Introduction
In the clinical practice, ischemia reperfusion
injury (IRI) occurs after the coronary artery is recanalized by
means of a medical intervention like a bypass graft. Myocardial
injury is aggravated after blood perfusion is restored to the
ischemic myocardium, leading to a series of ultrastructural
injuries, and altered functional metabolism and electrophysiology
(1,2). According to statistics of the World
Health Organization (WHO), acute coronary artery infarction will
become the major cause of human death from diseases by 2020
(3). The diagnosis and prevention of
myocardial ischemia reperfusion injury (MIRI) have become a hot
spot of clinical study. During ischemia reperfusion, myocardial
tissues get exposed to large concentrations of oxygen free
radicals, which damage the cell membranes via lipid peroxidation.
Malondialdehyde (MDA) is a metabolic product of lipid peroxidation
and it indirectly reflects the activity of oxygen free radicals on
tissues. Levels of superoxide dismutase (SOD), an important
antioxidant enzyme, can also be used to assess the oxidative state
of tissues. The occurrences of coronary thrombosis and coronary
artery spasm are the main reasons for IRI events. The strong
vasoconstrictive effect of endothelin-1 (ET-1) is known to trigger
myocardial ischemia, and thromboxane B2
(TXB2) is conducive to platelet aggregation and
vasoconstriction. The characteristics of blood rheology and
TXB2 changes in angina pectoris patients with IRI were
examined in this study, in order to investigate weather a
relationship with MIRI exists.
Materials and methods
General information
Forty patients with angina pectoris, admitted to
Beijing Shijitan Hospital from February 2014 to January 2015, and
treated with elective percutaneous coronary intervention (PCI),
were selected for the unstable angina (UA) group. All UA patients
had their diagnosis confirmed and had not had any related symptoms
for 48 h prior to the procedure. Forty patients deemed free from
coronary heart disease by coronary angiography during the same
period of time, were selected for the control group. Patients with
PCI or coronary angiography contraindications were excluded from
the study. The Ethics Committee of Beijing Shijitan Hospital
approved the study and the participants signed the informed consent
form. The patients with angina pectoris were divided into low-,
intermediate- and high-risk groups based on their medical history,
pain characteristics, clinical manifestations, electrocardiograms
and cardiac biomarkers taking into account the Guidelines for
Diagnosis and Treatment of Patients with Unstable Angina and
Non-ST-Segment Elevation Myocardial Infarction (2007 edition).
Test methods
Peripheral venous blood samples (10 ml) were
withdrawn from the patients in the UA group 1 day before and 1 day
after the PCI. The samples were divided into a 7 ml sample treated
with anticoagulant and a 3 ml sample without anticoagulant. All
samples were centrifuged at 3,000 rpm for 10 min at 4°C; then
plasma and serum were collected and stored at −80°C for later
examination.
Detection indexes
A MVIS2035 blood rheology analyzer (Chongqing
Tianhai Medical Equipment, Shandong, China) was used to detect
hemodynamic parameters (viscosities at high, medium and low shear
rates, plasma viscosity, as well as erythrocyte aggregation index).
Radioimmunoassay using the FM2000 γ-immunoassay counter (Hybribio,
Xi'an, China) was performed to measure the concentrations of serum
ET-1 and TXB2. The 3100 type automatic biochemical
analyzer (Hitachi, Tokyo, Japan) was used to detect the content of
SOD and MDA in plasma samples.
Statistical analysis
SPSS 18.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis of data. Measurement data are
presented as mean ± SD, and Chi-square test was applied for
analysis; analysis of variance was used for comparison between
groups and t-test for pairwise comparisons. A P<0.05 indicates a
statistically significant difference.
Results
Comparisons of clinical
characteristics in the two groups
Patients' characteristics such as age, sex, history
of hypertension and diabetes mellitus, body mass index (BMI) and
four items of blood-lipid tests [total cholesterol (TC),
triglyeride (TG), high density lipoprotein (HDL) and low density
lipoprotein (LDL)] were balanced and comparable between the two
groups (P>0.05) (Table I).
 | Table I.Comparisons of general information in
the two groups (mean ± SD, n=40). |
Table I.
Comparisons of general information in
the two groups (mean ± SD, n=40).
| Item | Control group | UA group | t/χ2 | P-value |
|---|
| Age (years) | 59.9±9.8 | 61.3±8.9 | 0.041 | 0.260 |
|
Male/female/(cases) | 25/15 | 23/17 | 0.032 | 0.222 |
|
Hypertension/(cases) | 17 | 20 | 0.055 | 0.296 |
| Diabetes
mellitus/(cases) | 9 | 12 | 0.064 | 0.318 |
| BMI
(kg/m2) | 25.01±2.28 | 26.86±3.09 | 1.382 | 0.320 |
| TC (mmol/l) | 4.35±1.02 | 4.41±0.92 | 1.483 | 0.431 |
| TG (mmol/l) | 1.60±0.96 | 1.48±0.64 | 1.568 | 0.443 |
| HDL (mmol/l) | 1.14±0.32 | 1.01±0.25 | 1.720 | 0.798 |
| LDL (mmol/l) | 2.54±0.82 | 2.54±0.62 | 1.711 | 0.713 |
Comparisons of hemodynamic parameters
in the two groups
The blood rheology in the UA group was manifested as
hyperviscosity. All the parameters compared, such as viscosity at
high, medium and low shear rates, and the erythrocyte aggregation
index, were highest in the UA group (after surgery), lower in the
UA group (before surgery), and lowest in the control group. There
were significant differences in pairwise comparisons (P<0.05)
(Table II).
 | Table II.Comparisons of blood rheology in the
two groups (mean ± SD, n=40). |
Table II.
Comparisons of blood rheology in the
two groups (mean ± SD, n=40).
| Group | Viscosity at high
shear rate (mPa/sec) | Viscosity at medium
shear rate (mPa/sec) | Viscosity at low
shear rate (mPa/sec) | Plasma viscosity
(mPa/sec) | Erythrocyte
aggregation index |
|---|
| Control group | 5.61±0.31 | 7.68±0.39 | 10.99±0.78 | 1.70±0.05 | 7.13±0.73 |
| UA group (before
surgery) |
9.84±0.21a |
9.78±0.37a |
15.25±0.41a |
2.18±0.16a |
8.13±0.63a |
| UA group (after
surgery) |
11.13±0.11a,b |
11.78±0.35a,b |
20.08±0.33a,b |
3.28±0.25a,b |
10.22±0.42a,b |
| F-value | 3.88 | 9.38 | 10.64 | 2.92 | 9.03 |
| P-value | 0.027 | 0.022 | 0.014 | 0.036 | 0.028 |
Comparisons of ET-1 and
TXB2 concentrations in the two groups
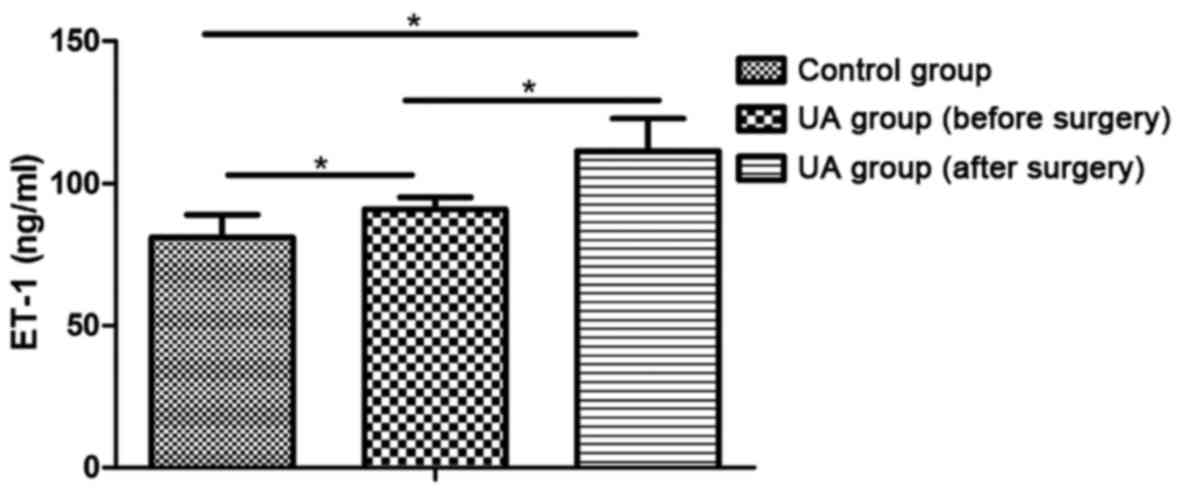
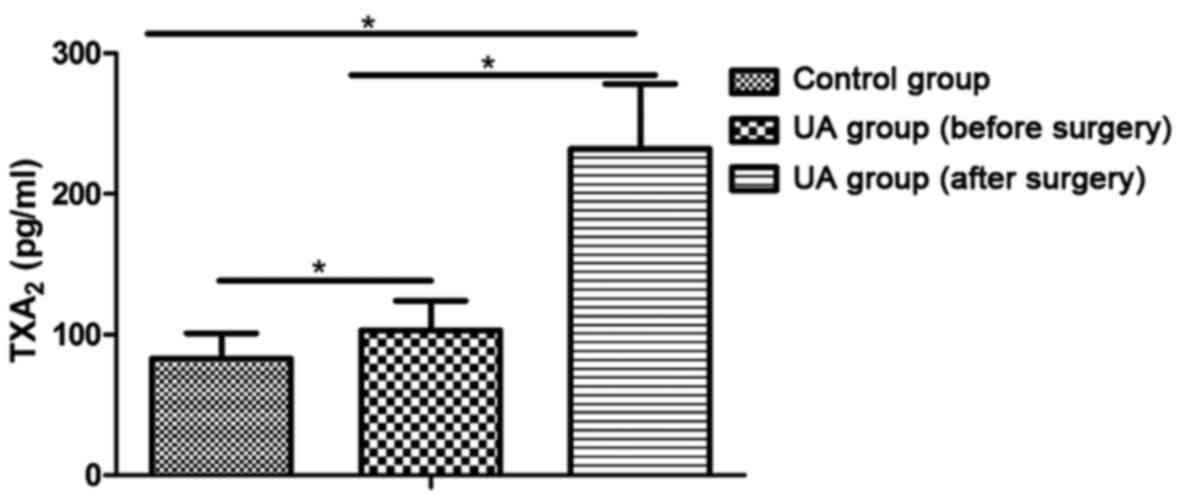
The levels of ET-1 and TXB2 in the UA
group were higher than those in the control group and they
increased further after surgery (P<0.05) (Figs. 1 and 2).
Comparisons of risk stratifications
and TXB2 levels in angina pectoris patients with
reperfusion injury
In the UA group, the serum TXB2
concentrations increased gradually along with the increase of risk
stratification, and the levels were significantly higher than those
in the patients in the control group (P<0.05) (Table III).
 | Table III.Comparisons of risk stratifications
and TXB2 levels in angina pectoris patients with
reperfusion injury (mean ± SD). |
Table III.
Comparisons of risk stratifications
and TXB2 levels in angina pectoris patients with
reperfusion injury (mean ± SD).
| Group | Cases | TXB2
(pg/ml) |
|---|
| Control group | 40 | 82±17 |
| Low-risk group | 10 | 177±27a |
| Intermediate-risk
group | 14 | 219±34a–c |
| High-risk group | 16 | 260±38a,b |
| F-value |
| 4.062 |
| P-value |
| 0.008 |
Comparisons of SOD and MDA content
between the two groups
The results for the activity of serum SOD showed the
UA group (after surgery) had the lowest levels, the UA group
(before surgery) had higher levels and then the control group
displayed the highest levels of all. On the other hand, the MDA
levels in the UA group (after surgery) were higher than those in
the UA group (before surgery), and those levels in turn were higher
than the levels in the control group; there were significant
differences in pairwise comparisons (P<0.05) (Table IV).
 | Table IV.Comparisons of SOD and MDA content in
the two groups (mean ± SD, n=40). |
Table IV.
Comparisons of SOD and MDA content in
the two groups (mean ± SD, n=40).
| Index | Control group | UA group (before
surgery) | UA group (after
surgery) | F-value | P-value |
|---|
| SOD (U/ml) |
119.8±23.9 |
90.5±20.3a |
61.9±7.5b,c | 3.08 | 0.041 |
| MDA (nmol/ml) |
1.7±0.4 |
2.8±0.8a |
4.9±1.5b,c | 8.03 | 0.033 |
Discussion
MIRI refers to a syndrome caused by inflammatory
responses, damage to endothelial cells, blood stream obstruction
and reperfusion arrhythmia as well as other injuries due to free
radical damage, calcium ion and leucocyte injuries (4).
Study has shown that the occurrence of
hypercoagulability in ischemic angina pectoris is closely related
to the adhesion and aggregation of platelets (5). TXA2 is a vasoconstrictive
factor with unstable activity, mainly synthesized and released by
platelet micro-particles. It can further activate the platelets on
the basis of inflammation, thus promoting the occurrence of
coronary artery spasm and formation of intravascular thrombosis
(6). TXB2 is a stable
metabolic product of TXA2 in plasma, and it can be used
to get a reflection of the actual level of TXA2
(7). For patients with unstable
angina pectoris, the content of TXB2 in plasma increases
because of the platelet adhesion caused by damage to the vascular
endothelium (8). The results of our
experiments showed that, in the UA group, the serum TXB2
concentration increased along with the rise in the risk
stratification; and the differences in comparisons were all
statistically significant (P<0.05). ET-1 is a bioactive peptide
with strong myocardial toxicity, which can reflect the secretory
function of the vessel's endothelium. It has intense
vasoconstrictive effects and can promote myocardial ischemia,
ventricular and vascular remodeling by activating relevant hormones
and accelerating the proliferation of vascular smooth muscle cells
(9–11). This study proved the ET-1 levels in
UA groups was higher than those in the control group, and the ET-1
level after the postoperative reperfusion injury was statistically
different from that before surgery (P<0.05). Our findings
suggest TXB2 may stimulate the interaction between
platelet activation and local inflammatory factors like ET-1 as
well as other endothelial secretory factors, thus creating a
vicious cycle after reperfusion injury.
Animal experiments have proven that severe coronary
stenosis can lead to massive production of free radicals and
aggregation of platelets (12).
Relevant studies have confirmed that when reperfusion injury occurs
in the ischemic myocardium, the production of oxygen free radicals
bursts in the body, proteins and lipids are oxidized and disabled,
and the activity of lysosomes is decreased, resulting in cell death
(13,14). SOD is an important antioxidant enzyme
in the myocardium, and oxygen free radicals can enhance the lipid
peroxidation by inhibiting the activity of SOD, thus causing
myocardial injury. When the blood supply is restored in the
ischemic myocardium, a large quantity of oxygen free radicals
produced in the tissues can damage the cell membranes through lipid
peroxidation, thus increasing membrane permeability, causing
transduction abnormalities of lipid signaling molecules and
inducing neutrophil accumulation and formation of microthrombi,
which can lead to no-reflow phenomenon and aggravated myocardial
injury (15–17). MDA is a metabolic product of lipid
peroxidation triggered by oxygen free radicals in myocardial cells,
which can lead to degeneration, senescence, mutation and death of
myocardial cells by promoting the cross-linking of nucleic acids,
proteins and phospholipid (18). Our
data showed that the activity of serum SOD was smallest in the UA
group (after surgery), higher in the UA group (before surgery) and
highest in the control group. Conversely, the MDA content was
highest in the UA group (after surgery), lower in the UA group
(before surgery) and lowest in the control group, with significant
differences in pairwise comparisons (P<0.05). After the
postoperative reperfusion injury, MDA and SOD levels changed
significantly compared with those before the surgery (P<0.01).
The reperfusion injury occurs in the ischemic myocardium as the
blood and oxygen supplies are restored. On the one hand, the
activity of xanthine oxidase in the body is strengthened (19); on the other, when the atheromatous
plaques are desquamated from the tunica intima, inflammatory
factors and the complement system are activated, and a large number
of oxygen free radicals are released (20). A large amount of SOD molecules are
required to eliminate the oxygen free radicals in the body;
therefore, the active enzyme sites available decline. Moreover,
excessive oxygen free radicals can induce extremely strong lipid
peroxidation, and consequently, the MDA content is increased
accordingly.
In conclusion, a hyperviscosity syndrome is present
in the blood rheology of patients with angina pectoris and IRI, and
the increasing TXB2 levels can be used as markers of
platelet activation and reference for clinical risk stratification,
providing great help in the prevention and assessment of disease
progression during treatment of IRI.
References
|
1
|
Jahania MS, Sanchez JA, Narayan P, Lasley
RD and Mentzer RM Jr: Heart preservation for transplantation:
Principles and strategies. Ann Thorac Surg. 68:1983–1987. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng X, Lian D, Wong A, Bygrave M, Ichim
TE, Khoshniat M, Zhang X, Sun H, De Zordo T, Lacefield JC, et al:
Novel small interfering RNA-containing solution protecting donor
organs in heart transplantation. Circulation. 120:1099–1107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopez AD and Murray CC: The global burden
of disease, 1990–2020. Nat Med. 4:1241–1243. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reffelmann T and Kloner RA: The
‘no-reflow’ phenomenon: Basic science and clinical correlates.
Heart. 87:162–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falk E: Coronary thrombosis: Pathogenesis
and clinical manifestations. Am J Cardiol. 68:28B–35B. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Saldeen T, Romeo F and Mehta JL:
Oxidized LDL upregulates angiotensin II type 1 receptor expression
in cultured human coronary artery endothelial cells: The potential
role of transcription factor NF-kappaB. Circulation. 102:1970–1976.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie MY, Lv Q, Wang J and Yin JB:
Assessment of myocardial segmental function with coronary artery
stenosis in multi-vessel coronary disease patients with normal wall
motion. Eur Rev Med Pharmacol Sci. 20:1582–1589. 2016.PubMed/NCBI
|
|
8
|
Venturinelli ML, Hovnan A, Soeiro AM,
Nicolau JC, Ramires JA, D'Amico EA and Serrano CV Jr: Platelet
activation in different clinical forms of the coronary artery
disease (role of P-selectin and others platelet markers in stable
and unstable angina). Arq Bras Cardiol. 87:446–450. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanagisawa M, Kurihara H, Kimura S, Tomobe
Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K and Masaki T: A novel
potent vasoconstrictor peptide produced by vascular endothelial
cells. Nature. 332:411–415. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamirisa P, Frishman WH and Kumar A:
Endothelin and endothelin antagonism: Roles in cardiovascular
health and disease. Am Heart J. 130:601–610. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakai S, Miyauchi T, Kobayashi M,
Yamaguchi I, Goto K and Sugishita Y: Inhibition of myocardial
endothelin pathway improves long-term survival in heart failure.
Nature. 384:353–355. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao SK, Ober JC, Gonenne A, Clubb FJ Jr,
Krishnaswami A, Ferguson JJ, Anderson HV, Gorecki M, Buja LM and
Willerson JT: Active oxygen species play a role in mediating
platelet aggregation and cyclic flow variations in severely
stenosed and endothelium-injured coronary arteries. Circ Res.
73:952–967. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li AX, Sun M and Li X: Withaferin-A
induces apoptosis in osteosarcoma U2OS cell line via generation of
ROS and disruption of mitochondrial membrane potential. Eur Rev Med
Pharmacol Sci. 21:1368–1374. 2017.PubMed/NCBI
|
|
14
|
Yorimitsu T and Klionsky DJ: Eating the
endoplasmic reticulum: Quality control by autophagy. Trends Cell
Biol. 17:279–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laskey WK: Brief repetitive balloon
occlusions enhance reperfusion during percutaneous coronary
intervention for acute myocardial infarction: A pilot study.
Catheter Cardiovasc Interv. 65:361–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanemoto Y, Nakase H, Akita N and Sakaki
T: Effects of anti-intercellular adhesion molecule-1 antibody on
reperfusion injury induced by late reperfusion in the rat middle
cerebral artery occlusion model. Neurosurgery. 51:1034–1042. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma X, Zhang X, Li C and Luo M: Effect of
postconditioning on coronary blood flow velocity and endothelial
function and LV recovery after myocardial infarction. J Interv
Cardiol. 19:367–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bayram E and Atalay C: Identification of
the culprit artery involved in inferior wall acute myocardial
infarction using electrocardiographic criteria. J Int Med Res.
32:39–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Werns SW, Shea MJ, Mitsos SE, Dysko RC,
Fantone JC, Schork MA, Abrams GD, Pitt B and Lucchesi BR: Reduction
of the size of infarction by allopurinol in the ischemic-reperfused
canine heart. Circulation. 73:518–524. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shandelya SM, Kuppusamy P, Weisfeldt ML
and Zweier JL: Evaluation of the role of polymorphonuclear
leukocytes on contractile function in myocardial reperfusion
injury. Evidence for plasma-mediated leukocyte activation.
Circulation. 87:536–546. 1993. View Article : Google Scholar : PubMed/NCBI
|