Introduction
Inflammatory bowel disease (IBD) usually leads to
severe diarrhea, pain, fatigue and weight loss, and sometimes
causes life-threatening complications (1). The incidence of IBD is increasing each
year, while the underlying molecular mechanisms remain to be fully
elucidated. It is well known that IBD results from a defective
mucosal immune response that ultimately promotes chronic
inflammation of the entire or part of the digestive tract (1,2). A
crucial event in the development of IBD is that the high
sensitivity to the bacterial microflora in the gastrointestinal
tract induces severe autoimmunity enteritis, in which regulatory T
(Treg) cells and the type 17 T-helper (Th17) cell subset are
involved (3,4). Treg cells, characteristically
expressing the transcription factor Forkhead box p3 (Foxp3), are
immunosuppressive and generally suppress or downregulate the
induction and proliferation of effector T cells (4). In T cell-induced mouse colitis, colonic
inflammation and damage are prevented by adoptive transfer of Treg
cells (5). Furthermore, Treg cells
regulate self-reactive lymphocytes via a variety of mechanisms,
including secretion of inhibitory cytokines such as interleukin
(IL)-10 and transforming growth factor-β (TGF-β), granzyme-mediated
cytolysis, cytotoxic T-lymphocyte-associated protein 4 induction,
metabolic disruption and dendritic cell targeting (6). By inducing and/or maintaining Foxp3
expression, TGF-β promotes the differentiation and maturation of
Treg cells that inhibit the unwanted immune response and dampen
inflammation after microbial infection (7). Mice deficient of IL-10 not only
developed colitis, but also colon cancer at a high rate (8).
Of note, TGF-β in conjunction with IL-6 also leads
to the production of Th17 cells, which predominantly produce IL-17,
a potent pro-inflammatory cytokine (9). The inflamed gastrointestinal mucosa of
patients with IBD exhibits an excessive infiltration of Th17 cells
and Th17-associated cytokines, including IL-17, IL-6, IL-23 and
interferon (IFN)-γ in colon tissues (10). Hence, the dysregulation of cytokines
driven by TGF-β modulates intercellular communications, and thus
has a crucial role in the pathogenesis of IBD. TGF-β, belonging to
the TGF-β superfamily, includes three major isoforms, TGF-β1, −2
and 3, secreted by a variety of cell types (11). Normally, a large amount of TGF-β1
produced in the gastrointestinal tract is in its latent precursor
form, requiring cleavage and dissociation for conversion to a
mature bioactive dimer (12).
Elevated expression of TGF-β1 was detected in colon tissues from
IBD patients (12). In mice,
overexpression of consecutively activated TGF-β1 by adenoviral
delivery into the local colon tissue causes intestinal fibrosis
(13), indicating a potential
therapeutic target in the prevention of fibrosis in IBD. However,
it has been demonstrated that overexpression of TGF-β1 induces
suppression of the Th1 cell response by upregulating IL-10 and
downregulating IL-12 receptor β2 chain (14), which has been proposed as a potential
therapy for IBD.
The role of TGF-β1 in inflamed colon disease is
complex, as it is a multi-functional cytokine, which negatively as
well as positively regulates the immune response. The present study
provided evidence that the therapeutic effect of dexamethasone may
depend on the local TGF-β1 levels in 2,4,6-trinitrobenzenesulfonic
acid (TNBS)-induced mouse colitis at least partially through
promoting the differentiation of Treg cells and thus altering the
balance of pro- and anti-inflammatory cytokines.
Materials and methods
TNBS-mediated induction and treatment
of colitis in mice
A total of 92 male BALB/c mice (weight, 22.0–25.6 g;
age, 8 weeks; Chinese Academy of Sciences, Beijing, China) were
used in all experiments and were randomly divided into different
groups of 4 animals each. All of the animal protocols were approved
by the Experimental Animal Ethics Committee of Peking University
People's Hospital (Beijing, China). All mice were given free to
access to water and food, and housed in a pathogen-free animal
facility maintained at 25°C with a relative humidity of 50±10%, and
illuminated by a 12-h light-dark cycle. For induction of colitis,
mice were placed individually in an induction chamber and
anaesthetised with 4% enflurane (Isoflo; Esteve Farma, Lisboa,
Portugal) in 100% oxygen at a delivery rate of 1.0 l/min until loss
of movement. Following anaesthesia, 2.5 mg TNBS (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) in 150 µl 50% ethanol was
administered through a 3.5 F catheter inserted ~4 cm to the rectum.
Control mice were treated with 150 µl 50% ethanol. Mice were
allowed to recover for 24 h, and thereafter, the colitic mice were
given a PBS enema containing 1×107, 1×108 or
1×109 pfu of adenovirus expressing full-length mouse
TGF-β1 (Vector Biolabs, Malvern, PA, USA). A proportion of the
colitic mice receiving adenoviral TGF-β1 were treated by oral
gavage with 5 mg/kg body weight of dexamethasone (Sigma-Aldrich;
Merck KGaA). All mice were sacrificed at the indicated
time-points.
Macroscopical scoring
Tissue samples were excised and damage was evaluated
by an investigator blinded to the grouping. As described previously
(15), macroscopical damage was
scored on a 0–10 scale (0, normal; 1, localized hyperemia, no
ulcers; 2, ulceration without hyperemia or bowl wall thickening; 3,
ulceration with inflammation at 1 site; 4, ulceration and
inflammation at 2 or more sites; 5, major sites of damage extended
>1 cm along length of colon; 6–10, when an area of damage
extended >2 cm along length of the colon, the score was
increased by 1 for each additional cm of involvement).
Myeloperoxidase (MPO) and alkaline
phosphatase (ALP) activity assay
Colon tissue (50 mg) was homogenized in 4 volumes of
ice cold lysis buffer (0.1% Nonidet P (NP)-40/PBS) using a Dounce
homogenizer, and then centrifuged at 13,400 × g for 5 min at 4°C.
The supernatant was collected, the MPO activity was determined by
using an MPO Activity detection kit (cat. no. ab111749; Abcam,
Cambridge, MA, USA) and values were expressed as the unit per g
tissue. For evaluation of ALP activity, colon tissue protein was
extracted with a lysis buffer composed of 20 mM Tris-HCl (pH 7.5),
150 mM NaCl and 1% Triton X-100. The enzymatic activity of ALP was
determined using a Diethanolamine assay (cat. no. AP0100;
Sigma-Aldrich; Merck KGaA) and expressed as units per mg protein of
tissue.
ELISA
The levels of TGF-β1 (cat. no. ab119557), IL-17
(cat. no. ab100702), IL-23 (cat. no. cat. no. ab119545), IL-10
(cat. no. ab100697), IL-6 (cat. no. ab100712) and IFN-γ (cat. no.
ab100690; all Abcam) as well as tumour necrosis factor (TNF)-α
(cat. no. 88-7324-22; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) were measured using ELISA according to the manufacturer's
protocol.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mesenteric lymph nodes
with an RNeasy Mini kit (Qiagen, Hilden, Germany), and then
reversely transcribed into complementary (c)DNA by using a cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.). The cDNA (2 µl) was
used to perform quantitative PCR with 1X SYBR-Green Mix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and 250 nM primers on a 7700
real-time PCR Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) for detecting the expression of various genes
with the following primers: RAR-related orphan receptor (ROR)γt
forward, 5′-TTTTCCGAGGATGAGATTGC-3′ and reverse,
5′-CTTTCCACATGCTGGCTACA-3′; Foxp3 forward,
5′-CAGCTCTGCTGGCGAAAGTG-3′ and reverse,
5′-TCGTCTGAAGGCAGAGTCAGGA-3′; TGF-β1 forward,
5′-TGACGTCACTGGAGTTGTACGG-3′ and reverse,
5′-GGTTCATGTCATGGATGGTGC-3′; GAP DH forward,
5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse,
5′-CCTGCTTCACCACCTTCTTGA-3′. The PCR program began with initial
denaturation at 95°C for 10 min followed by 40 cycles of 95°C for
15 sec and 58°C for 30 sec. The mRNA level was calculated using
2−ΔΔCt and normalized to GAPDH as described (16). Values are expressed as the fold
change of the control.
Flow cytometry
To quantify the amount of Th17 and Treg cells, the
mesenteric lymph nodes were cut into small pieces, incubated with
2.5 mM EDTA at 37°C with agitation for 20 min to remove cells,
minced and digested for 20 min at 37°C with 1 mg/ml collagenase
type VIII (Sigma-Aldrich; Merck KGaA). The T cells were isolated
using a Pan T-cell Isolation kit II (cat. no. 130-095-130; Miltenyi
Biotec, Bergisch Gladbach, Germany). The isolated T cells were
fixed and stained with anti-FoxP3 antibodies (cat. no. 130-098-119;
1:100; Miltenyi Biotec) or anti-IL-17A antibodies (cat. no.
130-103-007; 1:200; Miltenyi Biotec). Cell sorting analysis was
performed on a FACSCalibur flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
Western blot analysis
Total protein was extracted from colon tissue by
using radioimmunoprecipitation assay lysis buffer (150 mM NaCl, 1%
NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 50 mM Tris-HCl pH 7.4
and 10% glycerol) supplemented with protease and phosphatase
inhibitors (Roche Diagnostics, Basel, Switzerland). In total, 100
µg protein per lane was subjected to 10 or 15% SDS-PAGE and then
transferred to a nitrocellulose membrane (Thermo Fisher Scientific,
Inc.). Membranes were blocked for 1 h in Tris-buffered saline
containing 0.05% Tween-20 (TTBS) and 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) or non-fat milk. Thereafter, membranes
were incubated with the indicated primary antibodies (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight, including
mouse anti-TGFβ1 (cat. no. sc-130348; 1:600), mouse anti-caspase3
(cat. no. sc-136219; 1:1,000), mouse anti-cleaved caspase3 (cat.
no. sc-271028; 1:500), rabbit anti-Bim (cat. no. sc-11425; 1:200),
mouse anti-p38MAPK (cat. no. sc-7972; 1:1,000), rabbit
anti-phospho-p38MAPK (cat. no. sc-17852-R; 1:1,000), mouse anti-JNK
(cat. no. sc-7345; 1:500), anti-phospho-JNK (cat. no. sc-293136;
1:1,000), anti-c-Jun (cat. no. sc-166540; 1:800) and mouse
anti-β-actin (cat. no. sc-130300; 1:2,000). After 5 washes with
TTBS, membranes were incubated with horseradish
peroxidase-conjugated goat anti-mouse or rabbit immunoglobulin G
(cat. no. sc-2005 and sc-2004, repectively; 1:5,000; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Blots were
developed with an enhanced chemiluminescence detection kit (cat.
no. 32106; Pierce; Thermo Fisher Scientific, Inc.). The specific
band was scanned and then quantified with ImageJ 1.49 software
(National Institutes of Health, Bethesda, NJ, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical evaluation was performed by using one-way
analysis of variance (ANOVA) with Dunnett's multiple comparison
post hoc tests for comparing multiple time-points in the same group
or two-way ANOVA with Tukey's multiple comparison post-hoc tests
for comparing multiple groups at multiple time-points with Prism
4.0 software (GraphPad Inc., La Jolla, CA, USA). P≤0.05 was
considered to indicate a statistically significant difference.
Results
Effects of local TGF-β1 levels on
colon damage in TNBS-treated mice
In the present study, a mouse model of TNBS-induced
colitis was used to investigate the effect of local TGF-β1 levels
on the severity of colon inflammation. As described previously
(13), the TGF-β1 levels in colon
tissues were modulated by delivering different amounts of
adenovirus expressing full-length mouse TGF-β1. Colon TGF-β1 levels
were measured by using western blot and RT-qPCR. Compared with that
in the control group, the protein abundance of TGF-β1 in
TNBS-treated mice was significantly higher at days 2, 5 and 7, and
overexpression of TGF-β1 was detected in TNBS-treated mice
following adenoviral TGF-β1 delivery (Fig. 1A). In addition, in comparison with
those in the control group, the mRNA levels of TGF-β1 in
TNBS-treated mice were also significantly increased at days 2, 5
and 7. In TNBS-treated mice, adenoviral TGF-β1 delivery led to a
marked increase of TGF-β1 mRNA in a dose-dependent manner (Fig. 1B). The levels of the active form of
TGF-β1 were also assessed using ELISA. Of note, the activated
TGF-β1 levels in TNBS-treated mice were not obviously changed over
a time course of 14 days compared with those in the control group.
However, delivery of adenoviral TGF-β1 resulted in a dose-dependent
increase of activated TGF-β1 in TNBS-treated mice (Fig. 1C).
Colon damage was then evaluated by determining the
macroscopic mucosal damage score (Table
I). Compared with those in the control group, the colon damage
score in TNBS-treated mice increased significantly over all
time-points. In TNBS-treated mice, delivery of AdTGF-1
significantly decreased colon damage at days 2 and 5, and slightly
decreased the score at days 7 and 14; however, there was no
significant difference. By contrast, delivery of AdTGF-2 or AdTGF-3
significantly increased the colon damage score at days 2 and 14,
and had no obvious effect on colon damage in TNBS-treated mice at
days 5 and 7 (Table I).
 | Table I.Colonic damage score in mice with
TNBS-induced intestinal inflammation following administration of
AdTGF-β1. |
Table I.
Colonic damage score in mice with
TNBS-induced intestinal inflammation following administration of
AdTGF-β1.
| Time-point | CTL | TNBS | TNBS + AdTGF-1 | TNBS + AdTGF-2 | TNBS + AdTGF-3 |
|---|
| Day 2 | 0.3±0.18 |
5.6±0.23a |
4.2±0.12a,b |
7.3±0.24a,b |
8.3±0.14a,b |
| Day 5 | 0.5±0.17 |
8.6±0.23a | 6.1±0.19
a,b |
8.6±0.23a |
9.0±0.12a |
| Day 7 | 0.7±0.17 |
7.3±0.18a | 6.5±0.24
a |
7.8±0.12a |
8.2±0.11a |
| Day 14 | 0.6±0.12 |
6.1±0.17a |
5.8±0.23a |
7.4±0.11a,b |
7.7±0.18a,b |
The present study also assessed colon inflammation
responses by determining the activities of MPO and ALP in colon
tissue. MPO activity, directly associated with the neutrophil
content, is commonly used for quantification of inflammation
severity (17). Colonic inflammation
is also characterized by an increase in AP activity, which has been
mainly attributed to leucocyte activity (18). Compared with the control, a
significant increase of MPO activity was detected in TNBS-treated
mice at all time-points. Delivery of AdTGF-2 or AdTGF-3 further
enhanced MPO activity in TNBS-treated mice, while delivery of
AdTGF-1 markedly inhibited the TNBS-induced increase of MPO
activation at days 2 and 5 (Table
II). In comparison with the control, ALP activity also
increased significantly at all time-points in TNBS-treated mice.
Delivery of AdTGF-1 decreased TNBS-induced ALP activation at all
time-points, and AdTGF-2 decreased TNBS-induced ALP activation at
days 5, 7 and 14. However, ALP activity was not markedly affected
in TNBS-treated mice following AdTGF-3 delivery (Table III). These results suggested that
the colonic TGFβ-1 levels may influence the severity of colon
inflammation and damage in mice with TNBS-induced colitis.
 | Table II.Evaluation of myeloperoxidase
activities (U/g colon tissue) in mice with TNBS-induced intestinal
inflammation following administration of AdTGF-β1. |
Table II.
Evaluation of myeloperoxidase
activities (U/g colon tissue) in mice with TNBS-induced intestinal
inflammation following administration of AdTGF-β1.
| Time-point | CTL | TNBS | TNBS + AdTGF-1 | TNBS + AdTGF-2 | TNBS + AdTGF-3 |
|---|
| Day 2 | 6.63±0.83 |
138.05±9.87a |
81.83±4.01a,b |
175.43±6.54a,b |
215.43±9.94a,b |
| Day 5 | 6.07±0.76 |
95.53±2.82a |
70.67±3.48a,b |
156.00±7.58a,b |
182.00±5.35a,b |
| Day 7 | 6.87±0.64 |
75.44±3.12a |
56.67±2.44a |
93.33±3.53a |
158.67±4.67a,b |
| Day 14 | 6.67±0.67 |
49.45±4.08a |
34.07±4.19a |
80.40±2.69a,b |
110.77±5.82a,b |
 | Table III.Evaluation of ALP (U/mg protein of
colon tissue) activities in mice with TNBS-induced intestinal
inflammation following administration of AdTGF-β1. |
Table III.
Evaluation of ALP (U/mg protein of
colon tissue) activities in mice with TNBS-induced intestinal
inflammation following administration of AdTGF-β1.
| Time-point | CTL | TNBS | TNBS + AdTGF-1 | TNBS + AdTGF-2 | TNBS + AdTGF-3 |
|---|
| Day 2 | 0.507±0.052 |
1.150±0.065a |
0.660±0.057b |
1.000±0.058a |
1.212±0.043a |
| Day 5 | 0.510±0.085 |
1.775±0.085a |
0.700±0.029b |
0.816±0.044a,b |
1.935±0.062a |
| Day 7 | 0.527±0.037 |
1.583±0.044a |
0.722±0.032b |
0.800±0.058a,b |
1.462±0.055a |
| Day 14 | 0.537±0.052 |
1.496±0.055a |
0.830±0.047a,b |
0.700±0.058a,b |
1.332±0.064a |
Effects of colonic TGF-β1 levels on
dexamethasone efficacy in mice with TNBS-induced colon
inflammation
Following adenoviral TGF-β1 delivery, dexamethasone
was administered to TNBS-treated mice once a day by orogastric
gavage for 4 days. Compared with those in control mice, the colon
damage score in the TNBS group were significantly increased.
TNBS-induced colon damage was obviously alleviated by delivery of
AdTGF-1 but was not influenced by AdTGF-2 and AdTGF-3. Of note,
dexamethasone treatment significantly decreased the colon damage
score in TNBS-treated mice receiving AdTGF-1 or AdTGF-2, while it
had no effect in TNBS-induced mice receiving AdTGF-3 (Fig. 2A). Compared with that in the control
group, the ALP activity in TNBS-treated mice was significantly
increased. Delivery of AdTGF-1 or AdTGF-2 significantly prevented
TNBS-induced ALP activation, while AdTGF-3 had no effect on ALP
activity in TNBS-treated mice. Dexamethasone treatment did not
affect ALP activity in TNBS-induced mice receiving AdTGF-1 or
AdTGF-2, while it markedly decreased the ALP activity in
TNBS-induced mice receiving AdTGF-3 (Fig. 2B). In comparison with the control
group, a significant increase of the MPO activity was detected in
TNBS-treated mice, which was further increased by delivery of
AdTGF-2 or AdTGF-3. Compared with that in the TNBS group, the
activation level of MPO was decreased in TNBS-induced mice
receiving AdTGF-1. Of note, in TNBS-treated mice receiving AdTGF-1
or AdTGF-2, the MPO activity was decreased by dexamethasone
treatment in a significant manner. However, dexamethasone did not
affect the MPO activity in TNBS mice receiving AdTGF-3 (Fig. 2C). Accordingly, the local TGF-β1
levels not only affected colon damage but also influenced the
efficacy of dexamethasone in mice with TNBS-induced colitis.
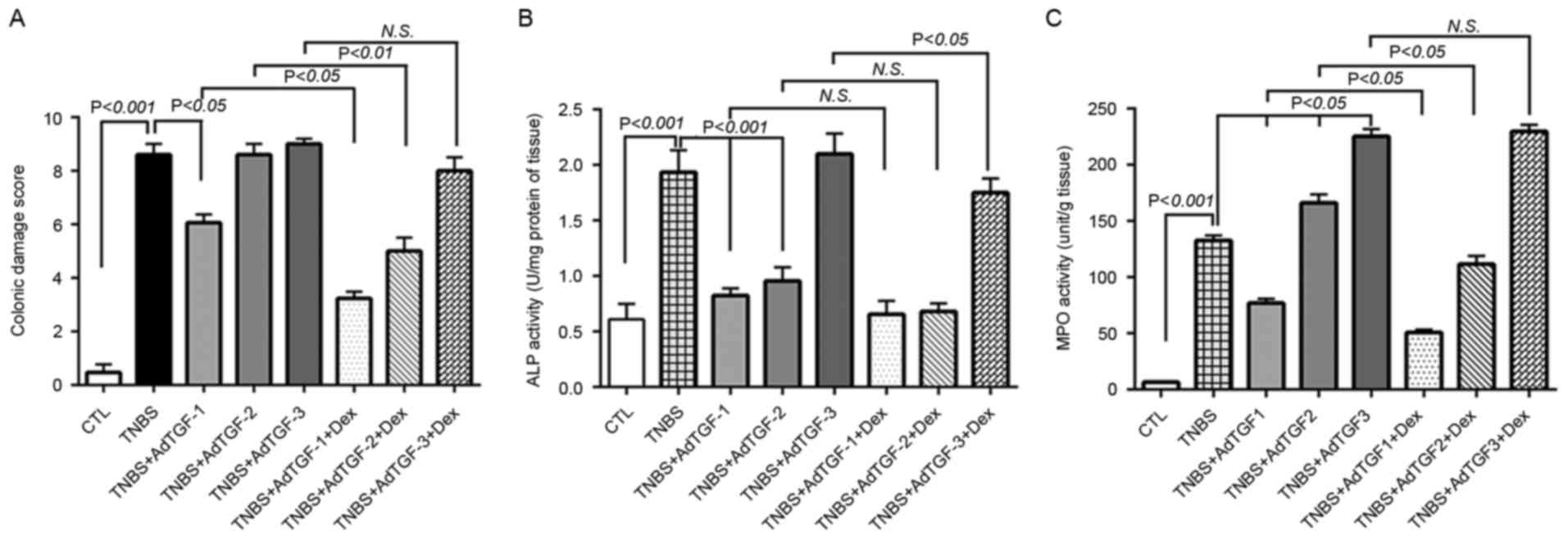 | Figure 2.Effects of local TGF-β1 levels on
dexamethasone efficacy in mice with TNBS-induced colitis. AdTGF-β1
was delivered to the colons of TNBS mice and dexamethasone (5 mg/kg
body weight) was given once a day by orogastric gavage for 4 days.
(A) The colonic damage score was determined to evaluate macroscopic
mucosal damage. (B) Colon tissue protein was extracted with a lysis
buffer composed of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl and 1%
Triton X-100, and ALP activity was determined. (C) Homogenates of
colon tissues were prepared and evaluated for MPO enzymatic
activity. Doses: AdTGF-1, 1×107 pfu; AdTGF-2,
1×108 pfu; AdTGF-3, 1×109 pfu. Values are
expressed as the mean ± standard deviation (n=4 per group). N.S.,
no significance; MPO, myeloperoxidase; ALP, alkaline phosphatase;
CTL, control; TNBS, 2,4,6-trinitrobenzenesulfonic acid; AdTGF,
adenovirus overexpressing transforming growth factor β1; Dex,
dexamethasone. |
Effects of local TGF-β1 levels and
dexamethasone treatment on colonic cytokine secretion in
TNBS-treated mice
The cytokine response is one of the major
characteristics in IBD (7). In the
present study, the levels of the Th1-associated cytokine IFN-γ, the
pro-inflammatory cytokines TNF-α and IL-6, the Th17-associated
cytokines IL-23 and IL-17, and the Th2-associated cytokine IL-10
were assessed in the homogenates of colonic tissues (Fig. 3). Compared with those in the control
group, the levels of IFN-γ, TNF-α, IL-6, IL-17 and IL-23 increased
significantly, while those of IL-10 decreased significantly in
TNBS-treated mice. Of note, delivery of AdTGF-1 further increased,
while that of AdTGF-3 further decreased the IL-10 levels in
TNBS-treated mice. IL-10 levels were decreased in TNBS-treated mice
following AdTGF-2 delivery. Dexamethasone treatment enhances the
effect with AdTGF-1 on the IL-10 levels in TNBS-treated mice.
Delivery of AdTGF-1 prevented TNBS-induced increases of IFN-γ,
TNF-α and IL-23, but had no significant effect on the levels of
IL-6 and IL-17. However, TNBS-induced increases of IFN-γ, IL-6 and
IL-23 were enhanced by AdTGF-2 and AdTGF-3. The levels of TNF-α and
IL-17 were further increased in TNBS-treated mice following AdTGF-3
delivery, while the effect of AdTGF-2 was not significant. Of note,
dexamethasone treatment caused a downregulation of the levels of
TNF-α, IL-6, IL-17 and IL-23 in TNBS-induced mice receiving
AdTGF-2, while it had no obvious effects in TNBS-treated mice
receiving AdTGF-1 or AdTGF-3. However, dexamethasone treatment led
to a downregulation of the levels of IFN-γ in TNBS-induced mice
receiving AdTGF-1 and AdTGF-2, and had no effects on IFN-γ levels
in TNBS mice receiving AdTGF-3. Therefore, the results of the
present study suggested that the local TGF-β1 levels may determine
the efficacy of dexamethasone in regulating the balance of
cytokines in mice with TNBS-induced colitis.
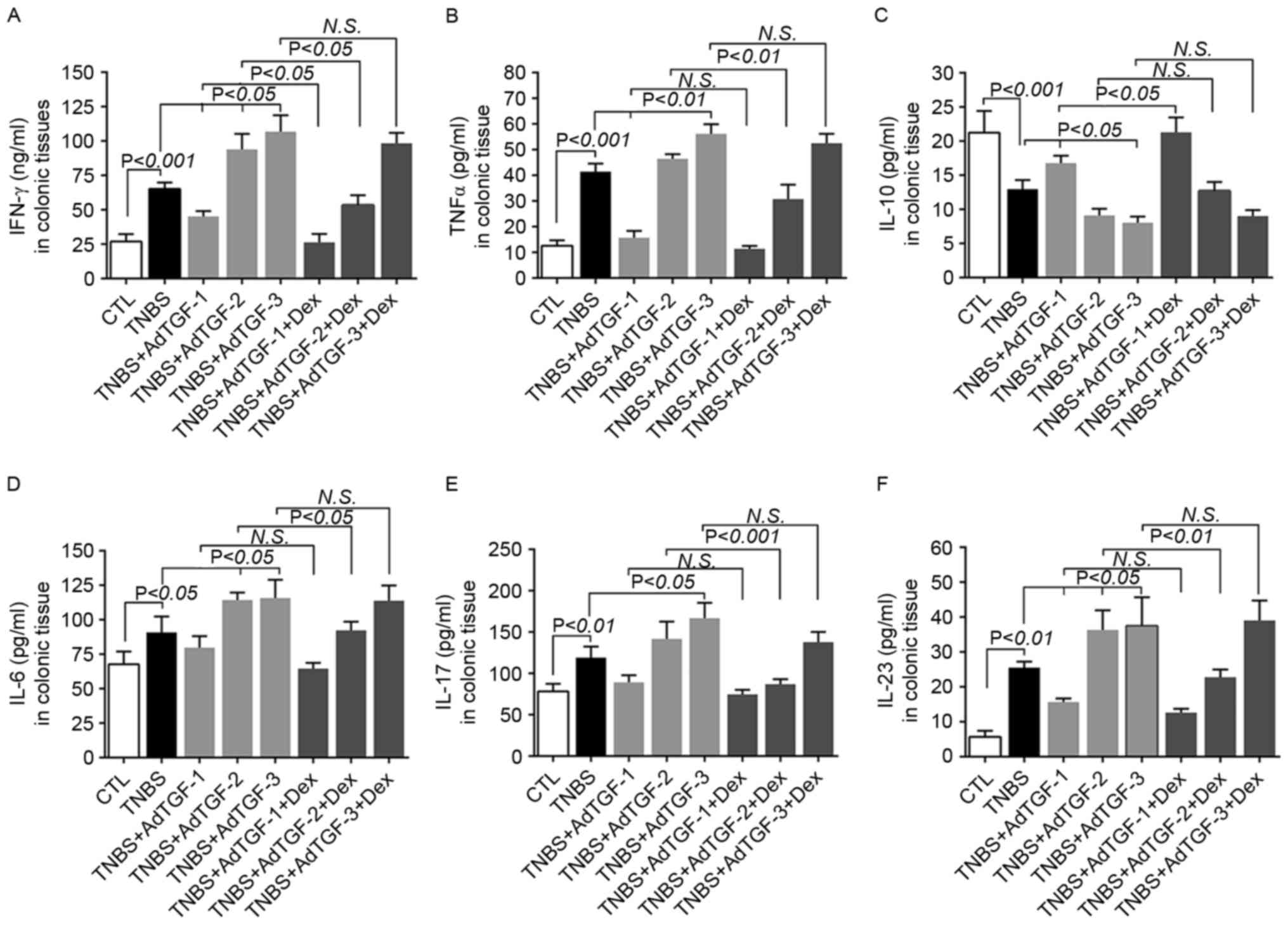 | Figure 3.Effects of local TGF-β1 levels and
dexamethasone treatment on colon cytokine secretion in TNBS-treated
mice. AdTGF-β1 was delivered to the colons of TNBS mice and
dexamethasone (5 mg/kg body weight) was given once a day by
orogastric gavage for 4 days. Homogenates of colonic tissues were
used to measure the levels of (A) IFN-γ, (B) TNF-α, (C) IL-10, (D)
IL-6, (E) IL-17 and (F) IL-23. Doses: AdTGF-1, 1×107
pfu; AdTGF-2, 1×108 pfu; AdTGF-3, 1×109 pfu.
Values are expressed as the mean ± standard deviation (n=4 per
group). N.S., no significance; IFN, interferon; IL, interleukin;
TNF, tumor necrosis factor; CTL, control; TNBS,
2,4,6-trinitrobenzenesulfonic acid; AdTGF, adenovirus
overexpressing transforming growth factor β1; Dex,
dexamethasone. |
Effects of local TGF-β1 levels and
dexamethasone treatment on regulating the balance of Th17 and Treg
cells in mesenteric lymph nodes of TNBS-treated mice
To investigate the role of Treg and Th17 cells in
mice with TNBS-induced colitis, the fraction of Treg and Th17 cells
in mesenteric lymph nodes was assessed. Furthermore, the levels of
transcription factors Foxp3 for Treg cells and RORγt for Th17 cells
were analyzed using RT-qPCR (Fig.
4A). Compared with those in the control, the mRNA levels of
RORγt increased significantly in TNBS-treated mice, which was
further increased by the delivery of AdTGF-3, but not by AdTGF-1 or
AdTGF-2. Dexamethasone treatment had no effect on the RORγt mRNA
levels in TNBS-induced mice receiving adenoviral TGF-β1. As
compared with the control, TNBS enema led to a significant decrease
of Foxp3 mRNA, which was inhibited by AdTGF-1. However, AdTGF-3
further decreased Foxp3 mRNA levels in TNBS-treated mice. Of note,
dexamethasone treatment prevented the reduction of Foxp3 mRNA in
TNBS-treated mice receiving AdTGF-2, and had an enhancing effect on
upregulating the Foxp3 mRNA levels with AdTGF-1 in TNBS-treated
mice (Fig. 4A).
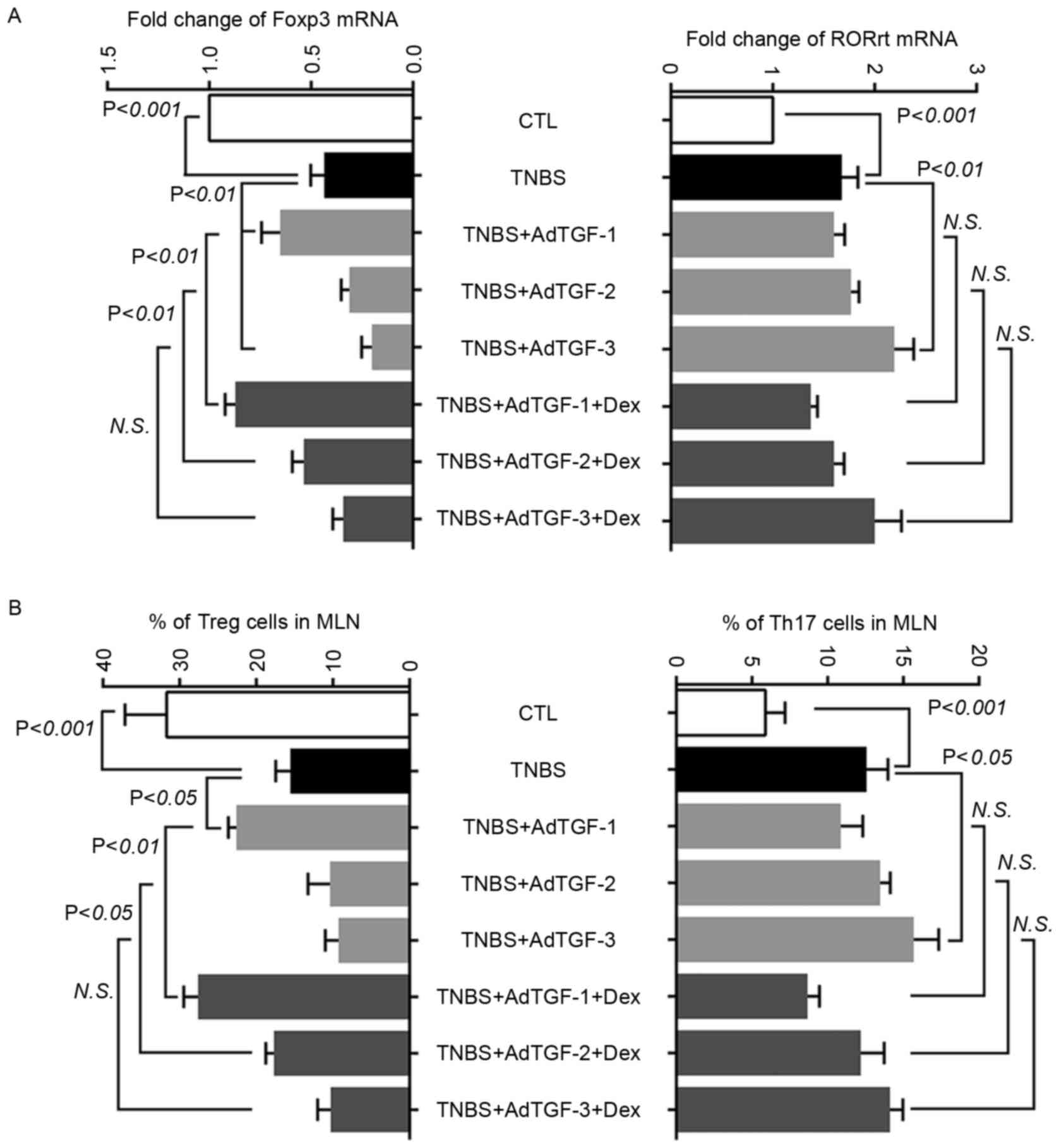 | Figure 4.Effects of local TGF-β1 levels and
dexamethasone treatment on regulating the balance of Th17 cells and
Tregs in mesenteric lymph nodes of TNBS-treated mice. AdTGF-β1 was
delivered to the colons of TNBS mice and dexamethasone (5 mg/kg
body weight) was given once a day by orogastric gavage for 4 days.
(A) Total RNA was extracted from mesenteric lymph nodes and
reversely transcribed to complementary DNA. Real time polymerase
chain reaction analysis was performed to evaluate the levels of
transcription factors Foxp3 and RORγt. (B) The fraction of Treg and
Th17 cells in mesenteric lymph nodes was analyzed using flow
cytometry. Doses: AdTGF-1, 1×107 pfu; AdTGF-2,
1×108 pfu; AdTGF-3, 1×109 pfu. Values are
expressed as the mean ± standard deviation (n=4 per group). N.S.,
no significance; MLN, mesenteric lymph nodes; CTL, control; TNBS,
2,4,6-trinitrobenzenesulfonic acid; AdTGF, adenovirus
overexpressing transforming growth factor β1; Dex, dexamethasone;
Foxp3, forkhead box p3; ROR, RAR-related orphan receptor; Treg,
T-regulatory cell; Th17 cell, type 17 T-helper cell. |
To understand the role of TGF-β1 levels and
dexamethasone treatment in regulating the balance of Treg and Th17
cells in TNBS-treated mice, the present study analyzed the fraction
of Treg and Th17 cells in mesenteric lymph nodes using flow
cytometry (Fig. 4B). Compared with
the control, the percentage of Th17 cells increased significantly
in TNBS-treated mice, which was further increased following AdTGF-3
delivery. Dexamethasone had no effects on the percentage of Th17
cells in TNBS mice receiving adenoviral TGF-β1. In addition, the
reduction of Treg cells in TNBS-treated mice was prevented by
AdTGF-1 delivery. However, delivery of AdTGF-2 and AdTGF-3 further
and significantly decreased the Treg cell population in TNBS mice.
Dexamethasone prevented the reduction of Treg cells in TNBS-treated
mice receiving AdTGF-2 and had an enhancing effect on upregulating
the percentage of Treg cells with AdTGF-1 (Fig. 4B). However, dexamethasone treatment
did not change the number of Treg cells in TNBS mice receiving
AdTGF-3. These results suggested that local TGF-β1 levels may
affect the balance of Treg and Th17 cells in TNBS-induced mice
colitis, and demonstrated that the efficacy of dexamethasone may be
influenced by the local TGF-β1 levels. In addition, dexamethasone
alleviated TNBS-induced colon damage predominantly by upregulating
Treg cells.
Effects of local TGF-β1 levels and
dexamethasone treatment on cytokines in mesenteric lymph nodes of
TNBS-treated mice
Homogenates were prepared from mesenteric lymph
nodes and the levels of cytokines IL-10 and IL-23 were determined
using ELISA (Fig. 5). Compared with
those in the control group, the levels of IL-23 increased
significantly in TNBS-treated mice and were further increased by
delivery of AdTGF-3, but not AdTGF-1 and AdTGF-2. Dexamethasone
treatment had no effects on the IL-23 levels in TNBS mice receiving
adenoviral TGF-β1. In comparison with the control, TNBS enema led
to a marked reduction of IL-10, which was prevented by AdTGF-1, but
not AdTGF-2 or AdTGF-3. Dexamethasone enhanced the effect of
AdTGF-1 delivery on increasing the IL-10 levels in TNBS-treated
mice. In TNBS mice receiving AdTGF-2 and AdTGF-3, the levels of
IL-10 were not altered following dexamethasone treatment. These
results indicated that the TGF-β1 levels determined the secretion
of IL-10 and IL-23, and that dexamethasone predominantly
upregulated IL-10 levels, which was associated with the levels of
TGF-β1.
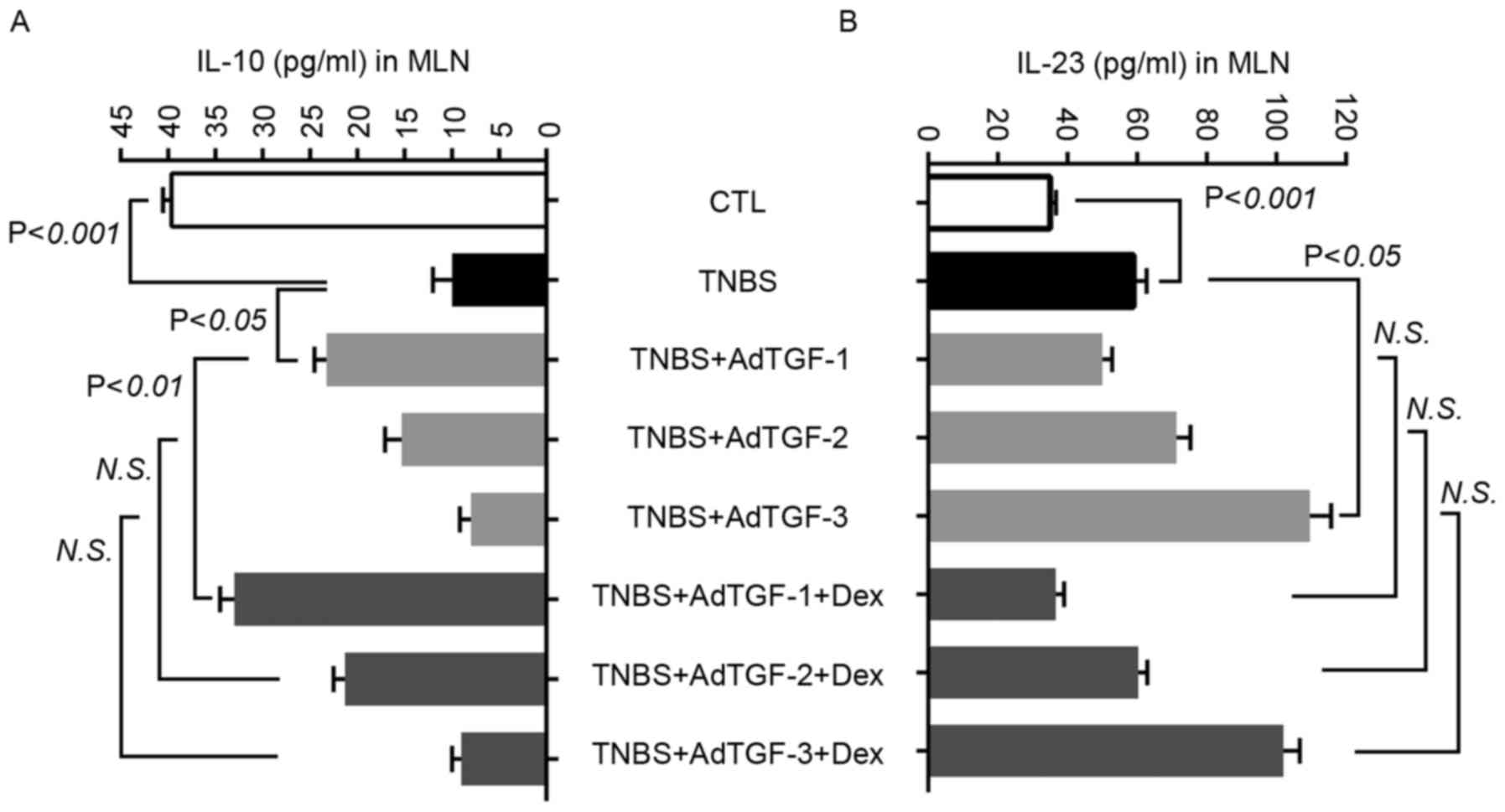 | Figure 5.Effects of local TGF-β1 levels and
dexamethasone treatment on the cytokine concentration in mesenteric
lymph nodes of TNBS-treated mice. AdTGF-β1 was delivered to the
colons of TNBS mice and dexamethasone (5 mg/kg body weight) was
given once a day by orogastric gavage for 4 days. Homogenates of
mesenteric lymph nodes were prepared and the levels of cytokines
(A) IL-10 and (B) IL-23 were determined using ELISA. Doses:
AdTGF-1, 1×107 pfu; AdTGF-2, 1×108 pfu;
AdTGF-3, 1×109 pfu. Values are expressed as the mean ±
standard deviation (n=4 per group). N.S., no significance; MLN,
mesenteric lymph nodes; CTL, control; TNBS,
2,4,6-trinitrobenzenesulfonic acid; AdTGF, adenovirus
overexpressing transforming growth factor β1; Dex, dexamethasone;
IL, interleukin. |
Effects of local TGF-β1 levels and
dexamethasone treatment on apoptosis of colon tissues in
TNBS-treated mice
The expression of the apoptotic proteins caspase3
and Bim was evaluated using western blot analysis (Fig. 6A). As compared with the control, the
levels of active caspase3 and Bim increased significantly in
TNBS-treated mice. TNBS-induced upregulation of activated caspase3
was significantly prevented by delivery of AdTGF. Of note,
dexamethasone treatment decreased the active caspase3 levels in
TNBS mice receiving adTGF1. In TNBS-treated mice, increases in Bim
were significantly inhibited by delivery of AdTGF-1 and AdTGF-2,
but not AdTGF-3. However, dexamethasone treatment had no effect on
the Bim levels in TNBS-induced mice receiving adenoviral TGF-β1.
The present study also evaluated the activity of the
p38MAPK/JNK/c-Jun signaling pathway (Fig. 6B). Compared with the control, the
TNBS-induced activation of p38MAPK, JNK and c-Jun was significantly
prevented by adenoviral TGF-β1 delivery, particularly by AdTGF-1
and AdTGF-2. Of note, dexamethasone treatment had an enhancing
effect on AdTGF-1 and AdTGF-2 by downregulating phospho-p38MAPK,
phospho-JNK and c-Jun in TNBS-treated mice. These results indicated
that dexamethasone may prevent colon tissue apoptosis by inhibiting
activation of the p38MAPK/JNK/c-Jun pathway, wherein the local
TGF-β1 levels have an important role.
 | Figure 6.Effects of local TGF-β1 levels and
dexamethasone treatment on colon tissue apoptosis in TNBS-treated
mice. AdTGF-β1 was delivered to the colons of TNBS mice and
dexamethasone (5 mg/kg body weight) was given once a day by
orogastric gavage for 4 days. Total protein was extracted from
colonic tissues using radioimmunoprecipitation assay lysis buffer.
Expression of (A) the apoptotic proteins caspase3 and Bim as well
as (B) p38MAPK/JNK/c-Jun pathway activity were assessed using
western blot analysis. Doses: AdTGF-1, 1×107 pfu;
AdTGF-2, 1×108 pfu; AdTGF-3, 1×109 pfu.
Values are expressed as the mean ± standard deviation (n=4 per
group). *P<0.05 vs. CTL; #P<0.05 vs. TNBS;
&P<0.05 vs. TNBS + AdTGF-1; $P<0.05
vs. TNBS + AdTGF-2. CTL, control; TNBS,
2,4,6-trinitrobenzenesulfonic acid; AdTGF, adenovirus
overexpressing transforming growth factor β1; Dex, dexamethasone;
MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal
kinase; SD, standard deviation. |
Discussion
Dexamethasone, a synthetic glucocorticoid with
enhanced potency, has been widely used as an anti-inflammatory
immunosuppressive agent in the treatment of IBD (19,20). In
experimental IBD models, it was demonstrated that dexamethasone and
prednisone suppressed the inflammatory response through a variety
of mechanisms, including decreasing the activity of nuclear
factor-κB and reducing heparanase and heat shock protein 70
expression (21,22). The present study investigated the
effects of local TGF-β1 levels on the anti-inflammatory role of
dexamethasone by focusing on the differentiation of Treg and Th17
cells in an experimental TNBS-induced IBD model. It was revealed
that the abundance of TGF-β1 in the colon increased significantly
in TNBS-treated mice compared with those in the control group,
whereas its activated level exhibited no difference between TNBS
and control mice over a 14-day period. Vallance et al
(13) reported that delivering
adenoviral vectors encoding spontaneously active TGF-β1 into the
colons of normal mice led to a severe and prolonged inflammatory
response as well as localized collagen deposition, leading to
progressive fibrosis. Actually, various amounts of TGF-β1 were
reported to be present in the colons of patients with IBD (10,12,23),
which may influence the efficacy of anti-inflammatory agents, since
TGF-β1 has disparate roles in the pathogenesis of IBD by either
stimulating or suppressing the immune system (24). To mimic the variability of TGF-β1
levels in human IBD patients, the present study delivered three
different doses of adenoviral vector encoding full-length murine
TGF-β1, not its active form, into the colons of TNBS mice.
Overproduced TGF-β1 and the increased active form were obtained in
a dose-dependent manner in TNBS mice following adenoviral TGF-β1
delivery. The present study then evaluated colonic damage as well
as MPO and ALP activity in TNBS mice receiving adenoviral TGF-β1
and dexamethasone. MPO and ALP enzymatic activity has been
considered a sensitive biochemical marker of colonic inflammation,
and their inhibition may be interpreted as a result of an
anti-inflammatory effect (17,18). The
present results indicated that local TGF-β1 levels modulate
TNBS-induced colon damage as well as MPO and ALP activation, and
that the efficacy of dexamethasone depends on the abundance of
local TGF-β1 in TNBS-induced mouse colitis. In a previous study,
TNBS-induced rat colitis was also ameliorated by intraperitoneal
gene therapy with plasmid DNA encoding native TGF-β (25).
In inflammation, TGF-β1 has a key regulatory role
and is required for the induction of the differentiation and
maturation of Treg cells that control the immune response (6). Alternatively, in the presence of IL-6,
TGF-β1 may initiate the production of Th17 cells that have a
crucial role in promoting and/or maintaining chronic inflammatory
diseases (26). Accordingly, the
cytokine profile of colonic tissues was assessed in the present
study. In TNBS mice, a significant elevation in pro-inflammatory
cytokines was detected, including TNF-α, IFN-γ, IL-6, IL-17 and
IL-23, while the anti-inflammatory cytokine IL-10 was significantly
reduced. The profile of cytokines in TNBS-treated mice was
significantly altered following delivery of adenoviral TGF-β1. The
low amount, AdTGF-1, significantly decreased the production of
pro-inflammatory cytokines, particularly IFN-γ, TNF-α and IL-23,
while it significantly increased the levels of IL-10. By contrast,
the high amount, AdTGF-3, exerted a reverse role in the regulation
of cytokine production. The present study also identified that
dexamethasone treatment had an enhancing effect with AdTGF-1 on
reducing cytokine production particularly on IFN-γ and IL-10. In
TNBS mice receiving AdTGF-2, the increases of IFN-γ, TNF, IL-6,
IL-17 and IL-23 were significantly prevented by dexamethasone
application. However, dexamethasone had no effects on the levels of
cytokines in TNBS mice receiving a high amount of AdTGF-3. The
present study suggested that the local TGF-β1 levels may affect the
severity of colon inflammation and determined their effect on the
efficacy of dexamethasone in the treatment of IBD. A study on
TNBS-induced rat colitis reported that prednisolone treatment
prevented the production of IL-6, IL-1, TNF-α and IFN-γ (22). Furthermore, dexmedetomidine, a
selective α2-adrenergic receptor agonist, led to amelioration of
TNBS-induced colitis in mice, likely by increasing IL-4 and IL-10
levels as well as reducing IL-23 levels (27). The cytokines that induce Th17 cell
lineage development likely include IL-6, IL-21, IL-1β and IL-23,
with a synergistic role for TGF-β1 (28). Th17 cells are thought to
predominantly produce IL-17, particularly IL-17A and IL-17F, as
well as IL-23 (28). IL-10 is the
major cytokine produced by Treg cells (6). Based on the alteration of the cytokine
profile, the present results suggested that Treg and Th17 cells may
be involved in TNBS-induced colitis. Of note, decreased IL-23
levels may facilitate the marked increase of IL-10 in TNBS-induced
mice treated with a low amount of TGF-β1. Although IL-23 promotes
the secretion of IL-17 from Th17 cells by binding to its receptor
on Th17 cells, Th17 cells deficient in IL-23 may secret the
anti-inflammatory cytokine IL-10 (29).
To analyze the alteration of the fraction of Treg
and Th17 cells, the present study assessed the mRNA levels of their
specific transcription factors Foxp3 and RORγt, and their
percentage in mesenteric lymph nodes. The mesenteric lymph nodes
lie between the layers of the mesentery, are hundreds in number and
help the body to fight disease. IBDs such as Crohn's disease or
ulcerative colitis are common inflammatory conditions linked to
mesenteric lymphadenitis (30).
Compared with the control, the present study identified increased
Th17 and decreased Treg cells in TNBS-treated mice. Furthermore,
adenoviral TGF-β1 delivery and dexamethasone treatment had no
effect on Th17 cells in TNBS-treated mice. However, delivery of a
low amount of AdTGF-β1 prevented the TNBS-induced reduction of Treg
cells, with a potential enhancing role for dexamethasone treatment.
Results consistent with this were obtained from the analysis of the
cytokines IL-10 and IL-23. The present study indicated that in
TNBS-treated mice, the balance of Th17 and Treg cells was disrupted
and the cytokine profile was therefore altered, and that
dexamethasone treatment mainly promoted the production of Treg
cells under low levels of TGF-β1.
The present study further evaluated the expression
levels of apoptotic proteins and the activation levels of the
p38MAPK/JNK/c-Jun signaling pathway in colon tissues. Immunoblot
analysis demonstrated that TNBS-induced upregulation of activated
caspase3 and Bim was inhibited by AdTGF-β1 delivery mainly at the
low concentration. Dexamethasone treatment further decreased the
levels of activated caspase3 in TNBS mice receiving adenoviral
TGF-β1, particularly at the low concentration, while it had no
obvious effect on Bim. Furthermore, activation of the
p38MAPK/JNK/c-Jun pathway was detected in TNBS-treated mice, which
was inhibited predominantly by AdTGF-1 and AdTGF-2. Dexamethasone
treatment further decreased the levels of phospo-p38MAPK,
phospho-JNK and c-Jun in TNBS mice receiving AdTGF-1 and AdTGF-2.
Accordingly, the present results suggested that the
p38MAPK/JNK/c-Jun pathway may be involved in the inhibitory
function of lows amounts of TGF-β1 delivered to the colons of mice
with TNBS-induced colon damage. In addition, dexamethasone may
protect the colon against damage through inhibition of the
p38MAPK/JNK/c-Jun pathway depending on the local levels of TGF-β1.
Similarly, a previous study detected elevated levels of active
caspase3 and phosphorylated p38MAPK in mice with TNBS-induced
colitis (31). In an in vitro
TNF-α-induced HT29 intestinal epithelial cell apoptosis model,
p38MAPK phosphorylation was increased (31). It was also reported that the JNK
inhibitor XG-102 protects against TNBS-induced mouse colitis, where
the production of TNF-α, expression of Bim, B-cell lymphoma
2-associated X protein and p53 as well as activation of caspase3
and JNK substrate c-Jun were significantly reduced (32).
Taken together, the results of the present study
demonstrated that low amounts of TGF-β1 delivered to the colon
alleviate the inflammatory response and damage of colon tissue,
mainly by restoring the levels of Treg cells in TNBS-treated mice.
The present study also provided evidence that the therapeutic
effect of dexamethasone may depend on the local levels of TGF-β1 in
TNBS-induced colitis at least partially through promoting the
differentiation of Treg cells and thus altering the balance of the
pro- and anti-inflammatory cytokines. In addition, dexamethasone
may protect the colon against damage through inhibition of the
p38MAPK/JNK/c-Jun pathway in the presence of low levels of
TGF-β1.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81170357 to P.Y.).
Availability of data and materials
Not applicable.
Authors' contributions
PY and YL designed the project and planned the
experiments. PY, NC and LS performed the experiments and analyzed
the data. TP and GC contributed to the animal sample preparation
and the interpretation of the results. PY wrote the manuscript with
support from NC and LS. All authors discussed the results and
contributed to the final manuscript.
Ethics approval and consent to
participate
All of the animal protocols were approved by the
Experimental Animal Ethics Committee of Peking University People's
Hospital (Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW and Kaplan GG: Increasing incidence and prevalence of the
inflammatory bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54.e42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Lange KM and Barrett JC: Understanding
inflammatory bowel disease via immunogenetics. J Autoimmun.
64:91–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaistha A and Levine J: Inflammatory bowel
disease: The classic gastrointestinal autoimmune disease. Curr
Probl Pediatr Adolesc Health Care. 44:328–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YK and Mazmanian SK: Has the
microbiota played a critical role in the evolution of the adaptive
immune system? Science. 330:1768–1773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powrie F, Leach MW, Mauze S, Caddle LB and
Coffman RL: Phenotypically distinct subsets of CD4+ T
cells induce or protect from chronic intestinal inflammation in C.
B-17 scid mice. Int Immunol. 5:461–471. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Himmel ME, Yao Y, Orban PC, Steiner TS and
Levings MK: Regulatory T-cell therapy for inflammatory bowel
disease: More questions than answers. Immunology. 136:115–122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neurath MF: Cytokines in inflammatory
bowel disease. Nat Rev Immunol. 14:329–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goettel JA, Scott Algood HM,
Olivares-Villagómez D, Washington MK, Chaturvedi R, Wilson KT, Van
Kaer L and Polk DB: KSR1 protects from interleukin-10
deficiency-induced colitis in mice by suppressing T-lymphocyte
interferon-γ production. Gastroenterology. 140:265–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strober W and Fuss IJ: Proinflammatory
cytokines in the pathogenesis of inflammatory bowel diseases.
Gastroenterology. 140:1756–1767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feagins LA: Role of transforming growth
factor-β in inflammatory bowel disease and colitis-associated colon
cancer. Inflamm Bowel Dis. 16:1963–1968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marafini I, Zorzi F, Codazza S, Pallone F
and Monteleone G: TGF-Beta signaling manipulation as potential
therapy for IBD. Curr Drug Targets. 14:1400–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vallance BA, Gunawan MI, Hewlett B, Bercik
P, Van Kampen C, Galeazzi F, Sime PJ, Gauldie J and Collins SM:
TGF-beta1 gene transfer to the mouse colon leads to intestinal
fibrosis. Am J Physiol Gastrointest Liver Physio. 289:G116–G128.
2005. View Article : Google Scholar
|
|
14
|
Kitani A, Fuss IJ, Nakamura K, Schwartz
OM, Usui T and Strober W: Treatment of experimental
(Trinitrobenzene sulfonic acid) colitis by intranasal
administration of transforming growth factor (TGF)-beta1 plasmid:
TGF-beta1-mediated suppression of T helper cell type 1 response
occurs by interleukin (IL)-10 induction and IL-12 receptor beta2
chain downregulation. J Exp Med. 192:41–52. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wallace JL and Keenan CM: An orally active
inhibitor of leukotriene synthesis accelerates healing in a rat
model of colitis. Am J Physiol. 258:G527–G534. 1990.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng L, Gao ZQ and Wang SX: A chronic
ulcerative colitis model in rats. World J Gastroenterol. 6:150–152.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sánchez de Medina F, Martinez-Augustin O,
González R, Ballester I, Nieto A, Gálvez J and Zarzuelo A:
Induction of alkaline phosphatase in the inflamed intestine: A
novel pharmacological target for inflammatory bowel disease.
Biochem Pharmacol. 68:2317–2326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friend DR: Review article: Issues in oral
administration of locally acting glucocorticosteroids for treatment
of inflammatory bowel disease. Aliment Pharmacol Ther. 12:591–603.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Mattos BR, Garcia MP, Nogueira JB,
Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM
and Simioni PU: Inflammatory bowel disease: An overview of immune
mechanisms and biological treatments. Mediators Inflamm.
2015:4930122015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scheinman RI, Gualberto A, Jewell CM,
Cidlowski JA and Baldwin AS Jr: Characterization of mechanisms
involved in transrepression of NF-kappa B by activated
glucocorticoid receptors. Mol Cell Biol. 15:943–953. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quaglio AE, Castilho AC and Di Stasi LC:
Experimental evidence of heparanase, Hsp70 and NF-κB gene
expression on the response of anti-inflammatory drugs in
TNBS-induced colonic inflammation. Life Sci. 141:179–187. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Del Zotto B, Mumolo G, Pronio AM,
Montesani C, Tersigni R and Boirivant M: TGF-beta1 production in
inflammatory bowel disease: Differing production patterns in
Crohn's disease and ulcerative colitis. Clin Exp Immunol.
134:120–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monteleone G, Boirivant M, Pallone F and
MacDonald TT: TGF-beta1 and Smad7 in the regulation of IBD. Mucosal
Immunology. 1 Suppl 1:S50–S53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giladi E, Raz E, Karmeli F, Okon E and
Rachmilewitz D: Transforming growth factor-beta gene therapy
ameliorates experimental colitis in rats. Eur J Gastroenterol
Hepatol. 7:341–347. 1995.PubMed/NCBI
|
|
26
|
Schmitt N and Ueno H: Regulation of human
helper T cell subset differentiation by cytokines. Curr Opin
Immunol. 34:130–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Erdogan Kayhan G, Gul M, Kayhan B, Gedik
E, Ozgul U, Kurtoglu EL, Durmus M and Ersoy MÖ: Dexmedetomidine
ameliorates TNBS-induced colitis by inducing immunomodulator
effect. J Surg Res. 183:733–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marwaha AK, Leung NJ, McMurchy AN and
Levings MK: TH17 cells in autoimmunity and immunodeficiency:
Protective or pathogenic? Front Immunol. 3:1292012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McGeachey MJ, Bak-Jensen KS, Chen Y, Tato
CM, Blumenschein W, McClanahan T and Cua DJ: TGF-beta and IL-6
drive the production of IL-17 and IL-10 by T cells and restrain
T(H)-17 cell-mediated pathology. Nat Immunol. 8:1390–1397. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hartog A, Belle FN, Bastiaans J, de Graaff
P, Garssen J, Harthoorn LF and Vos AP: A potential role for
regulatory T-cells in the amelioration of DSS induced colitis by
dietary non-digestible polysaccharides. J Nutr Biochem. 26:227–233.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D, Wang L, Yan L, Miao X, Gong C,
Xiao M, Ni R and Tang Q: Vacuolar protein sorting 4B regulates
apoptosis of intestinal epithelial cells via p38 MAPK in Crohn's
disease. Exp Mol Pathol. 98:55–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reinecke K, Eminel S, Dierck F, Roessner
W, Kersting S, Chromik AM, Gavrilova O, Laukevicience A, Leuschner
I, Waetzig V, et al: The JNK inhibitor XG-102 protects against
TNBS-induced colitis. PLoS One. 7:e309852012. View Article : Google Scholar : PubMed/NCBI
|




















