Introduction
Immunotherapy is a new avenue of cancer treatment
for a range of different cancer types. It is now understood that
the immune system is capable of recognizing and eliminating cancer
cells, but tumors evade and suppress host immune responses and
therefore persist and spread (1–3). During
the past few decades, anticancer immunotherapy has evolved from a
promising therapeutic option to a robust clinical reality. Many
immunotherapeutic regimens are now approved for use in cancer
patients, and many others are being investigated as standalone
therapeutic interventions or combined with conventional treatments
in clinical studies.
Mycobacterium smegmatis (M. smegmatis)
is a fast-growing saprophytic environmental bacterium, which is a
non-pathogenic and commensal genus (4,5). M.
smegmatis also has a number of properties such as growth
rapidily and can be transformed effectively with many genes, that
renders it an ideal vaccine vector. Further more, M.
smegmatis is reported to activate dendritic cells and trigger
CD8-mediated immune responses, and immunization with rM.S can
generate more durable memory T cells than intramuscular DNA
vaccination (6,7). These findings indicate the potential
role of mycobacteria as recombinant vaccine delivery vector.
Immunogenic target antigen is another crucial
element for developing a successful vaccine. The
melanoma-associated antigen A3 (MAGEA3) is a member of the large
cancer/testis antigens (CTA), which are frequently aberrantly
expressed in a wide range of cancer (8–12). MAGEA
gene family is regarded as a promising target of specific
immunotherapy because MAGEA is expressed mainly in cancers that
have acquired maliganat phenotypes and contribute towards
malignancy (13). MAGEA3 is an tumor
antigenic nonapeptide that is identified in various tumors and
associated with a broad set of HLA (human MHC locus) molecules
(14). Consequently, MAGEA3 antigen
is a genuinely selective target for tumor-specific active
immunotherapy.
It is well known that novel and effective adjuvants
can elicit stronger cellular and humoral adaptive immune responses
to antigenic targets. The expression of a particular CTA is limited
to only a subset of patients with a particular tumor type;
therefore, for human application, this is too weak to induce a
substantial response against difficult antigens. In order to expand
the number of patients and tumor types that can be treated, it is
necessary to expand the repertoire of antigens by this approach. We
developed another CTA, SSX2 (synovial sarcoma X breakpoint 2),
which is the primary member of the SSX family expressed in
different kinds of cancers inculding prostate, lung, breast and
multiple myeloma and pancreatic cancer (15–19).
SSX2 gene encodes for the human tumor-specific antigen HOM-MEL-40,
which is an immunogenic protein known to trigger spontaneous
antibody responses (20). The SSX2
protein can induce spontaneous immune responses. Therefore, the
development of vectors expressing SSX2 opens up a wide array of
possibilities in the immunotherapy of cancer.
In this study, we designed two fusion proteins with
different ligation sequences, MAGEA3-SSX2 and SSX2-MAGEA3, from
M. smegmatis for tumor immunotherapy and detected their
tumor therapeutic effect by mice tumor-burdened experiments.
Materials and methods
Bacterial strains and growth
conditions
The M. smegmatis strain MC2155 was
supplied by Yinlan Bo's Laboratory at the Fourth Military Medical
University (Xi'an, China). M. smegmatis cultures were grown
in 7H10 solid medium (7H10 solid medium contained 3 ml/l glycerin,
0.5 g/l Tween-80, 100 ml/l OADC and 19/l middle brook 7H10 agar
powder) and incubated at 37°C for 2–3 days; the medium was
supplemented with hygromycin (50 ng/ml) when selecting for the
recombinant plasmid. Escherichia coli cultures were grown in
Luriae-Bertani (LB) broth or plates (LB broth contained 10 g/l
trypeptone; 15 g/l NaCl; 5 g/l yeast extract; LB plates contained
10 g/l trypeptone; 15 g/l NaCl; 5 g/l yeast extract and 15 g/l agar
powder) and incubated at 37°C overnight; the media were
supplemented with ampicillin (100 µg/ml) when selecting for the
recombinant plasmid.
Plasmid and strain construction
The pDE22 vector was supplied by Yinlan Bo's
laboratory at the Fourth Military Medical University. The E.
coli strain DH5-α was purchased from MBI Fermentas (Vilnius,
Lithuania). The pUC57 vector was purchased from Tiangen (Beijing,
China). Taq DNA polymerase and Pst I endonuclease were obtained
from Takara Biotechnology Co., Ltd. (Dalian, China). BamHI
endonuclease, ClaI endonuclease, EcoRV endonuclease
and T4 DNA ligase were obtained from MBI Fermentas (Burlington, ON,
Canada). All other media components and chemicals used were of the
highest purity grade available commercially from Beijing Chemical
Plant, China.
Splicing overlap extension polymerase chain reaction
(SOE-PCR) primers were synthesized by Shanghai Bioengineering
Company (Shanghai, China). The MAGEA3 gene was cloned from DNA of
EC9706 cell via PCR using the primer pair: Sense primer
5′-GCCGATATCATGCCTCTTGAGCAGAGGAGTC-3′ and antisense primer
5′-GCTGCCGCCGCCGCCGCTGCC-3′. The SSX2 gene was cloned from DNA of
EC9706 cell via PCR using the primer pair: Sense primer
5′-GCCGATATCATGAACGGAGACGACGCCTTTC-3′ and antisense primer
5′-GCTGCCGCCGCCGCCGCTGCC-3′. The cloned genes MAGEA3 and SSX2 were
constructed from two kinds of different connection sequence gene
fragments, MAGEA3-SSX2 (MS) and SSX2-MAGEA3 (SM). The MAGEA3-SSX2
fragment was amplified using the primer pair: Sense primer
5′-CGGCGGCGGCGGCAGCATGCCTCTTGAGCAGAG-3′ and antisense primer
5′-CCATCGATTTACTCGTCATCTTCCTCAGGG-3′, and the SSX2-MAGEA3 fragment
was amplified using the primer pair: Sense primer
5′-CGGCGGCGGCGGCAGCATGAACGGAGACGACG-3′ and antisense primer
5′-CCATCGATTCACTCTTCCCCCTCTCTCAAA-3′. The MAGEA3-SSX2 and
SSX2-MAGEA3 fusion expression cassettes were generated using the
gap repair method as above, and a linker designed was used to
maintain the correct biological activity of both MAGEA3 and SSX2. A
verified clone with the correct sequence (AuGCT Biotechnology,
Beijing, China) was transferred into a pDE22 cloning vector, then
cut with the appropriate restriction endonucleases and inserted in
the E. coli-mycobacterium shuttle plasmid pDE22 construct.
Plasmid DNA was introduced into M. smegmatis by
electroporation using standard techniques (21) to generate the rM.S strain expressing
the two kinds of fusion protein MAGEA3-SSX2 and SSX2-MAGEA3.
Western blot analysis
To monitor the expression of the M. smegmatis
MAGEA3 and SSX2 transgenes, the rMS strains were grown in 7H10/ADC
until mid-log phase and blocked with 10% bovine serum albumin. The
lysate of grown rM.S was fractionated on 20% SDS-polyacrylamide
gels and blotted onto nitrocellulose filters (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The membranes were
blocked with 5% non-fat milk and incubated with a rabbit anti-human
MAGEA3 antibody (Abgent, Inc., San Diego, CA, USA) at a dilution of
1:100 and a rabbit anti-human SSX2 antibody (Abcam, Cambridge, MA,
USA) at a dilution of 1:200 or a mouse anti-β-actin monoclonal
antibody at a dilution of 1:2,000 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The membranes were subsequently incubated with
a goat anti-mouse or anti-rabbit horseradish peroxidase secondary
antibody (Sigma-Aldrich; Merck KGaA). The protein complexes were
detected using enhanced chemiluminescence reagents (Pierce; Thermo
Fisher Scientific, Inc.).
The production of antibodies against MAGEA3 and SSX2
in the blood of immunized mice was determined using the purified
MAGEA3 protein (Abnova, Walnut, CA, USA) or SSX2 protein (Abnova)
separated by SDS-PAGE. All experiments were carried out at least
three times.
Immunization of mice
Seven-week-old and specific pathogen-free male
BALB/c mice provided by the laboratory animal center of the Fourth
Military Medical University were used for immunogenicity studies.
All animal protocols were reviewed and approved by the
Institutional Animal Care and Use Committee of the Fourth Military
Medical University (ID11013). Mice were randomly divided into six
groups (6 per group) to receive subcutaneous injections as follows:
Normal control group (NC) received 0.2 ml saline/mouse, M.
smegmatis group infected with the M. smegmatis strain
and received 1×106 CFU empty pDE22 vector/mouse via the
tail vein, recombinant M. smegmatis MAGEA3 (rM.S-M) infected
with the M. smegmatis strain and transfected with
pDE22-MAGEA3 at a dose of 1×106 CFU/mouse, recombinant
M. smegmatis SSX2 (rM.S-S) infected with the M.
smegmatis strain and transfected with pDE22-SSX2 at a dose of
1×106 CFU/mouse, recombinant M. smegmatis
MAGEA3-SSX2 (rM.S-MS) infected with the M. smegmatis strain
and transfected with pDE22-MAGEA3-SSX2 at a dose of
1×106 CFU/mouse, recombinant M. smegmatis
SSX2-MAGEA3 (rM.S-SM) infected with the M. smegmatis strain
transfected with pDE22-SSX2-MAGEA3 at a dose of 1×106
CFU/mouse. Mice were immunized once every 5 days with rM.S for a
total of three times.
Immunotherapy in the tumor-bearing
mice
Seven-week-old, specific pathogen-free male BALB/c
nude mice provided by the laboratory animal center of the Fourth
Military Medical University were housed and monitored in a specific
pathogen-free environment with sterile food and water in our animal
facility. The human esophageal EC9706 cancer cell line, which was
MAGEA3 and SSX2 double-positive cancer cell, was maintained in
culture and prepared for injection as previously described
(22). EC9706 tumor cells were
cultured and inoculated subcutaneously into one site on the back
surface of each BALB/c nude mouse at a concentration of
1×106 cells. Mice were cultured and observed until an
obvious visible tumor appeared on the mouse back. Tumor-bearing
mice were randomly divided into six groups with 6 mice each. The
mice received the following different treatment: Normal control
group (NC) receiving 100 µl 0.9% saline/mouse, M. smegmatis
group, rM.S-M group, rM.S-S group, rM.S-MS group and rM.S-SM group
infected with the blood of the immunized mice from the same groups
as the above via the tail vein at a dose of 100 µl/mouse,
respectively.
In the present study, all data collection was
completed from 5 to 21 days after injection. The sizes of tumors
were measured using a digital caliper in three dimensions (L × W ×
H). The height of the tumors was determined by physically grasping
the tumor by its base. Tumor volume was calculated using the
following equation: Tumor Volume=(π·H (H2 + 3·(L+W/2)))/6. Mice
were euthanized by inhalation of CO2 gas on day 21 of
tumor growth. Tumors were dissected and weighed.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) from at least three independent experiments. Statistical
analysis was perforned using SPSS16.0 (SPSS, Inc., Chicago, IL,
USA). Student's t-test and χ2 test were used to analyze
the difference between different groups. The comparison of multiple
groups was carried out using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
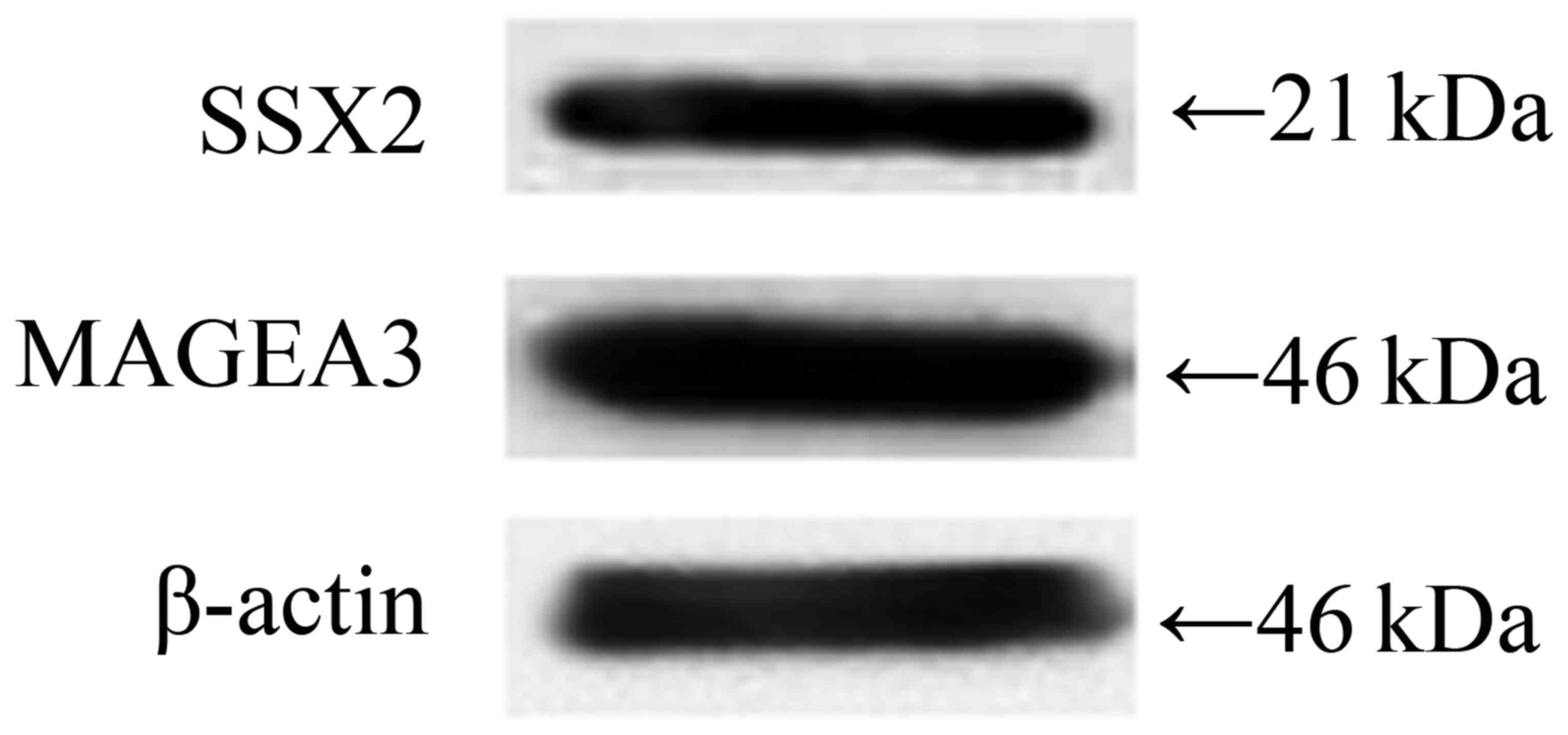
MAGEA3 and SSX2 were double-positive
expressed in human esophageal EC9706 cancer cell line
Firstly, we detected the protein expression level of
MAGEA3 and SSX2 in EC9706 cells, and the results showed that both
MAGEA3 and SSX2 were highly expressed (Fig. 1).
Construction of rM.S strains and the
expression of two fusion proteins
The optimized MAGEA3-SSX2 and SSX2-MAGEA3 fusion
segments were synthesized in the pUC57 vector by Shanghai Generay
Biotechnology Co., Ltd. (Shanghai, China) in BamHI and
EcoRV restriction sites at the 5′and 3′ends. Then, the
generated MAGEA3-SSX2 and SSX2-MAGEA3 fusion segments, along with
the vector, were digested with PstI and ClaI
restriction enzymes and ligated into pDE22 in the corresponding
enzyme site at the 5′and 3′ ends. Agarose gel electrophoresis of
the PstI and ClaI digested plasmid showed the
expected bands of 1,596 bp, representing the insert and pDE22,
respectively (Fig. 2A). Also,
sequencing showed that the target DNA was inserted correctly into
the multi-cloning site of pDE22.
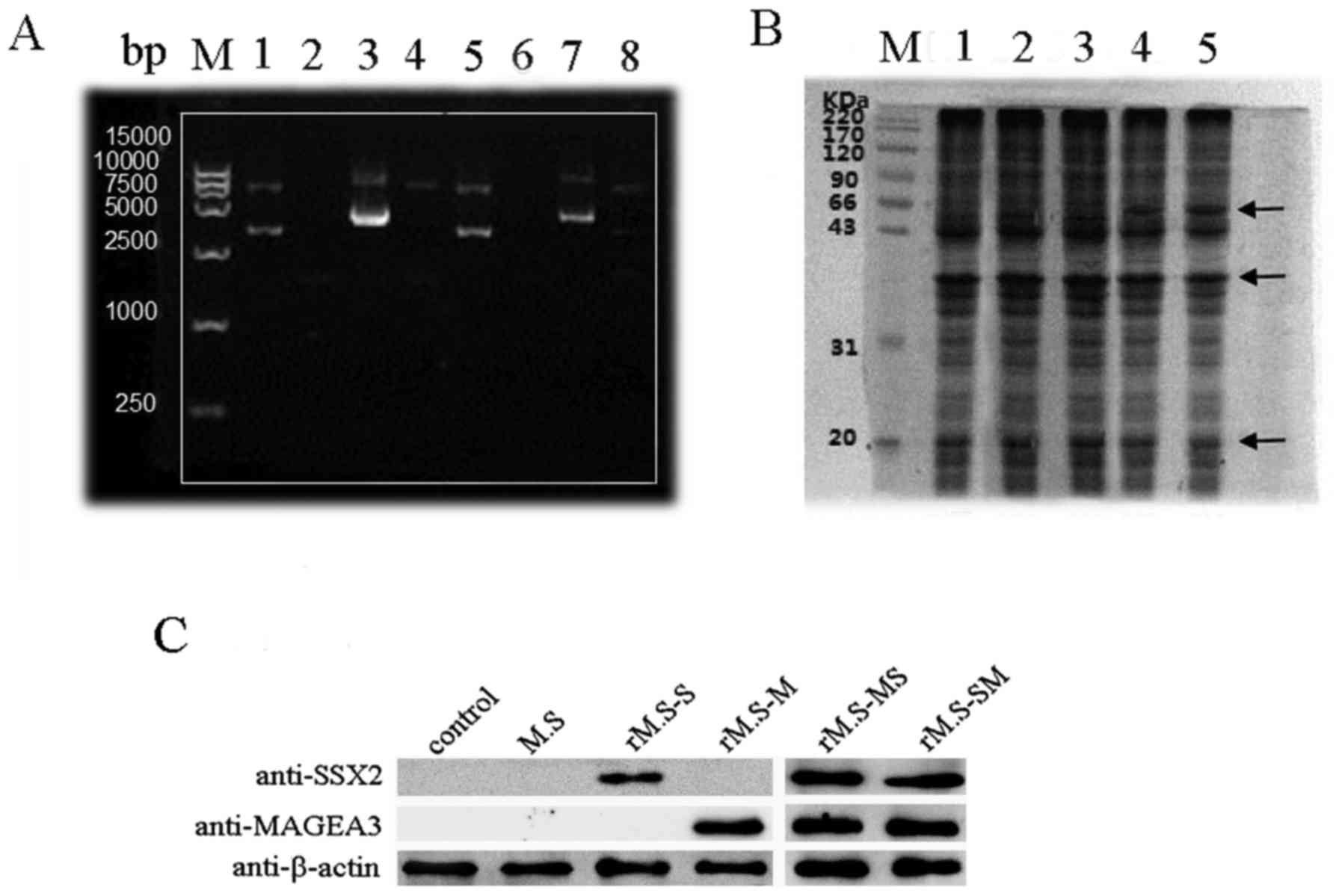 | Figure 2.Identification of successfully
constructed rM.S vaccine and detection the expression of
MAGEA3-SSX2 (MS) and SSX2-MAGEA3 (SM) fusion proteins in the rM.S.
(A) Agarose gel electrophoresis for the verification of recombinant
pDE22 vector. Lane M, DNA marker; line 1, the empty vector of
pDE22; line 2, rM.S; line 3, pDE22-MS; line 4, pDE22-MS digested
with EcoRV and Cla1; line 5, the empty vector of
pDE22; line 6, rM.S-SM; line 7, pDE22- SM; line 8, pDE22-SM
digested with EcoRV and Cla1. (B) SDS-PAGE showed the
expression of the MAGEA3 and SSX2 transgenes in the rM.S. Lane M,
protein marker; line 1, M. smegmatis; line 2, MAGEA3-M.
smegmatis; line 3, SSX2-M. smegmatis; line 4,
MAGEA3-SSX2-rM.S; line 5, SSX2-MAGEA3-rM.S. MAGEA3-SSX2 and
SSX2-MAGEA3 predicted molecular masses of 67 kDa (MAGEA3
approximately 46 kDa and SSX2 approximately 21 kDa). (C) Western
blot analysis of MAGEA3 and SSX2 transgenes expression in the rM.S.
MAGEA3, melanoma-associated antigen A3. rM.S, recombinant M.
smegmatis; rM.S-MS, recombinant M. smegmatis MAGEA3-SSX2; rM.S-SM,
recombinant M. smegmatis SSX2-MAGEA3; rM.S-S, recombinant M.
smegmatis-SSX2; rM.S-M, recombinant M. smegmatis-MAGEA3. |
The pDE22-based constructs were electroporated into
the fast-growing, non-pathogenic M. smegmatis strain to
obtain a rM.S strain that could be easily manipulated in most
laboratories. Following induction, MAGEA3-SSX2 and SSX2-MAGEA3 were
expressed and corresponded to their predicted molecular masses of
67 kDa (MAGEA3 approximately 46 kDa and SSX2 approximately 21 kDa)
on an SDS-PAGE gel (Fig. 2B).
Western blotting showed that the MAGEA3-SSX2 and SSX2-MAGEA3 were
recognized by the purchased rabbit anti-human SSX2 polyclonal
antibody and anti-human MAGEA3 polyclonal antibody (Fig. 2C).
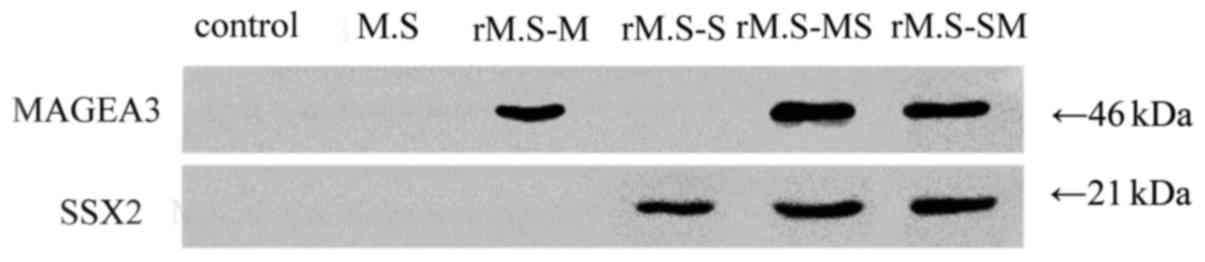
Antibody production in immunized
mice
Mice were immunized once every 5 days with rM.S for
a total of three times, as described in Methods. The expression of
fusion protein-specific antibody levels after final vaccination is
shown in Fig. 3. Immunized mice
blood fusion protein-specific antibodies levels were detected by
Western blot. Significantly specific specific antibodies levels
were observed in mice of the groups vaccinated with the rM.S
compared to the control group. In contrast, no specific antibodies
were expressed between the control group and the M.
smegmatis group.
The antitumor effect of the rM.S-MS
and rM.S-SM was better than that of the single rM.S-M or
rM.S-S
To establish the tumor-bearing mouse model, mice
were injected using 1×106 EC9706 cells into one site of
the ventral surface. Approximately 5 days later, the back surface
of every mouse formed a macroscopic and palpable tumor. Following
the detection of a palpable tumor, mice were treated daily by rM.S
injection with 100 µl/mouse or saline control. The rM.S-treated
tumor volumes were lower compared with the control group (Fig. 4A). As shown in Fig. 3, rM.S-M and rM.S-S-stimulated mice
group showed a slight decrease in tumor volume compared to the
decrease in tumor volume of the rM.S-MS- or rM.S-SM-stimulated mice
groups. There was no significant difference in tumor volume between
rM.S-MS or rM.S-SM mice. This was accompanied by a significant
reduction in the final tumor weight in the rM.S-treated mice.
Tumors were dissected and weighed on day 21 of tumor growth
(Fig. 4B). As shown in Fig. 3, thte rM.S-M- and rM.S-S-stimulated
mice groups showed a slight decrease in tumor weight compared to
the rM.S-MS- or rM.S-SM-stimulated mice groups. There was no
significant difference in tumor weight between rM.S-MS or rM.S-SM
mice. The result shows that MAGEA3 and SSX2 rM.S are better than
the single MAGEA3 M. smegmatis or single SSX2 M.
smegmatis for the demonstration of an antitumor effect. In
addition, there was no significant difference in the antitumor
effect of rM.S-MS or rM.S-SM.
Discussion
Mycobacterium smegmatis is a nonpathogenic
species of the genus Mycobacterium which is easily
manipulated to produce recombinant bacteria. Consequently, it is
widely used as a live vaccine against cancer (23,24).
rM.S has been used to express various proteins in studies. A
particular rM.S vaccine expressing a fusion protein containing
ESAT-6 and CFP10 induced higher humoral and cellular immunity than
the M. bovis BCG vaccination in a mouse model (25). Similarly, Xu et al reported
that fusion protein Ag85A-IL17A was expressed in rM.S vaccine which
attenuated allergic airwat inflammation (26). Here, we demonstrated two kinds of
novel vaccine vectors: rM.S-MS and rM.S-SM. rM.S-MS is a
recombinant non-pathogenic M. smegmatis MC2155
expressing the MAGEA3-SSX2 fusion protein; rM.S-SM is a rM.S
expressing the SSX2-MAGEA3 fusion protein. Moreover, the M.
smegmatis MC2155 strain has been shown to be
nonpathogenic following intravenous infections of SCID mice
(27); we also verified the
treatment effect of tumors in the tumor-bearing mouse model through
vaccine with recombinant strains.
We have shown that our recombinant vaccines rM.S-MS
and rM.S-SM are capable of inducing a specific immune response in
two different vaccination schemes (Fig.
2). This response probably reflects the immunogenicity of the
recombinant fusion protein when used in this vaccine vector. The
results of our group and others have confirmed that MAGEA3 and SSX2
tumor antigens with immunogenic and antigenic properties are
represented by the epitopes expressed in the recombinant fusion
protein (28–32). Both MAGEA3 and SSX2 belong to the
group of cancer-testis antigens (CTA); CTA are immunogenic antigens
with an expression that is largely restricted to testicular germ
cells and a variety of malignancies, making them attractive targets
for cancer immunotherapy (11,33). The
cancer-testis-X genes have been the principal targets of developing
immunotherapies (33). MAGEA3 and
SSX2 are all immunogenic CTA that have been shown to elicit
coordinated humoral and cell-mediated immune responses (33,34).
MAGEA3 is one of the best-characterized tumor
antigens. Due to its tumor-restricted expression pattern and its
recognition by both cytotoxic and helper T cells, it constitutes a
promising tumor antigen for anticancer immunotherapy. Roeder et
al demonstrated that MAGEA3 is a frequent tumor antigen of
metastasized melanoma (29).
Vansteenkiste et al demonstrated that MAGEA3 cancer
immunotherapy is an active immunotherapy that has been evaluated in
NSCLC (35). SSX gene products are
expressed in tumors of different histological types and can be
recognized by tumor-reactive CTLs from cancer patients. The
immunogenicity of SSX-2 has been previously corroborated by
detection of specific humoral and CD8+ T cell responses in cancer
patients (36). Ayyoub et al
also demonstrated that an SSX2-derived immunodominant T cell
epitope is recognized by CD4+ T cells from melanoma patients in
association with HLA-DR (37,38).
This is because MAGEA3 and SSX2 are able to trigger an immune
response in the tumor. The aim of our study is to build a fusion
protein composed of MAGEA3 and SSX2 and verify whether the fusion
proteins' antitumor immune responses are enhanced by either of the
two. In the present study, two fusion proteins were amplified in
M. smegmatis. ELISA was used to detect whether anti-fusion
protein antibodies are produced or not in vivo in immunized
mice after the injection of rM.S vaccine. In Fig. 2, rM.S-MS group mice blood and the
rM.S-SM group mice blood are able to produce both anti-MAGEA3 and
anti-SSX2 antibodies. There was no significant difference in
antibody concentration between the two groups. However, the
antibody concentration of the rM.S-MS and rM.S-SM groups was
significantly increased compared with the rM.S-M group and rM.S-S
group, which only produce single protein antibodies. The results
are in line with our expectations. Multiple-antigen combination
tests may be more useful for the development of diagnostic antibody
tests because of many antigens inducing serological responses.
The BALB/c mouse is the animal most commonly used as
an in vivo model for M. smegmatis infection and
constructed tumor-bearing mice model. The human esophageal EC9706
cancer cell line is a MAGEA3 and SSX2 double-positive tumor. In
this study, BALB/c mice were immunized with rM.S-M, rM.S-S, rM.S-MS
and rM.S-SM to provide experimental data to evaluate the effect of
these proteins in M. smegmatis; blood was obtained
containing the corresponding antibody to the treatment of
tumor-bearing mice. According to our data after immunization, we
extracted blood from the mice with the best antibody concentration
for the following experiment. Treatment with the rM.S inhibits
esophageal tumor growth in vivo in mice. The rM.S treated
tumor volumes and weight were significantly reduced compared with
the control group (Fig. 3).
In conclusion, in the current study we constructed
rM.S vaccines, rM.S-MS and rM.S-SM, and demonstrated the
immunogenicity of the vaccine. In addition, we demonstrated that
the rM.S vaccine can activate the immune system and enhance the
antitumor effect. The antitumor effect of the rM.S-MS and rM.S-SM
is better than that of the single rM.S-M or rM.S-S.
Acknowledgements
The authors are grateful to Yinlan Bo's Laboratory,
The Fourth Military Medical University, which made this work
possible. The authors would also like to thank the clinicians and
hospital staff who contributed to data collection for this
study.
Funding
The present research received funding from the
National Natural Science Foundation (grant nos. 31370926 and
81371774).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WJ and YX conceived and designed the experiments.
WJ, XL, JK, YL and YB performed the experiments. WJ and XL analyzed
the data. WJ, XL and JK contributed reagents/materials/analysis
tools. WJ and YX wrote the paper.
Ethics approval and consent to
participate
All animal protocols were reviewed and approved by
the Institutional Animal Care and Use Committee of the Fourth
Military Medical University (ID11013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lizée G, Cantu MA and Hwu P: Less yin,
more yang: Confronting the barriers to cancer immunotherapy. Clin
Cancer Res. 13:5250–5255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart TJ and Abrams SI: How tumours
escape mass destruction. Oncogene. 27:5894–5903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart TJ and Smyth MJ: Improving cancer
immunotherapy by targeting tumor-induced immune suppression. Cancer
Metastasis Rev. 30:125–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pierre-Audigier C, Jouanguy E, Lamhamedi
S, Altare F, Rauzier J, Vincent V, Canioni D, Emile JF, Fischer A,
Blanche S, et al: Fatal disseminated Mycobacterium smegmatis
infection in a child with inherited interferon gamma receptor
deficiency. Clin Infect Dis. 24:982–984. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sweeney KA, Dao DN, Goldberg MF, Hsu T,
Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen
B, et al: A recombinant Mycobacterium smegmatis induces
potent bactericidal immunity against Mycobacterium
tuberculosis. Nat Med. 17:1261–1268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Faassen H, Dudani R, Krishnan L and
Sad S: Prolonged antigen presentation, APC-, and CD8+ T cell
turnover during mycobacterial infection: Comparison with Listeria
monocytogenes. J Immunol. 172:3491–3500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cayabyab MJ, Hovav AH, Hsu T, Krivulka GR,
Lifton MA, Gorgone DA, Fennelly GJ, Haynes BF, Jacobs WR Jr and
Letvin NL: Generation of CD8+ T-cell responses by a recombinant
nonpathogenic Mycobacterium smegmatis vaccine vector
expressing human immunodeficiency virus type 1 Env. J Virol.
80:1645–1652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sang M, Lian Y, Zhou X and Shan B: MAGE-A
family: Attractive targets for cancer immunotherapy. Vaccine.
29:8496–8500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meek DW and Marcar L: MAGE-A antigens as
targets in tumour therapy. Cancer Lett. 324:126–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hofmann O, Caballero OL, Stevenson BJ,
Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A,
et al: Genome-wide analysis of cancer/testis gene expression. Proc
Natl Acad Sci USA. 105:20422–20427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: An expanding family of targets
for cancer immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gjerstorff MF, Andersen MH and Ditzel HJ:
Oncogenic cancer/testis antigens: Prime candidates for
immunotherapy. Oncotarget. 6:15772–15787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van den Eynde BJ and van der Bruggen P: T
cell defined tumor antigens. Curr Opin Immunol. 9:684–693. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gaugler B, Van den Eynde B, van der
Bruggen P, Romero P, Gaforio JJ, De Plaen E, Lethé B, Brasseur F
and Boon T: Human gene MAGE-3 codes for an antigen recognized on a
melanoma by autologous cytolytic T lymphocytes. J Exp Med.
179:921–930. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greve KB, Pøhl M, Olsen KE, Nielsen O,
Ditzel HJ and Gjerstorff MF: SSX2-4 expression in early-stage
non-small cell lung cancer. Tissue Antigens. 83:344–349. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bloom JE and McNeel DG: SSX2 regulates
focal adhesion but does not drive the epithelial to mesenchymal
transition in prostate cancer. Oncotarget. 7:50997–51011. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greve KB, Lindgreen JN, Terp MG, Pedersen
CB, Schmidt S, Mollenhauer J, Kristensen SB, Andersen RS, Relster
MM, Ditzel HJ and Gjerstorff MF: Ectopic expression of
cancer/testis antigen SSX2 induces DNA damage and promotes genomic
instability. Mol Oncol. 9:437–449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Zhou WB, Zhao Y, Liu XA, Ding Q,
Zha XM and Wang S: Cancer/testis antigen SSX2 enhances invasiveness
in MCF-7 cells by repressing ERα signaling. Int J Oncol.
40:1986–1994. 2012.PubMed/NCBI
|
|
19
|
Huang CJ, Chen RH, Vannelli T, Lee F,
Ritter E, Ritter G, Old LJ and Batt CA: Expression and purification
of the cancer antigen SSX2: A potential cancer vaccine. Protein
Expr Purif. 56:212–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Türeci O, Sahin U, Schobert I, Koslowski
M, Scmitt H, Schild HJ, Stenner F, Seitz G, Rammensee HG and
Pfreundschuh M: The SSX-2 gene, which is involved in the t(X;18)
translocation of synovial sarcomas, codes for the human tumor
antigen HOM-MEL-40. Cancer Res. 56:4766–4772. 1996.PubMed/NCBI
|
|
21
|
Bardarov S, Kriakov J, Carriere C, Yu S,
Vaamonde C, McAdam RA, Bloom BR, Hatfull GF and Jacobs WR Jr:
Conditionally replicating mycobacteriophages: A system for
transposon delivery to Mycobacterium tuberculosis. Proc Natl
Acad Sci USA. 94:10961–10966. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu H, Crawford D, Hutchinson KR, Youtz DJ,
Lucchesi PA, Velten M, McCarthy DO and Wold LE: Myocardial
dysfunction in an animal model of cancer cachexia. Life Sci.
88:406–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Young SL, Murphy M, Zhu XW, Harnden P,
O'Donnell MA, James K, Patel PM, Selby PJ and Jackson AM:
Cytokine-modified Mycobacterium smegmatis as a novel
anticancer immunotherapy. Int J Cancer. 112:653–660. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Y, Henning J and O'Donnell MA: Th1
cytokine-secreting recombinant Mycobacterium bovis bacillus
Calmette-Guérin and prospective use in immunotherapy of bladder
cancer. Clin Dev Immunol. 2011:7289302011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Peng P, Miao S, Zhao Y, Mao F,
Wang L, Bai Y, Xu Z, Wei S and Shi C: Recombinant Mycobacterium
smegmatis expressing an ESAT6-CFP10 fusion protein induces
anti-mycobacterial immune responses and protects against
Mycobacterium tuberculosis challenge in mice. Scand J Immunol.
72:349–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu W, Chen L, Guo S, Wu L and Zhang J:
Intranasal administration of recombinant Mycobacterium
smegmatis inducing IL-17A autoantibody attenuates airway
inflammation in a murine model of allergic asthma. PLoS One.
11:e01515812016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bange FC, Collins FM and Jacobs WR Jr:
Survival of mice infected with Mycobacterium smegmatis
containing large DNA fragments from Mycobacterium tuberculosis.
Tuber Lung Dis. 79:171–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kirkin AF, Dzhandzhugazyan KN and Zeuthen
J: Cancer/testis antigens: Structural and immunobiological
properties. Cancer Invest. 20:222–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roeder C, Schuler-Thurner B, Berchtold S,
Vieth G, Driesch Pv, Schuler G and Lüftl M: MAGE-A3 is a frequent
tumor antigen of metastasized melanoma. Arch Dermatol Res.
296:314–319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moeller I, Spagnoli GC, Finke J, Veelken H
and Houet L: Uptake routes of tumor-antigen MAGE-A3 by dendritic
cells determine priming of naïve T-cell subtypes. Cancer Immunol
Immunother. 61:2079–2090. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valmori D, Qian F, Ayyoub M, Renner C,
Merlo A, Gnjatic S, Stockert E, Driscoll D, Lele S, Old LJ and
Odunsi K: Expression of synovial sarcoma X (SSX) antigens in
epithelial ovarian cancer and identification of SSX-4 epitopes
recognized by CD4+ T cells. Clin Cancer Res. 12:398–404. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abate-Daga D, Speiser DE, Chinnasamy N,
Zheng Z, Xu H, Feldman SA, Rosenberg SA and Morgan RA: Development
of a T cell receptor targeting an HLA-A*0201 restricted epitope
from the cancer-testis antigen SSX2 for adoptive immunotherapy of
cancer. PLoS One. 9:e933212014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caballero OL and Chen YT: Cancer/testis
(CT) antigens: Potential targets for immunotherapy. Cancer Sci.
100:2014–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hemminger JA, Toland AE, Scharschmidt TJ,
Mayerson JL, Guttridge DC and Iwenofu OH: Expression of
cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and
CTAG2 in myxoid and round cell liposarcoma. Mod Pathol.
27:1238–1245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vansteenkiste J, Zielinski M, Linder A,
Dahabreh J, Gonzalez EE, Malinowski W, Lopez-Brea M, Vanakesa T,
Jassem J, Kalofonos H, et al: Adjuvant MAGE-A3 immunotherapy in
resected non-small-cell lung cancer: Phase II randomized study
results. J Clin Oncol. 31:2396–2403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bricard G, Bouzourene H, Martinet O,
Rimoldi D, Halkic N, Gillet M, Chaubert P, Macdonald HR, Romero P,
Cerottini JC and Speiser DE: Naturally acquired MAGE-A10- and
SSX-2-specific CD8+ T cell responses in patients with
hepatocellular carcinoma. J Immunol. 174:1709–1716. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ayyoub M, Hesdorffer CS, Metthez G,
Stevanovic S, Ritter G, Chen YT, Old LJ, Speiser D, Cerottini JC
and Valmori D: Identification of an SSX-2 epitope presented by
dendritic cells to circulating autologous CD4+ T cells. J Immunol.
172:7206–7211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ayyoub M, Stevanovic S, Sahin U, Guillaume
P, Servis C, Rimoldi D, Valmori D, Romero P, Cerottini JC,
Rammensee HG, et al: Proteasome-assisted identification of a
SSX-2-derived epitope recognized by tumor-reactive CTL infiltrating
metastatic melanoma. J Immunol. 168:1717–1722. 2002. View Article : Google Scholar : PubMed/NCBI
|