Introduction
Gastrointestinal stromal tumors (GISTs) were
initially classified by Mazur and Clark in 1983 (1), and represent a wide spectrum of
mesenchymal tumors in the entire gastrointestinal tract (2). GISTs are most commonly observed in the
stomach and small intestine, followed by the colon, rectum and
esophagus. They typically occur in individuals >50 years old and
the incidence of GISTs is 10–20 individuals in a million (3). A notable feature of GISTs is that
mutations within the KIT or platelet-derived growth factor
receptor (PDGFR) genes exist in the majority of cancer cells
(4). Normally, c-KIT activation
requires binding to its ligand, stem cell factor (SCF). Interaction
between c-KIT and SCF activates a cascade of downstream reactions,
whereas mutations in the KIT gene lead to uncontrolled
activation of the tyrosine kinase domain and promoted cell
proliferation (5). Activation of KIT
is crucial for the pathogenesis of the majority of GISTs (6), which makes this oncoprotein a potential
therapeutic target. Furthermore, imatinib and other tyrosine kinase
inhibitors are typically used to treat GISTs (7).
The downstream signaling pathways that are known to
be activated by KIT include the RAS/mitogen-activated protein
kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/protein
kinase B networks (8,9). Forkhead box protein O1 (FOXO1) is a
member of the FOXO transcription factors, which has been
demonstrated to be closely associated with cell apoptosis, DNA
damage or repair, cell autophagy, oxidative stress, angiogenesis
and sugar metabolism (10–12). Furthermore, the PI3K and MAPK
networks are key signaling pathways that regulate FOXO1 expression
(13). A previous study demonstrated
that high expression of FOXO1 is able to inhibit cell proliferation
(14). However, the potential
association of FOXO1 expression in GISTs remains unclear. The
present study was designed to investigate the regulatory mechanism
by which the PI3K and MAPK signaling pathways influence the
activity of FOXO1 and its downstream factors, B-cell lymphoma 2
(Bcl2) and Bcl-2-associated X protein (Bax).
Materials and methods
Reagents
The PI3K inhibitor, LY294002 hydrochloride (cat. no.
L9908) and the MAPK inhibitor, UO126 monoethanolate (cat. no. U126)
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Rabbit monoclonal antibody to c-Kit was purchased from Abcam (cat.
no. ab32363; Cambridge, MA, USA). Rabbit monoclonal antibodies to
FOXO1 (cat. no. 2880S), phosphorylated (p)-FOXO1 (s256, cat. no.
84192S), Bcl2 (cat. no. 4223S) and Bax (cat. no. 5023S) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Mouse anti-β-actin (cat. no. sc-47778), horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG; cat. no.
sc-2030) and HRP-conjugated goat anti-mouse IgG (cat. no. sc-2055)
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). Tetramethylrhodamine-conjugated donkey anti-human IgG (cat.
no. D110143) and cell counting kit-8 (CCK-8 kit; cat. no. E606335)
were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The
MaxVision immunohistochemistry kit (cat. no. KIT-5002) was
purchased from Fuzhou Maixin Biotech Co., Ltd. (Fuzhou, China).
DAPI (cat. no. 10236276001) was purchased from Roche Diagnostics
(Basel, Switzerland). The GIST-T1 gastrointestinal stromal tumor
cell line was purchased from Biowit Technologies, Ltd. (Shenzhen,
China) and the WI-38 normal lung fibroblast cell line was obtained
from Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China). The media used for cell culture [Dulbecco's modified
Eagle's medium (DMEM), minimum essential media (MEM) and fetal calf
serum] were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Cell culture and treatments
GIST-T1 cells, characterized in detail by Taguchi
et al (15), were cultured in
DMEM and WI-38 cells (ATCC® CCL-75™) in MEM. Both media
were supplemented with 10% fetal calf serum and maintained at 37°C
in a humidified atmosphere containing 5% CO2 and 95%
air. The GIST-T1 cells were maintained in the log-growth phase and
treatment groups were treated with 30 µM LY294002 (LY294002 group),
10 µM UO126 (UO126 group) or 30 µM LY294002 + 10 µM UO126 (LY+UO
group) at 37°C for 24 h. An equal amount of dimethylsulfoxide
(DMSO) was added into the control group and incubated at 37°C for
24 h.
Immunocytochemistry and
immunofluorescence staining
GIST-T1 cells were seeded into six-well chamber
slides at a density of 1×104 cells/well and allowed to
adhere. Subsequently, 1 ml medium was added to each well and
cultures were maintained at 37°C for 24 h, then washed and fixed
with acetone at 4°C for 20 min. Cells were permeabilized with 0.5%
Triton X-100 for 20 min. To inhibit endogenous peroxidase activity,
slides were incubated at 37°C with 3% H2O2
for 10 min, washed with PBS and blocked with 5% bovine serum
albumin (Biosharp, Anhui, China) at room temperature for 30 min.
Excess serum was removed with filter paper, and the primary c-Kit
antibody (1:500) was added and incubated at 4°C overnight.
Following washing three times with PBS for 5 min each, the
HRP-labeled Goat Anti-Mouse IgG (1:50) or DyLight 405-labeled Goat
Anti-Mouse IgG (1:200) was added and incubated at room temperature
for 30 min and washed three times with PBS for 5 min each. For
peroxidase staining, color was developed using
3,3′-diaminobenzidine, slides were washed to terminate the reaction
and then nuclei were counterstained with hematoxylin at room
temperature for 30 sec. For immunofluorescence staining, nuclei
were counterstained with DAPI at room temperature for 15 min,
slides were dried and then sealed with neutral resin. Slides were
observed under a light or a fluorescence microscope (magnification,
×400) and images were captured.
CCK-8 cell proliferation assay
A single-cell suspension of GIST-T1 was seeded into
96-well plates at 1×105 cells per well and treated with
inhibitors as described above. Following treatment for 0, 12, 24,
36 or 48 h, 10 µl CCK-8 reagent was added, samples were incubated
at 37°C and 5% CO2 for 1 h and agitated gently to ensure
any precipitate was dissolved. The absorbance of each well was
measured with a microplate reader, at an absorption wavelength of
450 nm. With the measured optical densities (ODs), the following
formula was used: Inhibition ratio (%)=(OD value of control
group-OD value of experimental group)/OD value of control group
×100.
Western blotting
Cell cultures were treated as described above. At
the end of the culture period, the total protein was extracted from
the cells in 100 µl extraction buffer (10 mM Tris, 1 mM EDTA, 1%
SDS, 0.1% Triton X-100 containing complete protease inhibitor
cocktail). A bicinchoninic acid protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.) was used to estimate the protein content
of the lysate with bovine serum albumin used as the standard. SDS
loading buffer [63 mM Tris-HCl, 10% glycerol, 2% SDS, 0.0025%
bromophenol blue, (pH 6.8)] was added following normalization of
the concentration of protein in each sample with 1% SDS. Protein
lysates (30 µg) were separated by 8% SDS-PAGE and
electrophoretically transferred onto PVDF membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% non-fat dry
milk (w/v) in TBST buffer (10 mM Tris, 150 mM NaCl, 0.05% Tween-20)
at room temperature for 1 h, and incubated overnight with primary
antibodies against FOXO1 (1:1,000), p-FOXO1 (s256; 1:1,000), Bcl2
(1:1,000), Bax (1:1,000) or β-actin (1:2,000) at 4°C. Subsequently,
blots were washed three times with TBST and incubated with
HRP-conjugated secondary antibody against IgG (1:3,000) at room
temperature for 1 h. Enhanced chemiluminescence substrate followed
by exposure within an EpiChemi II darkroom (UVP, Inc., Upland, CA,
USA) was used for visualization of the protein bands. Quantity One
software v4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to analyze the bands and quantify the signal.
Apoptosis assay by flow cytometry
Cultured GIST-T1 cells were treated and collected as
described above. Apoptosis was analyzed to investigate whether
LY294002 and UO126 were able to enhance cell killing sensitivity.
The apoptotic rates were measured using a flow cytometric assay.
Cell labeling was performed using Annexin V conjugated to
fluorescein isothiocyanate (FITC), which binds to
phosphatidylserine exposed on the surface membrane of cells
undergoing apoptosis. Cells were collected by trypsinization,
washed twice with PBS and centrifuged at 500 × g, room temperature
for 5 min. The cells were then suspended in 500 µl PBS and
incubated with 5 µl Annexin V-FITC (Annexin V-FITC Apoptosis
Detection Kit; Beyotime Institute of Biotechnology, Haimen, China)
and 10 µl (20 µg/ml) propidium iodide (PI) solution (Beyotime
Institute of Biotechnology) at room temperature for 20 min in the
dark. The samples were then measured using a flow cytometer with
FACS 101 software (BD Biosciences, Franklin Lakes, NJ, USA). For
each sample, 105 fluorescence signals were measured.
Cell cycle distribution assay by flow
cytometry
GIST-T1 cells were treated and collected as
described above, then fixed in 75% ethanol for 2 h at 4°C. Samples
were rehydrated with PBS and incubated with 500 µl (200 µg/ml) PI
solution for 30 min at room temperature. For each sample the
percentages of cells in the G0/G1, S and G2/M phases of the cell
cycle were calculated using a flow cytometer with FACS software.
For each sample, 10,000 fluorescence signals were measured.
Statistical methods for data
analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis of experimental data. The results are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Differences between groups were determined by one-way analysis of
variance with Dunnett's post hoc test.
Results
Confirmation of cell type by
immunocytochemistry
Immunocytochemical staining of cultured GIST-T1
cells with an anti-c-Kit antibody revealed brown granules in the
cytoplasm (Fig. 1). The presence of
this gastrointestinal stromal tumor cell marker indicated that the
cells cultured in the present study were from a gastrointestinal
stromal tumor.
Cell proliferation is inhibited by
signaling inhibitors
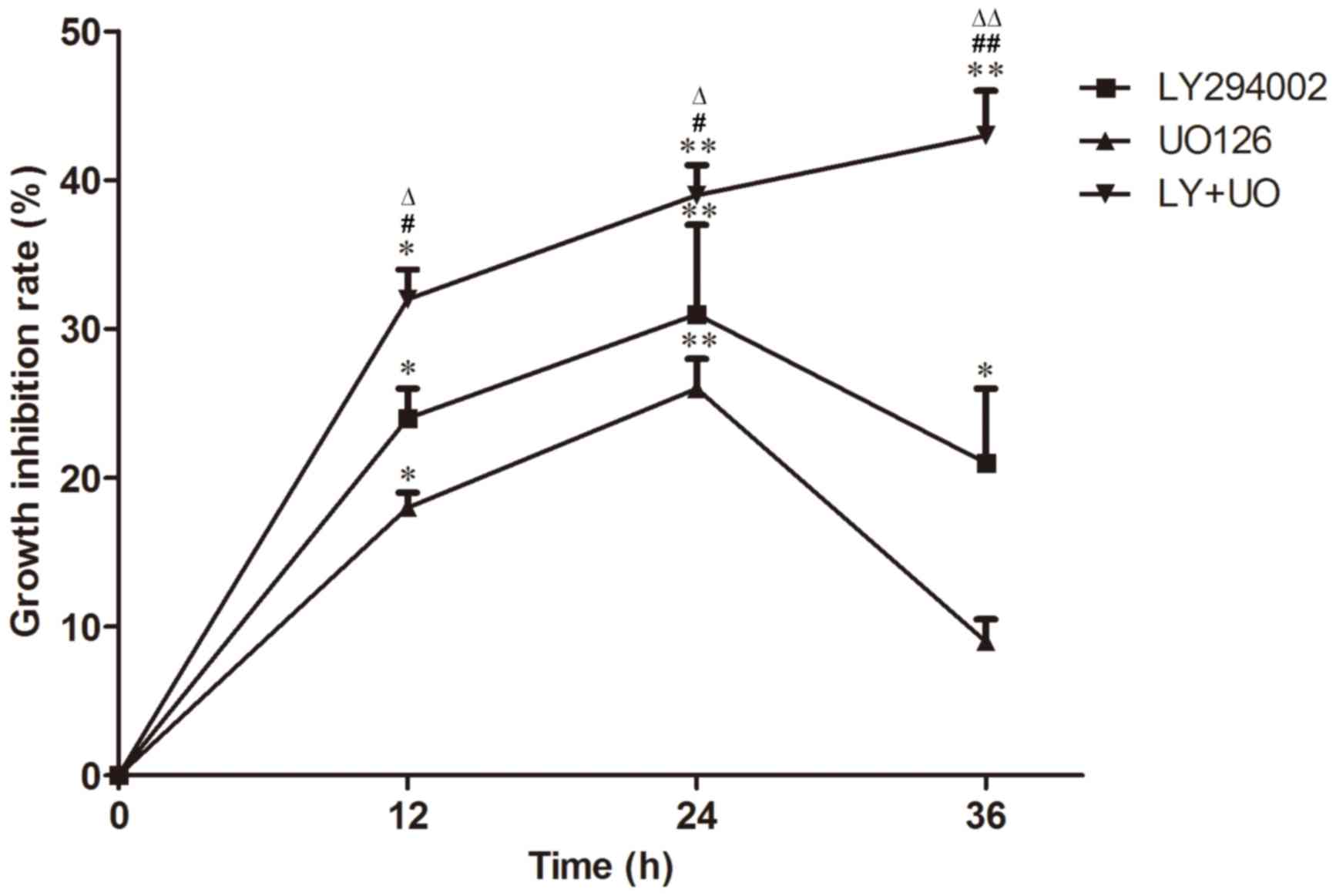
CCK-8 staining revealed that the inhibition of cell
proliferation was enhanced with treatment time in the LY+UO group
(P<0.05; Fig. 2). Inhibition of
cell proliferation inhibition in the LY+UO group was more
pronounced compared with groups treated with either inhibitor alone
(P<0.05; Fig. 2). Furthermore,
maximal inhibition was observed when cultures were treated with
LY294002 or UO126 for 24 h, whereas inhibition peaked at 36 h in
the LY+UO group, at which time-point the majority of cells were
dead (Fig. 2).
Western blotting analysis
Western blotting revealed that the expression of
FOXO1 and Bax significantly decreased, whereas p-FOXO1 and Bcl2
significantly increased in GIST-T1 cells compared with the normal
human fibroblasts WI-38 (P<0.05; Fig.
3A). To confirm that protein expression was regulated via the
PI3K or MAPK pathways, the cells were treated with pathway
inhibitors. The protein expression of p-FOXO1 and Bcl2 was
significantly decreased (both P<0.05), whereas Bax (P<0.05)
increased in cells treated with LY294002, UO126 or both compared
with controls. The reduction in the expression of p-FOXO1 and Bcl2
and the increase in protein expression of Bax were more marked in
the LY+UO group; however, total FOXO1 protein expression was not
significantly different among the groups treated with LY294002,
UO126, LY+UO or DMSO (Fig. 3B).
 | Figure 3.(A) Western blotting was used to
analyze the protein expression of FOXO1, p-FOXO1, Bcl2 and Bax in
GIST-T1 and WI-38 cells (normal human fibroblasts). *P<0.05 vs.
WI-38 cells. (B) Western blotting was used to analyze protein
expression of FOXO1, p-FOXO1, Bcl2 and Bax in cells treated with
LY294002 and UO126 alone or in combination. *P<0.05 vs. DMSO.
Error bars indicate the standard deviations. FOXO1, forkhead box
protein O1; p, phosphorylated; Bcl2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; DMSO, dimethylsulfoxide; LY+UO,
LY294002 + UO126. |
Immunofluorescence staining of FOXO1
distribution
Immunofluorescence staining revealed that FOXO1 was
widely distributed in the nucleus and cytoplasm in the DMSO group.
Treatment of GIST-T1 cells with LY294002 or UO126 resulted in
cytoplasmic levels of FOXO1 being markedly reduced, with a
corresponding increase in nuclear FOXO1. Furthermore the degree of
FOXO1 translocation to the nucleus in the LY+UO group was more
pronounced than in either single-inhibitor group (Fig. 4).
Apoptosis is induced by signaling
inhibitors
Cells in the control group exhibited an apoptosis
rate of 1.17±0.21%. In the LY294002-treatment group the apoptosis
rate was 2.05±0.32% whereas in the UO126-treated group it was
2.66±0.53%. Additionally, in the LY+UO group the apoptosis rate was
3.82±0.47%. These results demonstrated a significantly increased
apoptosis rate (P<0.05) when treating GIST-T1 cells with
LY294002 or UO126, and the effect of combined treatment was higher
than that of either inhibitor alone (Fig. 5).
Signaling inhibitors alter the cell
cycle distribution
Flow cytometry revealed that the proportion of G0/G1
phase cells in the LY294002, UO126 and LY+UO groups was 79.78±5.27,
73.73±4.86 and 87.28±6.27%, respectively. These proportions were
increased significantly compared with the control group at
65.43±4.35% (all P<0.05). The percentages of S phase cells in
three treatment groups were 18.65±2.17, 26.27±3.14 and 12.6±1.73%,
respectively. The percentages were also significantly decreased
compared with the control group (32.17±3.10%; P<0.05). The
effect of combination treatment was significantly greater than
either inhibitor alone (Fig. 6; all
P<0.05).
Discussion
The pathogenesis of GISTs is closely associated with
uncontrolled cell proliferation and inhibition of apoptosis
(16). The PI3K and MAPK pathways
serve a vital role in the pathogenesis of GISTs (17). Hyperactivation of the PI3K pathway is
associated with constitutive autophosphorylation of c-KIT and
cancer growth (9). The present study
confirmed that the PI3K and MAPK pathways were associated with cell
proliferation and apoptosis, and the cell cycle of GISTs.
Furthermore, a previous study indicated that the activation of
these pathways may be differential. However, the PI3K pathway
appears to be activated to a greater extent than the MAPK pathway
in wild-type GIST tissues and PDGFR mutants (17). Additionally, activation of the PI3K
pathway, rather than the MAPK pathway, was also associated with
drug-resistance to imatinib in GISTs (18). The results of the present study
demonstrated that the PI3K inhibitor, LY294002, had a stronger
growth inhibition effect than the MAPK inhibitor, UO126. However,
the regulatory effect of p-FOXO1, Bcl2 and Bax was more evident by
UO126 than by LY249002. The difference between phenotypes and
molecular expression indicated that regulation of other molecules
may also be responsible.
The PI3K and MAPK signaling pathways are important
regulators of FOXO1 (13). FOXO1 is
an important transcription factor that is associated with the
regulation of cell proliferation and apoptosis. The results
revealed, that compared with normal fibroblasts, total FOXO1 was
suppressed and p-FOXO1 was increased in GIST cells. These results
indicated that regulation of FOXO1 was associated with GISTs.
Furthermore, a recent study also reported that downregulation of
FOXO1 promoted cell proliferation in cervical cancer (11). The activity of FOXO1 may be regulated
by phosphorylation, acetylation and other modifications (11). The results of the present study
indicated that the PI3K and MAPK pathways regulated FOXO1 by
phosphorylation rather than by changing the expression level.
Similar results have also been reported previously where studies
have demonstrated that the PI3K and MAPK inhibitors were able to
inhibit the phosphorylation of FOXO1 and its translocation to the
nucleus, and thereby inhibit the transcriptional activity of FOXO1
on downstream factors (19–21).
Previous studies have demonstrated that FOXO1 is
associated with the regulation of Bcl2, Bax and other
apoptosis-related factors (13,22).
Furthermore, overexpression of Bcl2 inhibits apoptosis, whereas
overexpression of Bax is able to promote apoptosis (23). Phosphorylated FOXO1 is able to
upregulate Bcl2 and downregulate Bax expression to inhibit
apoptosis (24,25). Consistent with those previous
reports, an increased Bcl2 expression and suppressed Bax expression
along with increased p-FOXO1 was also observed in GIST-T1 cells in
the present study. However, treating GIST-T1 cells with LY249002
and UO126 was able to inhibit the activation of FOXO1 into p-FOXO1,
and thereby lead to increased apoptosis rate and arrested cell
cycle at the G0/G1 phase.
In conclusion, regulation of FOXO1 by the PI3K and
MAPK pathways was associated with the pathogenesis of GISTs. The
downregulation of total FOXO1 and hyperactivation of p-FOXO1 was
associated with cell proliferation, apoptosis rate and altered cell
cycle in GIST-T1 cells. Furthermore, the phosphorylation of FOXO1
was activated by the PI3K and MAPK pathways. Therefore, further
study of the role of the transcription factor FOXO1 in GISTs is
required, as it may provide a novel direction for the development
of targeted therapy drugs.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Fujian in China (grant no. 2015J01522).
References
|
1
|
Mazur MT and Clark HB: Gastric stromal
tumors: Reappraisal of histogenesis. Am J Surg Pathol. 7:507–519.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors. Gastroenterol Clin North Am. 42:399–415. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duensing A, Heinrich MC, Fletcher CD and
Fletcher JA: Biology of gastrointestinal stromal tumors: KIT
mutations and beyond. Cancer Invest. 22:106–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sako H, Fukuda K, Saikawa Y, Nakamura R,
Takahashi T, Wada N, Kawakubo H, Takeuchi H, Ohmori T and Kitagawa
Y: Antitumor effect of the tyrosine kinase inhibitor nilotinib on
gastrointestinal stromal tumor (GIST) and imatinib-resistant GIST
cells. PLoS One. 9:e1076132014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al: Efficacy and safety of regorafenib for advanced
gastrointestinal stromal tumours after failure of imatinib and
sunitinib (GRID): An international, multicentre, randomised,
placebo-controlled, phase 3 trial. Lancet. 381:295–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossi F, Ehlers I, Agosti V, Socci ND,
Viale A, Sommer G, Yozgat Y, Manova K, Antonescu CR and Besmer P:
Oncogenic Kit signaling and therapeutic intervention in a mouse
model of gastrointestinal stromal tumor. Proc Natl Acad Sci USA.
103:pp. 12843–12848. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel S: Exploring novel therapeutic
targets in GIST: Focus on the PI3K/Akt/mTOR pathway. Curr Oncol
Rep. 15:386–395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Yang J, Liao W, Liu X, Zhang H,
Wang S, Wang D, Feng J, Yu L and Zhu WG: Cytosolic FoxO1 is
essential for the induction of autophagy and tumour suppressor
activity. Nat Cell Biol. 12:665–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prasad SB, Yadav SS, Das M, Govardhan HB,
Pandey LK, Singh S, Pradhan S and Narayan G: Down regulation of
FOXO1 promotes cell proliferation in cervical cancer. J Cancer.
5:655–662. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto M, Pocai A, Rossetti L, Depinho
RA and Accili D: Impaired regulation of hepatic glucose production
in mice lacking the forkhead transcription factor Foxo1 in liver.
Cell Metab. 6:208–216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ai J, Duan J, Lv X, Chen M, Yang Q, Sun H,
Li Q, Xiao Y, Wang Y, Zhang Z, et al: Overexpression of FoxO1
causes proliferation of cultured pancreatic beta cells exposed to
low nutrition. Biochemistry. 49:218–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taguchi T, Sonobe H, Toyonaga S, Yamasaki
I, Shuin T, Takano A, Araki K, Akimaru K and Yuri K: Conventional
and molecular cytogenetic characterization of a new human cell
line, GIST-T1, established from gastrointestinal stromal tumor. Lab
Invest. 82:663–665. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu KH, Tsai HW, Lin PW, Hsu YS, Lu PJ and
Shan YS: Anti-apoptotic effects of osteopontin through the
up-regulation of Mcl-1 in gastrointestinal stromal tumors. World J
Surg Oncol. 12:1892014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ríos-Moreno MJ, Jaramillo S, Díaz-Delgado
M, Sánchez-León M, Trigo-Sánchez I, Padillo JP, Amérigo J and
González-Cámpora R: Differential activation of MAPK and
PI3K/AKT/mTOR pathways and IGF1R expression in gastrointestinal
stromal tumors. Anticancer Res. 31:3019–3025. 2011.PubMed/NCBI
|
|
18
|
Heinrich MC, Corless CL, Blanke CD,
Demetri GD, Joensuu H, Roberts PJ, Eisenberg BL, von Mehren M,
Fletcher CD, Sandau K, et al: Molecular correlates of imatinib
resistance in gastrointestinal stromal tumors. J Clin Oncol.
24:4764–4774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sykes SM, Lane SW, Bullinger L,
Kalaitzidis D, Yusuf R, Saez B, Ferraro F, Mercier F, Singh H,
Brumme KM, et al: AKT/FOXO signaling enforces reversible
differentiation blockade in myeloid leukemias. Cell. 146:697–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asada S, Daitoku H, Matsuzaki H, Saito T,
Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y and Fukamizu
A: Mitogen-activated protein kinases, Erk and p38, phosphorylate
and regulate Foxo1. Cell Signal. 19:519–527. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiang L, Banks AS and Accili D: Uncoupling
of acetylation from phosphorylation regulates FoxO1 function
independent of its subcellular localization. J Biol Chem.
285:27396–27401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moriishi T, Kawai Y, Komori H, Rokutanda
S, Eguchi Y, Tsujimoto Y, Asahina I and Komori T: Bcl2 deficiency
activates FoxO through Akt inactivation and accelerates osteoblast
differentiation. PLoS One. 9:e866292014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teijido O and Dejean L: Upregulation of
Bcl2 inhibits apoptosis-driven BAX insertion but favors BAX
relocalization in mitochondria. FEBS Lett. 584:3305–3310. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SJ, Winter K, Nian C, Tsuneoka M, Koda
Y and McIntosh CH: Glucose-dependent insulinotropic polypeptide
(GIP) stimulation of pancreatic beta-cell survival is dependent
upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB)
signaling, inactivation of the forkhead transcription factor Foxo1,
and down-regulation of bax expression. J Biol Chem.
280:22297–22307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muranen T, Selfors LM, Worster DT,
Iwanicki MP, Song L, Morales FC, Gao S, Mills GB and Brugge JS:
Inhibition of PI3K/mTOR leads to adaptive resistance in
matrix-attached cancer cells. Cancer Cell. 21:227–239. 2012.
View Article : Google Scholar : PubMed/NCBI
|