusuzaki K, Araki N,
Seto M, Yoshikawa H and Uchida A: Calcium hydroxyapatite ceramic
implants in bone tumor surgery. A long-term follow-up study. J Bone
Joint Surg Br. 86:719–725. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoogendoorn HA, Renooij W, Akkermans LM,
Visser W and Wittebol P: Long-term study of large ceramic implants
(porous hydroxyapatite) in dog femora. Clin Orthop Relat Res.
281–288. 1984.PubMed/NCBI
|
|
17
|
Ozawa M: Experimental study on bone
conductivity and absorbability of the pure β-TCP. J Jpn Soc
Biomater. 13:167–175. 1995.(In Japanese).
|
|
18
|
Ozawa M, Tanaka T, Morikawa S, Chazono M
and Fujii K: Clinical study of the pure β-tricalcium
phosphate-Reports of 167 cases. J East Jpn Orthop Traumatol.
12:409–413. 2000.(In Japanese).
|
|
19
|
Saito M, Shimizu H, Beppu M and Takagi M:
The role of beta-tricalcium phosphate in vascularized periosteum. J
Orthop Sci. 5:275–282. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka T, Chazono M and Komaki H: Clinical
application of beta-tricalcium phosphate in human bone defects.
Jikeikai Med J. 53:55–53. 2006.
|
|
21
|
Tanaka T, Kumagae Y, Saito M, Chazono M,
Komaki H, Kikuchi T, Kitasato S and Marumo K: Bone Formation and
Resorption in Patients After Implantation of beta-Tricalcium
Phosphate blocks with 60% and 75% Porosity in Opening-Wedge High
Tibial Osteotomy. J Biomed Mater Res B Appl Biomater. 86:453–459.
2007.
|
|
22
|
Dong J, Uemura T, Shirasaki Y and Tateishi
T: Promotion of bone formation using highly pure porous beta-TCP
combined with bone marrow-derived osteogenitor cells. Biomaterials.
23:4493–4502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamasaki N, Hirao M, Nanno K, Sugiyasu K,
Tamai N, Hashimoto N, Yoshikawa H and Myoui A: A comparative
assessment of synthetic ceramic bone substitutes with different
composition and microstructure in rabbit femoral condyle model. J
Biomed Mater Res B Appl Biomater. 91:788–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ng AM, Tan KK, Phang MY, Aziyati O, Tan
GH, Isa MR, Aminuddin BS, Naseem M, Fauziah O and Ruszymah BH:
Differential osteogenic activity of osteoprogenitor cells on HA and
TCP/HA scaffold of tissue engineered bone. J Biomed Mater Res A.
85:301–312. 2007.
|
|
25
|
Kitsugi T, Yamamoto T, Nakamura T, Kotani
S, Kokubo T and Takeuchi H: Four calcium phosphate ceramics as bone
substitutes for non-weight-bearing. Biomaterials. 14:216–224. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Finkemeier CG: Bone-grafting and
bone-graft substitutes. J Bone Joint Surg Am. 84-A:1–464.
2002.PubMed/NCBI
|
|
27
|
Chazono M, Tanaka T, Komaki H and Fujii K:
Bone formation and bioresorption after implantation of injectable
beta-tricalcium phosphate granules-hyaluronate complex in rabbit
bone defects. J Biomed Mater Res A. 70:542–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yokoyama K, Matsuba D, Adachi-Akahane S,
Takeyama H, Tabei I, Suzuki A, Shibasaki T, Iida R, Ohkido I,
Hosoya T and Suda N: Dihydropyridine- and voltage-sensitive Ca2+
entry in human parathyroid cells. Exp Physiol. 94:847–855. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Black DM and Schafer AL: The search for
the optimal anabolic osteoporosis therapy. J Bone Miner Res.
28:2263–2265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakamura T, Sugimoto T, Nakano T,
Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H,
Nishizawa Y, et al: Randomized teriparatide [human parathyroid
hormone (PTH) 1–34] once-weekly efficacy research (TOWER) trial for
examining the reduction in new vertebral fractures in subjects with
primary osteoporosis and high fracture risk. J Clin Endocrinol
Metab. 97:3097–3106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hock JM and Gera I: Effects of continuous
and intermittent administration and inhibition of resorption on the
anabolic response of bone to parathyroid hormone. J Bone Miner Res.
7:65–72. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hodsman AB, Bauer DC, Dempster DW, Dian L,
Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski
WP, et al: Parathyroid hormone and teriparatide for the treatment
of osteoporosis: A review of the evidence and suggested guidelines
for its use. Endocr Rev. 26:688–703. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andreassen TT, Ejersted C and Oxlund H:
Intermittent parathyroid hormone (1–34) treatment increases callus
formation and mechanical strength of healing rat fractures. J Bone
Miner Res. 14:960–968. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andreassen TT, Fledelius C, Ejersted C and
Oxlund H: Increases in callus formation and mechanical strength of
healing fractures in old rats treated with parathyroid hormone.
Acta Orthop Scand. 72:304–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Knopp E, Troiano N, Bouxsein M, Sun BH,
Lostritto K, Gundberg C, Dziura J and Insogna K: The effect of
aging on the skeletal response to intermittent treatment with
parathyroid hormone. Endocrinology. 146:1983–1990. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aleksyniene R, Thomsen JS, Eckardt H,
Bundgaard KG, Lind M and Hvid I: Parathyroid hormone PTH(1–34)
increases the volume, mineral content and mechanical properties of
regenerated mineralizing tissue after distraction osteogenesis in
rabbits. Acta Orthop. 80:716–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mashiba T, Burr DB, Turner CH, Sato M,
Cain RL and Hock JM: Effects of human parathyroid hormone (1–34),
LY333334, on bone mass, remodeling, and mechanical properties of
cortical bone during the first remodeling cycle in rabbits. Bone.
28:538–547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaback LA, do Y Soung, Naik A, Geneau G,
Schwarz EM, Rosier RN, O'Keefe RJ and Drissi H: Teriparatide (1–34
human PTH) regulation of osterix during fracture repair. J Cell
Biochem. 105:219–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Glover SJ, Eastell R, McCloskey EV, Rogers
A, Garnero P, Lowery J, Belleli R, Wright TM and John MR: Rapid and
robust response of biochemical markers of bone formation to
teriparatide therapy. Bone. 45:1053–1058. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okuda T, Ioku K, Yonezawa I, Minagi H,
Kawachi G, Gonda Y, Murayama H, Shibata Y, Minami S, Kamihira S, et
al: The effect of the microstructure of beta-tricalcium phosphate
on the metabolism of subsequently formed bone tissue. Biomaterials.
28:2612–2621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okuda T, Ioku K, Yonezawa I, Minagi H,
Gonda Y, Kawachi G, Kamitakahara M, Shibata Y, Murayama H, Kurosawa
H and Ikeda T: The slow resorption with replacement by bone of a
hydrothermally synthesized pure calcium-deficient hydroxyapatite.
Biomaterials. 29:2719–2728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gonda Y, Ioku K, Shibata Y, Okuda T,
Kawachi G, Kamitakahara M, Murayama H, Hideshima K, Kamihira S,
Yonezawa I, et al: Stimulatory effect of hydrothermally synthesized
biodegradable hydroxyapatite granules on osteogenesis and direct
association with osteoclasts. Biomaterials. 30:4390–4400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ikeda T, Kasai M, Suzuki J, Kuroyama H,
Seki S, Utsuyama M and Hirokawa K: Multimerization of the receptor
activator of nuclear factor-kappaB ligand (RANKL) isoforms and
regulation of osteoclastogenesis. J Biol Chem. 278:47217–47222.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Parfitt AM, Drezner MK, Glorieux FH, Kanis
JA, Malluche H, Meunier PJ, Ott SM and Recker RR: Bone
histomorphometry: Standardization of nomenclature, symbols, and
units. Report of the ASBMR Histomorphometry Nomenclature Committee.
J Bone Miner Res. 2:595–610. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Daugaard H, Elmengaard B, Andreassen TT,
Baas J, Bechtold JE and Soballe K: The combined effect of
parathyroid hormone and bone graft on implant fixation. J Bone
Joint Surg Br. 93:131–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Compston JE: Skeletal actions of
intermittent parathyroid hormone: Effects on bone remodelling and
structure. Bone. 40:1447–1452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leupold JA, Barfield WR, An YH and
Hartsock LA: A comparison of ProOsteon, DBX, and collagraft in a
rabbit model. J Biomed Mater Res B Appl Biomater. 79:292–297. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Castellani C, Zanoni G, Tangl S, van
Griensven M and Redl H: Biphasic calcium phosphate ceramics in
small bone defects: Potential influence of carrier substances and
bone marrow on bone regeneration. Clin Oral Implants Res.
20:1367–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Roberts WE, Turley PK, Brezniak N and
Fielder PJ: Implants: Bone physiology and metabolism. CDA J.
15:54–61. 1987.PubMed/NCBI
|
|
50
|
Johansson C and Albrektsson T: Integration
of screw implants in the rabbit: A 1-year follow-up of removal
torque of titanium implants. Int J Oral Maxillofac Implants.
2:69–75. 1987.PubMed/NCBI
|
|
51
|
Baker D, London RM and O'Neal R: Rate of
pull-out strength gain of dual-etched titanium implants: A
comparative study in rabbits. Int J Oral Maxillofac Implants.
14:722–728. 1999.PubMed/NCBI
|
|
52
|
Dahlin C, Sennerby L, Lekholm U, Linde A
and Nyman S: Generation of new bone around titanium implants using
a membrane technique: An experimental study in rabbits. Int J Oral
Maxillofac Implants. 4:19–25. 1989.PubMed/NCBI
|
|
53
|
Mori H, Manabe M, Kurachi Y and Nagumo M:
Osseointegration of dental implants in rabbit bone with low mineral
density. J Oral Maxillofac Surg. 55:351–361. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pasquier G, Flautre B, Blary MC, Anselme K
and Hardouin P: Injectable percutaneous bone biomaterials: An
experimental study in a rabbit model. J Mater Sci Mater Med.
7:683–690. 1996. View Article : Google Scholar
|
|
55
|
Lu JX, Gallur A, Flautre B, Anselme K,
Descamps M, Thierry B and Hardouin P: Comparative study of tissue
reactions to calcium phosphate ceramics among cancellous, cortical,
and medullar bone sites in rabbits. J Biomed Mater Res. 42:357–367.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dodde R II, Yavuzer R, Bier UC, Alkadri A
and Jackson IT: Spontaneous bone healing in the rabbit. J Craniofac
Surg. 11:346–349. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shimazaki K and Mooney V: Comparative
study of porous hydroxyapatite and tricalcium phosphate as bone
substitute. J Orthop Res. 3:301–310. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Eggli PS, Müller W and Shenk RK: Porous
hydroxyapatite and tricalcium phosphate cylinders with two
different pore size ranges implanted in the cancellous bone of
rabbits. A comparative histomorphometric and histologic study of
bony ingrowth and implant substitution. Clin Orthop Relat Res.
127–138. 1988.PubMed/NCBI
|
|
59
|
Uzawa T, Hori M, Ejiri S and Ozawa H:
Comparison of the effects of intermittent and continuous
administration of human parathyroid hormone(1–34) on rat bone.
Bone. 16:477–484. 1995.PubMed/NCBI
|
|
60
|
Hirano T, Burr DB, Cain RL and Hock JM:
Changes in geometry and porosity in adult, ovary-intact rabbits
after 5 months treatment with LY333334 (hPTH 1–34). Calcif Tissue
Int. 66:456–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pettway GJ, Schneider A, Koh AJ, Widjaja
E, Morris MD, Meganck JA, Goldstein SA and McCauley LK: Anabolic
actions of PTH(1–34): Use of a novel tissue engineering model to
investigate temporal effects on bone. Bone. 36:959–970. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Komatsubara S, Mori S, Mashiba T, Nonaka
K, Seki A, Akiyama T, Miyamoto K, Cao Y, Manabe T and Norimatsu H:
Human parathyroid hormone(1–34) accelerates the fracture healing
process of woven to lamellar bone replacement and new cortical
shell formation in rat femora. Bone. 36:678–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Corsini MS, Faraco FN, Castro AA, Onuma T,
Sendyk WR and Shibli JA: Effect of systemic intermittent
administration of human parathyroid hormone (rhPTH[1-34]) on the
resistance to reverse torque in rabbit tibiae. J Oral Implantol.
34:298–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yamamoto Y, Washimi Y, Kanaji A, Tajima K,
Ishimura D and Yamada H: The effect of bisphosphonate and
intermittent human parathyroid hormone 1–34 treatments on cortical
bone allografts in rabbits. J Endocrinol Invest. 35:139–145.
2012.PubMed/NCBI
|
|
65
|
Tanaka T, Komaki H, Chazono M and Fujii K:
Use of a biphasic graft constructed with chondrocytes overlying a
beta-tricalcium phosphate block in the treatment of rabbit
osteochondral defects. Tissue Eng. 11:331–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sugimoto T, Nakamura T, Nakamura Y, Isogai
Y and Shiraki M: Profile of changes in bone turnover markers during
once-weekly teriparatide administration for 24 weeks in
postmenopausal women with osteoporosis. Osteoporos Int.
25:1173–1180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ikeda T, Yamaguchi A, Yokose S, Nagai Y,
Yamato H, Nakamura T, Tsurukami H, Tanizawa T and Yoshiki S:
Changes in biological activity of bone cells in ovariectomized rats
revealed by in situ hybridization. J Bone Miner Res. 11:780–788.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Martin TJ and Sims NA: Osteoclast-derived
activity in the coupling of bone formation to resorption. Trends
Mol Med. 11:76–81. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kondo N, Ogose A, Tokunaga K, Ito T, Arai
K, Kudo N, Inoue H, Irie H and Endo N: Bone formation and
resorption of highly purified beta-tricalcium phosphate in the rat
femoral condyle. Biomaterials. 26:5600–5608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Walker EC, McGregor NE, Poulton IJ,
Pompolo S, Allan EH, Quinn JM, Gillespie MT, Martin TJ and Sims NA:
Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation
required for normal bone remodeling. J Bone Miner Res.
23:2025–2032. 2008. View Article : Google Scholar : PubMed/NCBI
|