Introduction
Defective megakaryopoiesis is responsible for
various types of blood disorders (1). Therefore, it is regarded as a crucial
source for treating thrombocytopenia, acute megakaryocytic leukemia
(M7), myeloproliferative diseases and myelodysplastic syndrome
(2–4). Understanding the molecular mechanisms
of megakaryocytic differentiation is important for developing novel
therapies for hematopoietic malignancy with defective MKs. Previous
data has demonstrated that protein arginine methyltransferase 1
(PRMT1) has high expression in leukemia, particularly in M7
(5). RNA binding motif protein 15
(RBM15) is required for the long-term maintenance of the
homeostasis of hematopoietic stem cells and for MK differentiation
(6,7). RBM15 stability is controlled by PRMT1
via CNOT4-mediated ubiquitylation (8). To investigate the biological
significance of PRMT1-mediated methylation of RBM15, the present
study analyzed the role of the PRMT1-RBM15 axis in
megakaryopoiesis. The present study demonstrated that human
umbilical cord blood cluster of differentiation (CD)34+
cells differentiated into mature MKs when they were cultured in
high thrombopoitin (TPO) medium. With MK maturation, the PRMT1
protein level decreased, while the RBM15 protein level increased.
Overexpression of PRMT1 and knock down of RBM15 blocked the
maturation of MKs due to the reduced RBM15 protein level. A PRMT1
inhibitor rescued the PRMT1-blocked megakaryocytic differentiation.
These results provide evidence for a novel role of PRMT1 in the
negative regulation of megakaryocytic differentiation. Targeting
PRMT1 may be a valuable therapeutic method for defective MK
diseases.
Materials and methods
Purification of cord blood
CD34+ cells
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Human umbilical cord blood was collected from 3
healthy pregnant women aged 28, 30 and 33 years old in The Second
Affiliated Hospital of Zhengzhou University from October 2015 to
April 2016. Informed content was provided from each patient.
Following separation with Ficoll gradient as previously mentioned
(9), the CD34+ cells were
purified with anti-CD34 magnetic beads (Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany) in accordance with the manufacturer's
protocol. Subsequently, the cells were cultured at 37°C for 48 h in
Iscove's modified Dulbecco's medium (IMDM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 20% serum
substitutes (BIT; Stemcell Technologies, Inc., Vancouver, BC,
Canada) and a cytokine mixture [20 ng/ml TPO, 20 ng/ml
interleukin-6, 100 ng/ml stem cell factor (SCF) and 10 ng/ml Flt3
ligand (PeproTech, Inc., Rocky Hill, NJ, USA)] (10). The purification achieved 95% purity
for CD34+ cells according to fluorescence-activated cell
sorting (FACS) analysis. The cells were collected by centrifugation
(300 × g at room temperature for 5 min), the supernatant was
aspirated and the cells were washed twice with iced PBS. The cells
were blocked with Human BD Fc Block™ (cat. no. 564219;
BD Biosciences, Franklin Lakes, NJ, USA) at a dilution of 1:100 and
incubated at room temperature for 30 min. A total of 1 µl anti-CD34
fluorescein isothiocyanate-conjugated antibodies (cat. no. 560238;
1:100; BD Biosciences) was added to 100 µl PBS containing 1% Fetal
Bovine Serum (FBS), the cells were cultured in this medium at room
temperature for 30 min. Cells were then analyzed using a BD
LSRFortessa™ machine with BD FACSDiva™
software (version 8.0) (both BD Biosciences) and FlowJo software
(version 7.6.5; FlowJo LLC, Ashland, OR, USA). To induce MK
differentiation, the expanded CD34+ cells were cultured
at 37°C in IMDM medium supplemented with 20% BIT with cytokine
mixture (TPO 100 and 2 ng/ml SCF). The expression of CD41 and CD42
was analyzed by flow cytometry 10 days after TPO stimulation. To
further investigate the function of PRMT1 in megakaryocytic
differentiation, a PRMT1 inhibitor DB75 (Sigma-Aldrich; Merck KGaA)
was used to treat the cells overexpressing PRMT1 protein and
cultured in the MK differentiation medium. For DB75 treatment, 20
µM DB75 was added to the medium for 10 days, which was every 3
days.
Virus production and transduction
The lentiviral vector, pTripZ (Open Biosystems;
Dharmacon), was engineered to express PRMT1 by replacing the shRNA
cassette with the PRMT1 coding sequence to achieve robust PRMT1
expression in hematopoietic cells. A total of 15 µg lentivirus
plasmids expressing the cDNA of PRMT1 V2, short hairpin (sh)RBM15#1
and shRBM15#2 (11,12) were transfected into 293T cells
(American Type Culture Collection, Manassas, VA, USA) with helper
plasmids. The 293T cells were cultured in Dulbecco's modified
Eagle's medium with 10% FBS at 37°C in an incubator with 5% CO2.
Calcium phosphate reagent [in 250 ml: 2D-Glucose (0.5 g), HEPES
(2.5 g) KCl (0.18 g) NaCl (4.0 g) and Na2HPO4
(0.05 g)] was used for virus production, as previously described
(13). Transduced positive
CD34+ cells were selected with 1 µg/ml puromycin.
Flow cytometry analysis
The cells were collected following centrifugation
(300 × g at room temperature for 5 min), the supernatant was
aspirated and the cells were washed twice with iced 2 ml PBS
containing 1% FBS. The cells were then blocked with Human BD Fc
Block™ (1:100) at room temperature for 30 min. A total
of 1 µl anti-CD41 (cat. no. 559777) and 1 µl anti-CD42 (cat. no.
555471) antibodies (both 1:100; BD Biosciences) was added to 1% FBS
in 100 µl PBS, and incubated with the cells at room temperature for
30 min. The BD FACSDiva software and BD LSRFortessa™
were used for data collection, and FlowJo software was used for
data analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to measure the mRNA expression
levels. Total RNA was isolated from all transfected and
non-transfected CD34+ cells using an RNeasy Micro kit
(cat. no. 74004; Qiagen GmbH, Hilden, Germany). Total RNA samples
(2 µg) were reverse transcribed using a SuperScript®
First-Strand Synthesis System for RT-PCR (cat. no. 11904-018;
Thermo Fisher Scientific, Inc.). qPCR was performed using a
SYBR® Green Real-time PCR Master Mix-Plus kit (Thermo
Fisher Scientific, Inc.). The RT-qPCR conditions were as follows:
An initial denaturation step at 94°C for 10 min, 35 cycles of 94°C
for 30 sec, 62°C for 30 sec and 68°C for 1 min, and a final
extension step at 68°C for 7 min. The samples were kept at 4°C. The
primer sequences used are listed in Table I. The copy number was normalized to
GAPDH levels using the 2-ΔΔCq method (14).
 | Table I.Primers used in reverse-transcription
quantitative polymerase chain reaction. |
Table I.
Primers used in reverse-transcription
quantitative polymerase chain reaction.
| Gene | Direction | Primer sequence
(5′-3′) |
|---|
| Protein arginine
methyltransferase 1 | Forward |
CCAGTGGAGAAGGTGGACAT |
|
| Reverse |
CTCCCACCAGTGGATCTTGT |
| RNA binding motif
protein 15 | Forward |
TCCCACCTTGTGAGTTCTCC |
|
| Reverse |
GTCAGCGCCAAGTTTTCTCT |
| GAPDH | Forward |
TGCACCACCAACTGCTTAGC |
|
| Reverse |
GGCATGGACTGTGGTCATGAG |
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde at room
temperature for 1 h and permeabilized with 0.25% Triton X-100. The
cells were then incubated with anti-PRMT1 (cat. no. 2449; Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-RBM15 (cat.
no. ab70549; Abcam, Cambridge, MA, USA) antibodies (both 1:100) for
2 h at room temperature. The cells were blocked with 5% Bovine
Serum Albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
room temperature for 30 min. Following 1% FBS in PBS washing, the
cells were stained with AlexaFluor-488-conjugated goat anti-rabbit
immunoglobulin G antibodies (1:1,000; cat. no. 14705; Cell
Signaling Technology, Inc.) at room temperature for 30 min and
counterstained with 4,6-diamidino-2-phenylindole at room
temperature for 30 min. Cell images were obtained at room
temperature using confocal microscopy (Nikon AIR+; Nikon
Corporation, Tokyo, Japan) with the magnification at ×400.
Statistical analysis
Data are presented as mean ± standard deviation from
three independent experiments. Data was statistical analysis using
GraphPad Prism software (version 6; GraphPad Software, Inc., La
Jolla, CA, USA). One-way analysis of variance followed by the
Student Newman-Keuls test was used to assess differences between
multiple groups of data, and a Student's t-test was used to compare
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Upon TPO stimulation, human umbilical
cord blood CD34+ cells differentiate into mature
MKs
To investigate the detailed role of PRMT1 and RBM15
in the process of megakaryocytopoiesis, CD34+ cells were
isolated from human umbilical cord blood cells. These cells gave
rise to CD41+CD42+ when grown in medium
containing TPO and SCF (Fig. 1A).
The PRMT1 mRNA level decreased and RBM15 mRNA level increased
during MK differentiation (Fig. 1B).
On day 10, intracellular immunostaining was conducted with
anti-PRMT1 and anti-RBM15 antibodies. The results demonstrated that
PRMT1-positive cells were markedly reduced at day 10 compared to
day 0, while RBM15-positive cells increased during MK
differentiation (Fig. 1C).
Consistently, it was demonstrated that intracellular PRMT1 protein
level was markedly reduced at day 10 compared to day 0 according to
FACS analysis, while the intracellular protein concentration of
RBM15 was elevated (Fig. 1D). These
data suggest that the PRMT1 and RBM15 protein levels are
dynamically regulated during normal MK differentiation.
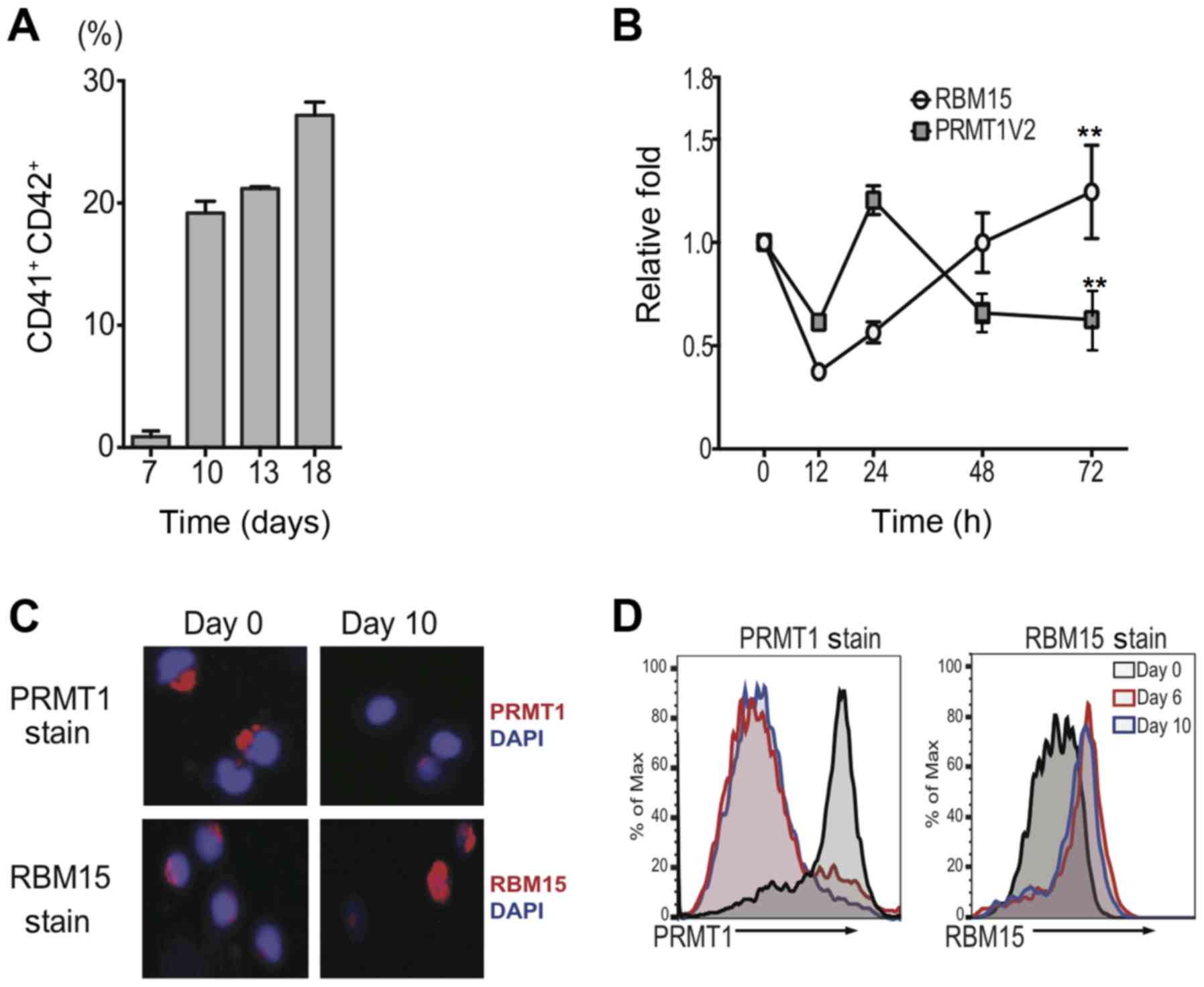 | Figure 1.Upon TPO stimulation, human umbilical
cord blood CD34+ cells differentiate into mature
megakaryocytes. (A) FACS analysis of CD41 and CD42 double-positive
cells cultured in medium containing high TPO on days 7, 10, 13 and
18. (B) Changes to RBM15 and PRMT1 expression at 12, 24, 48 and 72
h of TPO treatment. The mRNA levels were calculated as the mean ±
standard deviation from three independent experiments. (C)
Intracellular immunostaining with anti-PRMT1 and anti-RBM15
antibodies (magnification, ×400). (D) PRMT1 and RBM15 protein
levels were detected by FACS analysis in the course of
megakaryocyte differentiation. Results are displayed as histogram
overlays. **P<0.01 vs. 0 h. CD, cluster of differentiation;
FACS, fluorescence-activated cell sorting; RBM15, RNA binding motif
protein 15; PRMT1, protein arginine methyltransferase 1; PRMT1V2,
PRMT1 V2 isoform; DAPI, 4,6-diamidino-2-phenylindole; TPO,
thrombopoitin. |
PRMT1 blocks the differentiation of
human umbilical cord blood CD34+ cells into mature
MKs
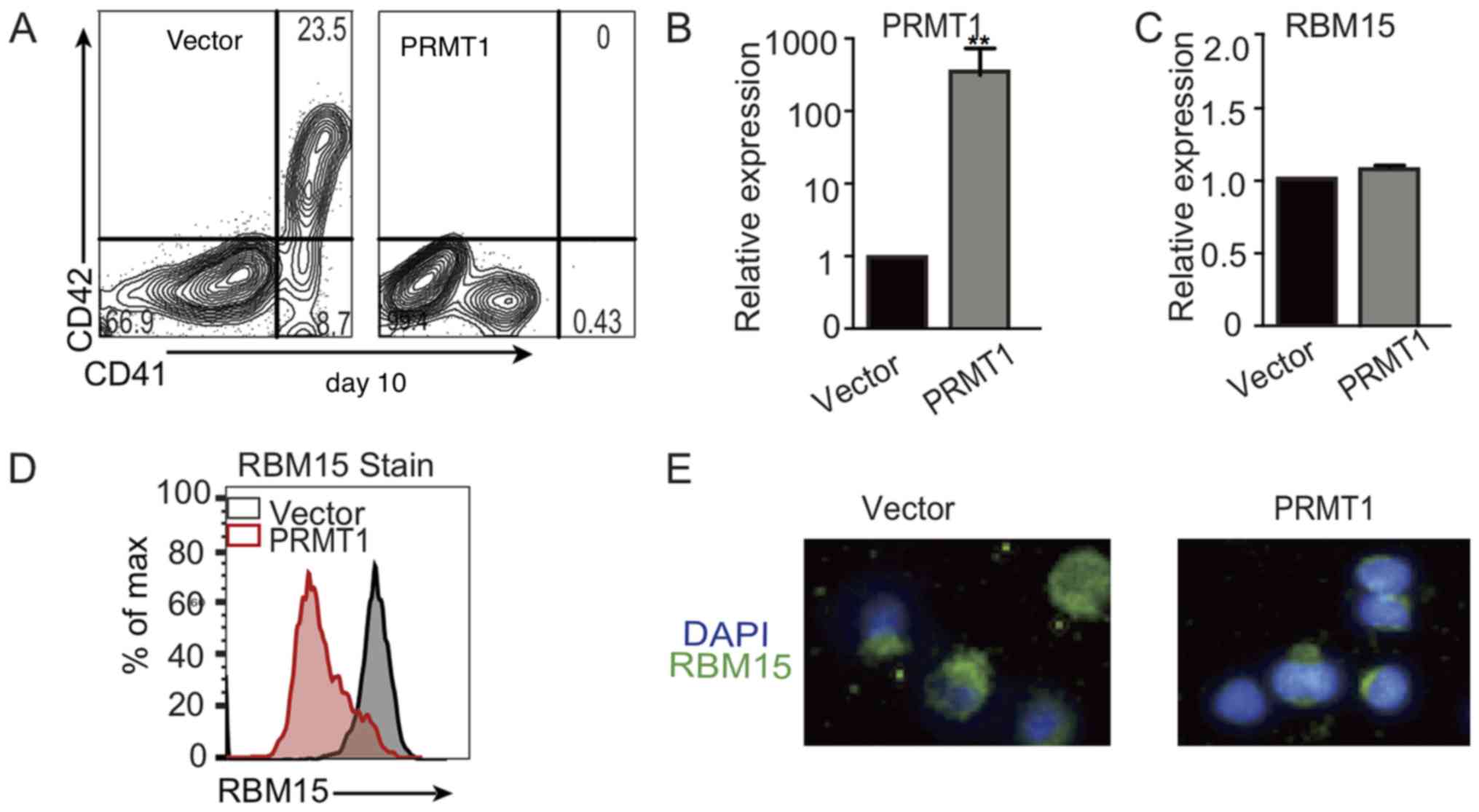
To validate the role of PRMT1 in the process of
megakaryocytopoiesis, CD34+ cells were transduced with
lentivirus expressing PRMT1. FACS analysis demonstrated that, on
day 10, CD41+CD42+ double-positive cells were
markedly reduced when cells were cultured in high TPO medium
(Fig. 2A). RT-qPCR analysis
demonstrated that PRMT1 mRNA expression levels were significantly
increased in CD34+ cells transfected with the vector or
PRMT1 lentivirus and cultured in CD34+ cells medium
(Fig. 2B). However, the RBM15 mRNA
level did not change significantly in cells transfected with the
vector or PRMT1 lentivirus (Fig.
2C). FACS analysis of PRMT1-overexpressing CD34+
cells validated that the RBM15 protein level was markedly reduced
compared with the level in the cells transfected with the control
vector (Fig. 2D). Immunofluorescence
microscopy with anti-RBM15 antibody staining further confirmed that
the RBM15 protein level was reduced in PRMT1-overexpressing cells
(Fig. 2E). These data suggest that
PRMT1 blocks the differentiation of human umbilical cord blood
CD34+ cells into mature MKs due to the decreased RBM15
protein level. RBM15 is important for MK differentiation.
Knockdown of RBM15 produces fewer
mature MKs
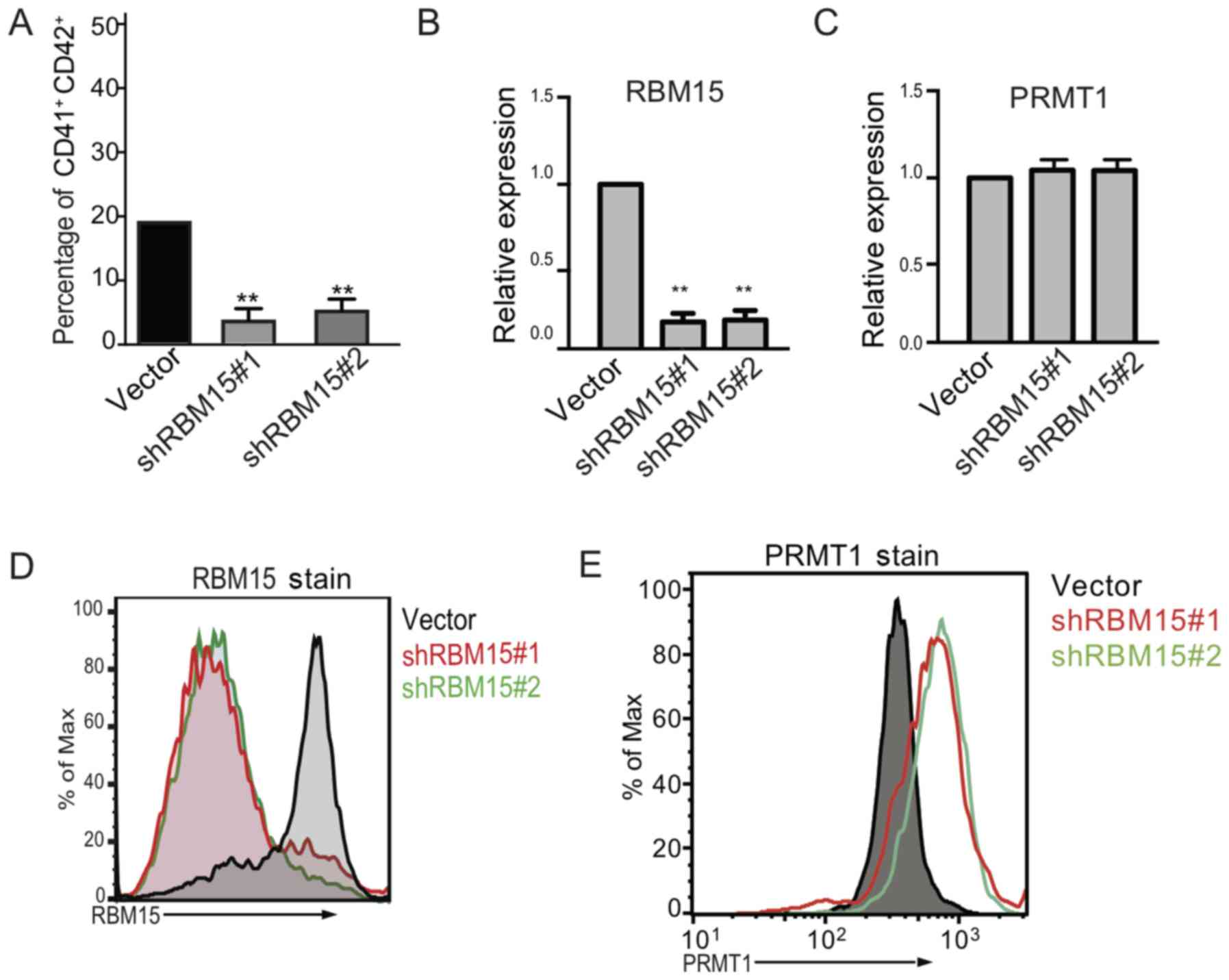
To further validate the importance of RBM15 in
regulating MK differentiation, RBM15 expression was knocked down
using shRNA in human cord blood CD34+ cells and the
cells were then cultured in MK differentiation medium. It was
demonstrated that knocking down RBM15 caused a significant decrease
in the CD41+ and CD42+ double-positive cells
compared with those treated with the vector control (Fig. 3A). The RBM15 mRNA expression level
was significantly decreased following RBM15 knock down compared
with the vector group, however, the PRMT1 mRNA expression level was
not significantly affected (Fig. 3B and
C). Flow cytometry demonstrated that the expression level of
RBM15 was decreased (Fig. 3D)
following knock down of RBM15, while PRMT1 increased (Fig. 3E). These data indicate that the
PRMT1-RBM15 axis regulates megakaryocytic differentiation at the
protein level, and the reduction of RBM15 expression produces fewer
mature MKs.
PRMT1 inhibitor rescues PRMT1-blocked
MK differentiation
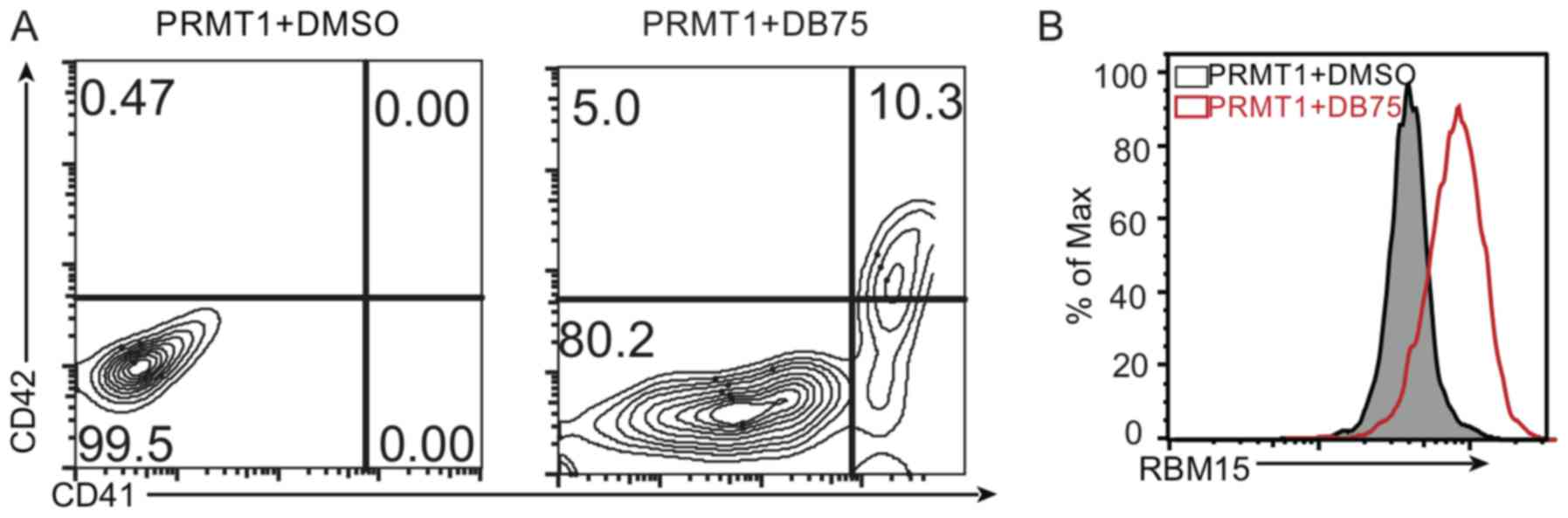
To further investigate the function of PRMT1 in
megakaryocytic differentiation, DB75 was used to treat the cells
overexpressing PRMT1 protein and cultured in the MK differentiation
medium. It was observed that the CD41+CD42+
double-positive cells were increased following treatment with PRMT1
inhibitor compared to the number of double-positive cells observed
following dimethyl sulfoxide (DMSO) treatment on day 10 (Fig. 4A). Additionally, the RBM15 protein
level increased following treatment with PRMT1 inhibitor compared
with that observed following DMSO treatment (Fig. 4B). These results demonstrate that
PRMT1 expression affects MK differentiation via regulating the
RBM15 protein level. These data provide a novel role of the
PRMT1-RBM15 axis in regulating megakaryocytic differentiation.
To further investigate the function of PRMT1 in
megakaryocytic differentiation, a PRMT1 inhibitor, DB75
(Sigma-Aldrich; Merck KGaA), was used to treat the cells
overexpressing PRMT1 protein and cultured in the MK differentiation
medium.
Discussion
Normal MK differentiation may be divided into two
phases: From human stem cells (HSC) to MK progenitor cells and from
MK progenitor cells to high polyploidy MK cells (15). Defective megakaryopoiesis is
responsible for various types of blood disorders (1). Thus, MKs are regarded as a crucial
source for treating thrombocytopenia, acute megakaryocytic leukemia
(M7), myeloproliferative diseases and myelodysplasia syndrome,
which affect a wide range of patients (2–4).
Understanding the molecular mechanisms of megakaryocytic
differentiation is important for developing novel therapies for
hematopoietic malignance with defective MKs.
PRMT1 serves critical roles in various cellular
processes, such as RNA splicing (16). PRMT1 is highly expressed in acute
myeloid and lymphoid leukemia, as well as in solid tumors (17,18).
Therefore, PRMT1-mediated MK blockage may contribute to
leukemogenesis. PRMT1 promotes the production of MK-erythroid
progenitor cells; however, PRMT1 has to be turned off to generate
mature, polyploidy CD41+CD42+ MK cells
(8). The present study demonstrated
that PRMT1 negatively modulated differentiation toward the
megakaryocytic lineage in human CD34+ hematopoietic
cells. PRMT1 activity is downregulated during megakaryocytic
differentiation. Immunostaining and flow cytometry analysis were
used in the present study to demonstrate, for the first time (to
the best of our knowledge), that the PRMT1 protein level is
dynamically regulated during differentiation down to the MK lineage
with primary cells. The dynamic regulation of PRMT1 concentration
reciprocally regulates the RBM15 protein concentration. In the
future, to identify upstream signals that are responsible for
regulating PRMT1 activity, it is crucial to fully understand how
hematopoiesis is regulated.
RBM15 is required to maintain the homeostasis of
long-term hematopoietic stem cells and the differentiation of MK
cells (10,11). RBM15 protein stability is controlled
by PRMT1 (5). RBM15 is involved in
chromosome translocation t(1;22), which produces the
RBM15-megakaryoblastic leukemia 1 (MKL1) fusion protein associated
with acute (A)MKL (19,20). PRMT1 is upregulated in AMKL (21). Consistent with RBM15 knock down in
human primary cells, RBM15 knockdown in mice produces a low
percentage of mature MKs (22).
Recently, the incidence of hematological malignancies has been
increasing, which is problematic for many families (23). Therefore, novel methods for treatment
are required. Targeting the RBM15/PRMT1 pathway may be a novel
therapeutic approach for related hematological malignancies.
Dysregulation of the PRMT1-RBM15 pathway may be a
common mechanism in hematological malignancies. The present study
identified a novel role of PRMT1 in the negative regulation of
human megakaryocytic differentiation via regulating the RBM15
protein level. DB75 is a PRMT1 inhibitor that has been demonstrated
to kill some leukemia cells (24).
The present study demonstrated that DB75 rescues PRMT1-blocked MK
differentiation via promoting RBM15 expression. Our previous data
indicated that PRMT1 methylates RBM15 at R578 and promotes RBM15
protein level degradation via ubiquitylation in a leukemia cell
line (8). The present study also
suggested that PRMT1 reduces RBM15 expression in human stem cells,
which may contribute to defective megakaryopoiesis. Therefore,
targeting the RBM15/PRMT1 pathway may be a novel therapeutic
approach for related hematological malignancies.
Acknowledgements
The present project was supported by grants from the
Nature Science Foundation of China (NSFC; grant nos. 81270570 and
81470365) to YL and a fellowship from China Scholarship Council
received by SJ.
References
|
1
|
Bourquin JP, Subramanian A, Langebrake C,
Reinhardt D, Bernard O, Ballerini P, Baruchel A, Cavé H, Dastugue
N, Hasle H, et al: Identification of distinct molecular phenotypes
in acute megakaryoblastic leukemia by gene expression profiling.
Proc Natl Acad Sci USA. 103:pp. 3339–3344. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Je EM, Yoo NJ, Kim YJ, Kim MS and Lee SH:
Mutational analysis of splicing machinery genes SF3B1, U2AF1 and
SRSF2 in myelodysplasia and other common tumors. Int J Cancer.
133:260–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto S, Toyama D, Yatsuki H,
Higashimoto K, Soejima H and Isoyama K: Acute megakaryocytic
leukemia (AMKL, FAB;M7) with Beckwith-Wiedemann syndrome. Pediatr
Blood Cancer. 55:733–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han Y, Tang Y, Chen J, Liang J, Ye C, Ruan
C and Wu D: Low-dose decitabine for patients with thrombocytopenia
following allogeneic hematopoietic stem cell transplantation: A
pilot therapeutic study. JAMA Oncol. 1:249–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zolotukhin AS, Uranishi H, Lindtner S,
Bear J, Pavlakis GN and Felber BK: Nuclear export factor RBM15
facilitates the access of DBP5 to mRNA. Nucleic Acids Res.
37:7151–7162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao N, Laha S, Das SP, Morlock K, Jesneck
JL and Raffel GD: Ott1 (Rbm15) regulates thrombopoietin response in
hematopoietic stem cells through alternative splicing of c-Mpl.
Blood. 125:941–948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Tran NT, Su H, Wang R, Lu Y, Tang
H, Aoyagi S, Guo A, Khodadadi-Jamayran A, Zhou D, et al: Cross-talk
between PRMT1-mediated methylation and ubiquitylation on RBM15
controls RNA splicing. eLife. 4:pii: e079382015. View Article : Google Scholar
|
|
9
|
Chang HC, Jones OW, Bradshaw C, Sarkar S
and Porreco RP: Enhancement of human amniotic cell growth by
Ficoll-Paque gradient fractionation. In Vitro. 17:81–90. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Z, Augustin J, Chang C, Hu J, Shah K,
Chang CW, Townes T and Jiang H: The DPY30 subunit in SET1/MLL
complexes regulates the proliferation and differentiation of
hematopoietic progenitor cells. Blood. 124:2025–2033. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horiuchi K, Kawamura T, Iwanari H, Ohashi
R, Naito M, Kodama T and Hamakubo T: Identification of Wilms' tumor
1-associating protein complex and its role in alternative splicing
and the cell cycle. J Biol Chem. 288:33292–33302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JH and Skalnik DG: Rbm15-Mkl1
interacts with the Setd1b histone H3-Lys4 methyltransferase via a
SPOC domain that is required for cytokine-independent
proliferation. PLoS One. 7:e429652012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vu LP, Perna F, Wang L, Voza F, Figueroa
ME, Tempst P, Erdjument-Bromage H, Gao R, Chen S, Paietta E, et al:
PRMT4 blocks myeloid differentiation by assembling a
methyl-RUNX1-dependent repressor complex. Cell Rep. 5:1625–1638.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Y, Smith E, Ker E, Campbell P, Cheng
EC, Zou S, Lin S, Wang L, Halene S and Krause DS: Role of
RhoA-specific guanine exchange factors in regulation of endomitosis
in megakaryocytes. Dev Cell. 22:573–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bedford MT and Clarke SG: Protein arginine
methylation in mammals: Who, what, and why. Mol Cell. 33:1–13.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mathioudaki K, Papadokostopoulou A,
Scorilas A, Xynopoulos D, Agnanti N and Talieri M: The PRMT1 gene
expression pattern in colon cancer. Br J Cancer. 99:2094–2099.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang YI, Hua WK, Yao CL, Hwang SM, Hung
YC, Kuan CJ, Leou JS and Lin WJ: Protein-arginine methyltransferase
1 suppresses megakaryocytic differentiation via modulation of the
p38 MAPK pathway in K562 cells. J Biol Chem. 285:20595–20606. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Z, Morris SW, Valentine V, Li M,
Herbrick JA, Cui X, Bouman D, Li Y, Mehta PK, Nizetic D, et al:
Fusion of two novel genes, RBM15 and MKL1, in the t(1;22) (p13;
q13) of acute megakaryoblastic leukemia. Nat Genet. 28:220–221.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mercher T, Coniat MB, Monni R, Mauchauffe
M, Nguyen Khac F, Gressin L, Mugneret F, Leblanc T, Dastugue N,
Berger R and Bernard OA: Involvement of a human gene related to the
Drosophila spen gene in the recurrent t(1;22) translocation of
acute megakaryocytic leukemia. Proc Natl Acad Sci USA. 98:pp.
5776–5779. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baldwin RM, Morettin A, Paris G, Goulet I
and Côté J: Alternatively spliced protein arginine
methyltransferase 1 isoform PRMT1v2 promotes the survival and
invasiveness of breast cancer cells. Cell Cycle. 11:4597–4612.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mercher T, Raffel GD, Moore SA, Cornejo
MG, Baudry-Bluteau D, Cagnard N, Jesneck JL, Pikman Y, Cullen D,
Williams IR, et al: The OTT-MAL fusion oncogene activates
RBPJ-mediated transcription and induces acute megakaryoblastic
leukemia in a knockin mouse model. J Clin Invest. 119:852–864.
2009.PubMed/NCBI
|
|
23
|
Chihara D, Ito H, Matsuda T, Shibata A,
Katsumi A, Nakamura S, Tomotaka S, Morton LM, Weisenburger DD and
Matsuo K: Differences in incidence and trends of haematological
malignancies in Japan and the United States. Br J Haematol.
164:536–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan L, Yan C, Qian K, Su H, Kofsky-Wofford
SA, Lee WC, Zhao X, Ho MC, Ivanov I and Zheng YG: Diamidine
compounds for selective inhibition of protein arginine
methyltransferase 1. J Med Chem. 57:2611–2622. 2014. View Article : Google Scholar : PubMed/NCBI
|