Introduction
Severe acute pancreatitis (SAP) is an acute, severe
pancreatic inflammation with a mortality rate of 30% (1). Currently, the management of SAP remains
controversial, primarily due to poor understanding of its
pathogenesis. Generally, SAP patients undergo conservative
management at the early phase, and removal of the peripancreatic
necrotic tissue at the late phase (2). Nevertheless, recent studies have
suggested that the incidence of SAP is associated with pancreatic
duct obstruction and hypertension, and hence surgical treatment of
SAP should focus on pressure reduction and drainage (3). Herein, we present the cases of 3 SAP
patients who were successfully treated in 2016 via endoscopic
pancreatic stenting at the early phase and nasopancreatic drainage
at the late phase.
Case reports
Ethics approval
The present study was approved by the Research
Ethics Committee of the General Hospital of Ningxia Medical
University (Yinchuan, China). Each patient signed an informed
consent for permission to use the clinical data and images.
Case 1
A 44-year-old male was admitted to the General
Hospital of Ningxia Medical University due to severe upper
abdominal pain, heavy sweat and increased respiratory and heart
rate. The patient had elevated biliary and liver enzyme level,
white blood cell (WBC) count of 20.90×109/l, neutral
granulocyte ratio (NEUT%) of 81.1%, amylase of 3,879.6 U/l, lipase
of 20,000 U/l, APACHE II score of 10, and oxygenation index of 149.
The patient was diagnosed with severe acute biliary pancreatitis.
He underwent emergency endoscopic operation. Endoscopic
sphincterotomy (EST) was performed. A wire was placed into the
pancreatic duct and a 6-cm-long 6-Fr-sized stent was inserted over
the wire. A large amount of pancreatic fluid containing protein
plugs was removed. A second wire was placed into the bile duct, and
a nasobiliary duct was placed along the guide wire. The patient was
then given non-invasive ventilation and conventional infusion
therapy for anti-infection, acid suppression, inhibition of enzyme
secretion and liver protection. The patient had restored
respiratory function and urination, WBC of 11.88×109/l
and NEUT% of 90.6% on day 1 postoperatively, and began oral feeding
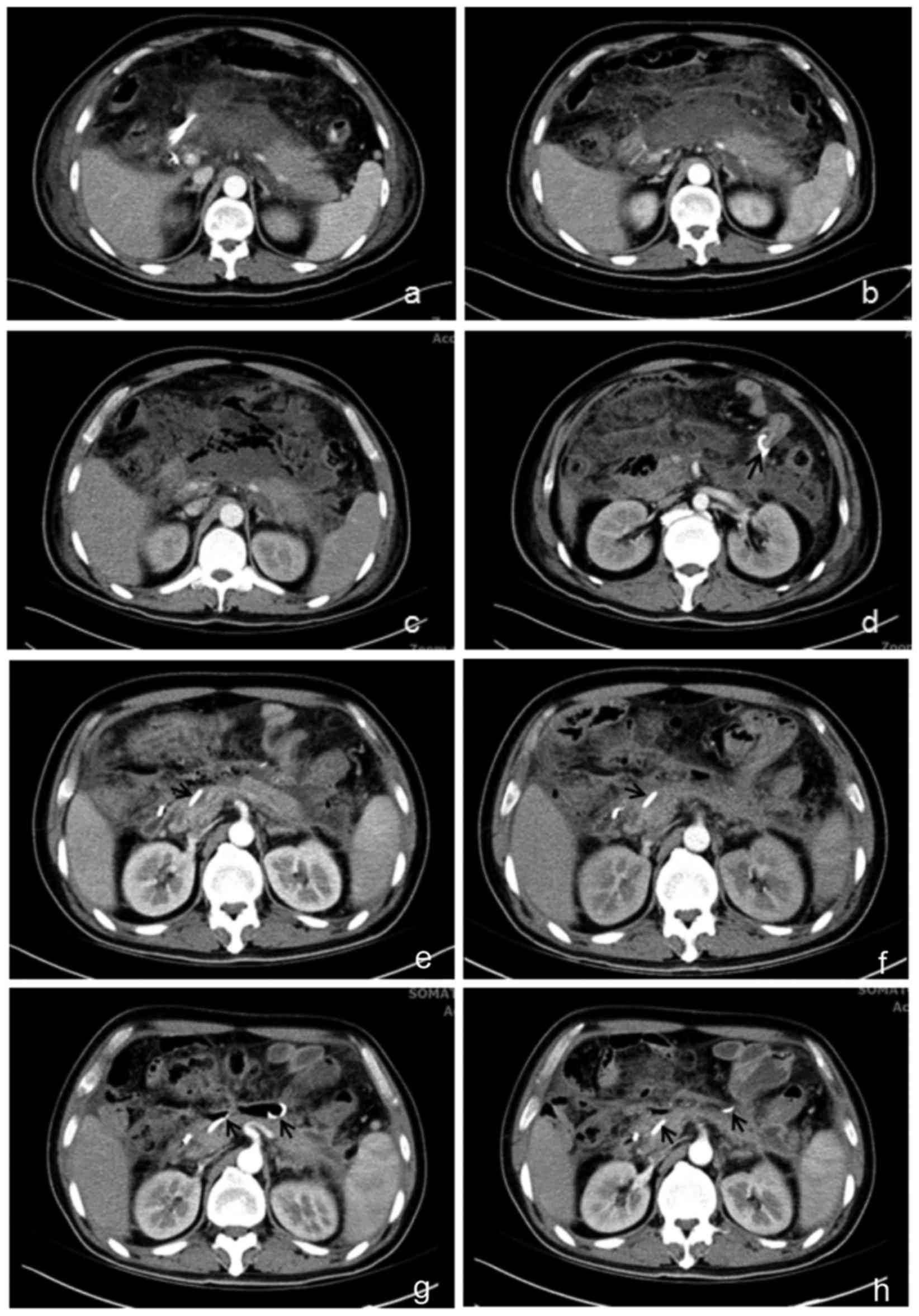
on day 7. Computed tomography (CT) scan showed progressive
pancreatic necrosis and acute peripancreatic fluid accumulation
(Fig. 1a and b). However, the
patient developed fever and increased WBC, and his condition was
not improved by anti-infection treatment. The pancreatic duct stent
was suspected to be blocked, and endoscopic operation was performed
again on day 14 to replace the old pancreatic duct stent with an
8-cm-long 7-Fr-sized stent. WBC dropped to 12.07×109/l,
and NEUT% was 88.8% immediately after the operation. At 1 week
after the second stenting, abdominal enhanced CT showed
peripancreatic encapsulated effusion and infective necrosis. The
pancreatic duct stent had slipped into duodenum (Fig. 1c and d). The patient then underwent
nasopancreatic drainage. Purulent pancreatic fluid (30 ml) was
removed during the operation and daily postoperatively (10–30 ml).
The patient was given antibiotics treatment according to the
antibiotic susceptibility of bile cultures. The nasopancreatic duct
fell off on day 12 after the first nasopancreatic duct drainage,
and the patient underwent endoscopic surgery to insert a new
nasopancreatic duct. Dark red purulent pancreatic fluid was removed
during the surgery (100 ml, Fig. 2a)
and daily postoperatively (50–200 ml, Fig. 2b). The fluid became clear after 1
week (Fig. 2c). CT scan showed the
gradually decreased peripancreatic infection necrosis (Fig. 1e-h). Patient was cured and discharged
at 28 days after the last surgery. The disease duration was 71
days.
Case 2
A 30-year-old female was admitted to the hospital
due to 19-day obvious abdominal pain. The patient had fever
(38.5°C), oliguria, increased heart rate and breathing, WBC of
11.67×109/l, NEUT% of 77.9%, oxygenation index of 181,
and APACHE II score of 8. CT scan revealed acute necrotizing
pancreatitis with extensive peripancreatic fluid. The patient was
diagnosed with severe acute idiopathic pancreatitis, and underwent
endoscopic operation to place a 6-cm-long 5-Fr-sized pancreatic
duct stent and a nasobiliary duct. Pancreatic fluid containing
protein plugs was removed. The patient was given conventional
infusion treatment. On day 1 postoperatively, patient's blood test,
amylase and lipase levels, respiratory and heart rate, and oxygen
saturation returned to normal range. APACHE II score was 2. On day
2, the patient was allowed oral feeding. On day 3, the patient
developed intermittent fever and increased blood parameters, which
were gradually returned to normal range after a 5-day
anti-infection therapy. CT suggested acute necrotizing pancreatitis
with markedly alleviated pancreatic fluid collection. The patient
was discharged from the hospital on day 8 as per her own request,
but was admitted back into our hospital due to severe abdominal
pain and high fever (39°C) on day 13. The patient had WBC of
34.23×109/l, NEUT% of 89.4%, and APACHE II score of 13.
CT scan suggested necrotizing pancreatitis with peripancreatic
encapsulated effusion and infective necrosis. She underwent
endoscopic surgery to remove the pancreatic duct stent and to
perform nasopancreatic drainage. Chylous pancreatic fluid was
removed during the operation (120 ml) and daily postoperatively
(50–100 ml/day). She was given postoperative anti-infection therapy
and non-invasive ventilation (oxygenation index of 198). On day 1
after drainage, the patient had greatly relieved abdominal pain,
WBC of 14.15×109/l, NEUT% of 78.9%, APACHE II score of
7, and normal respiratory and heart rate. The patient was allowed
oral feeding on day 2. The respiratory function was gradually
restored, and the body temperature and blood test results gradually
went back to normal range. CT scan suggested progressive reduction
of peripancreatic infection necrosis and complete removal of
pancreatic fluid collections on day 30 after drainage. The tube was
pulled out and the patient was discharged from the hospital. The
disease duration was 58 days, including 34 days of
hospitalization.
Case 3
A 60-year-old female with serum amylase of 1,493.6
U/l, WBC of 10.06×109/l, NEUT% of 90.5%, APACHE II score
of 8, and oxygenation index of 179 was diagnosed with severe acute
idiopathic pancreatitis in the hospital. She underwent emergency
endoscopic operation immediately after admission to place a
pancreatic duct stent and a nasobiliary duct. Pancreatic fluid
containing protein plugs were removed. The patient was given
postoperative conventional infusion therapy and non-invasive
ventilation. Respiratory function and urination were gradually
restored. Blood results and body temperature returned to normal,
and the patient began oral feeding on day 12. On day 22, the
patient developed high fever and elevated WBC despite the
anti-infection therapy. CT scan suggested peripancreatic
encapsulated effusion. The patient then underwent nasopancreatic
drainage. A large amount of white pus (100 ml) was removed during
the operation and daily postoperatively (50–150 ml). Abdominal
enhanced CT scan at day 7, 14 and 30 showed that peripancreatic
encapsulated effusion was clearly reduced. The patient was
discharged after 68 days of hospitalization. The disease duration
was 88 days since the onset.
Discussion
Traditionally, the best surgical intervention timing
for SAP is at 4 weeks after onset when walled-off necrosis (WON)
and infection has developed (4). The
surgical intervention includes percutaneous drainage or endoscopic
drainage followed by infectious necrotic tissue removal. We treated
3 SAP patients via endoscopic pancreatic stenting at the early
phase and nasopancreatic drainage at the late phase, and achieved
great outcomes. Our cases suggested that the indicated surgical
regime might be a promising strategy for the treatment of SAP.
Organ failure induced by severe systemic
inflammatory response is known as the most important cause of death
in the early phase of SAP (4).
Therefore, the main treatment goal at this stage is to control the
systemic inflammatory response. Patients are generally treated by
conservative infusion because early surgical intervention often
aggravate the inflammatory response, leading to an increased
mortality rate (5,6). Endoscopic operation does not create
extra trauma or cause the diffusion of peripancreatic fluid, and
thus is operable even in SAP patients with severe organ dysfunction
(7). More importantly, we found
viscous pancreatic fluid or even ‘protein plugs’ which, if not
removed, might clog the duct and aggravate the conditions. In this
study, after pancreatic duct obstruction was removed by surgery,
the inflammatory indexes (APACHE II score, WBC, body temperature,
heart rate) were improved in all patients, suggesting that the
clearance of pancreatic duct obstruction is beneficial for the
early control of systemic inflammatory response in SAP.
Most aseptic necrotizing pancreatitis can be cured
by conservative treatments. Nevertheless, peripancreatic infectious
necrosis occurs in 25–70% of SAP patients (6), and has become the primary cause of
death in the late phase of SAP (8).
Currently, a step-up approach of percutaneous drainage is the main
surgical approach (4). However,
approaches such as transmural or percutaneous image guided drainage
(9), and necrotic tissue removal via
abdominal or video assisted retroperitoneal debridement (VARD)
(10) need to create an additional
channel for the drainage of infection necrosis. Reportedly, 31–44%
of patients with acute necrotizing pancreatitis had pancreatic duct
rupture (11–13). For these patients, drainage through
the duodenal papillary pancreatic duct can enter the necrotic
effusion cavity directly through the ruptured pancreatic duct, so
as to achieve the drainage without an additional wound. Studies
have shown that this method can improve the cure rate of
peripancreatic hydrops, prevent recurrence of peripancreatic
hydrops and repair the pancreatic duct rupture (14–16), and
reduce the risk of hemorrhage and digestive tract leakage caused by
a puncture (17).
In the current 3 cases, nasopancreatic duct drainage
was performed and a large amount of infection necrosis was removed
during and after the surgery. Patients' conditions were greatly
improved, and inflammation and peripancreatic infectious necrosis
were gradually alleviated and eventually disappeared, indicating
that this surgical intervention is feasible for the treatment of
late necrotizing pancreatitis. Imaging findings suggested that the
infection necrotic cavities were linked to the nasopancreatic duct,
which might explain the efficacy of nasopancreatic duct drainage in
infection necrotizing pancreatitis.
Although the pathogenesis of acute pancreatitis has
not been fully elucidated yet, studies have suggested pancreatic
duct obstruction and hypertension as key events of both biliary and
non-biliary acute pancreatitis (18,19).
Moreover, pancreatic duct hypertension is positively correlated
with the severity of acute pancreatitis (20), leading to pancreatic ischemia
necrosis and severe pancreatitis (21,22).
Consistently, our cases had pancreatic duct obstruction during both
early and late phase, confirming the critical role of pancreatic
duct obstruction and hypertension in SAP. A prompt and effective
drainage of the pancreatic duct reduced the intra-duct pressure,
and thereby greatly improved the healing of SAP. None of the 3
patients were aggravated by pancreatitis after ERCP. We did not
perform pancreatogram during the operation, because acute
pancreatitis was associated with damage to the main pancreatic duct
and gland vesicle, and pressure on the pancreatic duct during
pancreatogram could directly lead to rupture of the main pancreatic
duct or gland vesicle, thus inducing postoperative pancreatitis
after ERCP and aggravating acute pancreatitis (23,24). The
clear diagnosis of the 3 patients led to avoidance of unnecessary
pancreatic duct angiography and reduced the incidence of
pancreatitis after ERCP. Pancreatic duct stenting is an important
prevention method for postoperative pancreatitis after ERCP, and
even an important remedy for postoperative pancreatitis after ERCP
(25). In our study, pancreatic duct
catheterization was used to treat acute pancreatitis, and these two
factors prevented the occurrence of postoperative pancreatitis.
In summary, we reported on 3 SAP cases that were
successfully treated via pancreatic stent at the early phase and
nasopancreatic drainage at the late phase. Results suggested that
the indicated treatment strategy might alleviate the systemic
inflammatory reaction via removal of pancreatic duct obstruction,
and thus improve the healing of SAP, although multicenter clinical
trials are needed.
Acknowledgements
Not applicable.
Funding
This study was supported by the Technology Support
Program of Ningxia (2015KJHM40).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW and QW interpreted the CT results. JS and WY
acquired and analyzed the general data of the patients. PL, ZW and
CT performed the surgery. PY and JL were responsible for the
inflammatory indexes analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the General Hospital of Ningxia Medical
University (Yinchuan, China). Each patient signed an informed
consent for permission to use the clinical data and images.
Patient consent for publication
All patients signed an informed consent for
permission to use the clinical data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beger HG and Rau BM: Severe acute
pancreatitis: Clinical course and management. World J
Gastroenterol. 13:5043–5051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mier J, León EL, Castillo A, Robledo F and
Blanco R: Early versus late necrosectomy in severe necrotizing
pancreatitis. Am J Surg. 173:71–75. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang C, Wang B, Xie B, Liu H and Chen P:
Treatment of severe acute pancreatitis through retroperitoneal
laparoscopic drainage. Front Med. 5:302–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Santvoort HC, Besselink MG, Bakker OJ,
Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF,
van Eijck CH, Bollen TL, et al: Dutch Pancreatitis Study Group: A
step-up approach or open necrosectomy for necrotizing pancreatitis.
N Engl J Med. 362:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zerem E, Imamović G, Sušić A and Haračić
B: Step-up approach to infected necrotising pancreatitis: A 20-year
experience of percutaneous drainage in a single centre. Dig Liver
Dis. 43:478–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Baal MC, van Santvoort HC, Bollen TL,
Bakker OJ, Besselink MG and Gooszen HG: Dutch Pancreatitis Study
Group: Systematic review of percutaneous catheter drainage as
primary treatment for necrotizing pancreatitis. Br J Surg.
98:18–27. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uhl W, Warshaw A, Imrie C, Bassi C, McKay
CJ, Lankisch PG, Carter R, Di Magno E, Banks PA, Whitcomb DC, et
al: International Association of Pancreatology: IAP guidelines for
the surgical management of acute pancreatitis. Pancreatology.
2:565–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Besselink MG, van Santvoort HC,
Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH,
Schaapherder AF and Gooszen HG: Dutch Acute Pancreatitis Study
Group: Timing and impact of infections in acute pancreatitis. Br J
Surg. 96:267–273. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hollemans RA, van Brunschot S, Bakker OJ,
Bollen TL, Timmer R, Besselink MG and van Santvoort HC: Dutch
Pancreatitis Study Group: Minimally invasive intervention for
infected necrosis in acute pancreatitis. Expert Rev Med Devices.
11:637–648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horvath K, Freeny P, Escallon J, Heagerty
P, Comstock B, Glickerman DJ, Bulger E, Sinanan M, Langdale L,
Kolokythas O, et al: Safety and efficacy of video-assisted
retroperitoneal debridement for infected pancreatic collections: A
multicenter, prospective, single-arm phase 2 study. Arch Surg.
145:817–825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neoptolemos JP, London NJ and Carr-Locke
DL: Assessment of main pancreatic duct integrity by endoscopic
retrograde pancreatography in patients with acute pancreatitis. Br
J Surg. 80:94–99. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lau ST, Simchuk EJ, Kozarek RA and
Traverso LW: A pancreatic ductal leak should be sought to direct
treatment in patients with acute pancreatitis. Am J Surg.
181:411–415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uomo G, Molino D, Visconti M, Ragozzino A,
Manes G and Rabitti PG: The incidence of main pancreatic duct
disruption in severe biliary pancreatitis. Am J Surg. 176:49–52.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trevino JM, Tamhane A and Varadarajulu S:
Successful stenting in ductal disruption favorably impacts
treatment outcomes in patients undergoing transmural drainage of
peripancreatic fluid collections. J Gastroenterol Hepatol.
25:526–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhasin DK and Rana SS: Combining
transpapillary pancreatic duct stenting with endoscopic transmural
drainage for pancreatic fluid collections: Two heads are better
than one! J Gastroenterol Hepatol. 25:433–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shrode CW, Macdonough P, Gaidhane M,
Northup PG, Sauer B, Ku J, Ellen K, Shami VM and Kahaleh M:
Multimodality endoscopic treatment of pancreatic duct disruption
with stenting and pseudocyst drainage: How efficacious is it? Dig
Liver Dis. 45:129–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hookey LC, Debroux S, Delhaye M,
Arvanitakis M, Le Moine O and Devière J: Endoscopic drainage of
pancreatic-fluid collections in 116 patients: A comparison of
etiologies, drainage techniques, and outcomes. Gastrointest Endosc.
63:635–643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harvey MH, Wedgwood KR, Austin JA and
Reber HA: Pancreatic duct pressure, duct permeability and acute
pancreatitis. Br J Surg. 76:859–862. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siqin D, Wang C, Zhou Z and Li Y: The key
event of acute pancreatitis: Pancreatic duct obstruction and bile
reflux, not a single one can be omitted. Med Hypotheses.
72:589–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujiwara H: Pressure measurement in
pancreatic duct and biliary duct system in dogs with acute
pancreatitis. Kobe J Med Sci. 37:47–55. 1991.PubMed/NCBI
|
|
21
|
Shi CX, Chen JW, Carati CJ, Schloithe AC,
Toouli J and Saccone GT: Effects of acute pancreatic duct
obstruction on pancreatic perfusion: Implication of acute
pancreatic duct decompression. Scand J Gastroenterol. 37:1328–1333.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arendt T: Bile-induced acute pancreatitis
in cats. Roles of bile, bacteria, and pancreatic duct pressure. Dig
Dis Sci. 38:39–44. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Varadarajulu S, Rana SS and Bhasin DK:
Endoscopic therapy for pancreatic duct leaks and disruptions.
Gastrointest Endosc Clin N Am. 23:863–892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He QB, Xu T, Wang J, Li YH, Wang L and Zou
XP: Risk factors for post-ERCP pancreatitis and hyperamylasemia: A
retrospective single-center study. J Dig Dis. 16:471–478. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi QQ, Ning XY, Zhan LL, Tang GD and Lv
XP: Placement of prophylactic pancreatic stents to prevent
post-endoscopic retrograde cholangiopancreatography pancreatitis in
high-risk patients: A meta-analysis. World J Gastroenterol.
20:7040–7048. 2014. View Article : Google Scholar : PubMed/NCBI
|