Introduction
MicroRNAs (miRNAs) are a family of endogenous
non-coding RNAs that account for >3% of all human genes
(1). miRNAs, were previously
considered as junk RNAs. However, previous studies indicated that
they are involved in the regulation of the stability and
translation of up to 60% of coding mRNAs in humans (2,3). Through
direct binding to the 3′-untranslated region (3′-UTR) of target
coding mRNAs, miRNAs repress the expression of their target genes
and affect a variety of essential biological processes (4–6). In
addition, miRNAs play significant roles in various pathological
processes including cardiovascular diseases, viral diseases, immune
diseases and cancer (7–11). Emerging studies showed that miRNAs
are abundant in the nervous system and are correlated with several
nervous system pathologies, including neurodevelopmental
abnormalities, neurodegenerative disorders and nerve injuries
(7,12,13).
Nerve injury is a common clinical issue that induces
nerve tissue damage and target organ dysfunction, and is generally
classified into two categories: Central nerve injury and peripheral
nerve injury, in accordance with the injury site. While central
nerve injuries regenerate poorly, the functional recovery of
peripheral nerve injuries are significantly better (14,15). A
major reason for the high regeneration abilities of injured
peripheral nerves is the presence of Schwann cells. Schwann cells
are unique types of glial cells in the peripheral nervous system,
and support neurons by forming myelin under physiological
conditions (16,17). After peripheral nerve injury, Schwann
cells proliferate and migrate to the injury site, engulf axon and
myelin debris, and form bands of Bungner to guide the directional
regrowth of axons (18). Therefore,
factors that promote Schwann cell proliferation and migration may
contribute to the repair and regeneration of injured peripheral
nerves.
Numerous miRNAs have been identified to be
differentially expressed in the proximal nerve segment after
peripheral nerve injury (19,20).
These differentially expressed miRNAs have been reported to
modulate various phenotypic processes of Schwann cells, including
apoptosis, proliferation, migration, differentiation and
myelination; processes which may affect peripheral nerve
regeneration (21–23). In a previous study, a total of 98
novel miRNAs have been discovered and functionally annotated in the
rat sciatic nerve segment after sciatic nerve transection (24). These novel miRNAs were perfectly
mapped to the rat genome, with their hairpin structures and low
free energy levels obtained (24).
Here, we focused on miR-sc6, a newly identified miRNA, and examined
its effects on Schwann cell proliferation and migration, and
determined the target genes of miR-sc6.
Materials and methods
Animal surgery
The present study was approved by the Administration
Committee of Experimental Animals in (Jiangsu, China; no.
20170302-016). Sprague Dawley (SD) rats were obtained from the
Experimental Animal Center (animal license nos. SCXK (Su) 2014-0001
and SYXK (Su) 2012-0031). A total of 30 adult male SD rats (weight,
180–220 g; age, 2 months) were randomly separated into 5 groups (at
0, 1, 4, 7 and 14 days after nerve injury) with 6 rats/group, and
used for sciatic nerve transection as previously described
(24). Briefly, after anesthesia, 10
mm of rat sciatic nerve was exposed, lifted, and resected at the
site proximal to the division of tibial and common peroneal nerves.
After the surgical incisions were closed, animals were housed in
temperature- and humidity-controlled environment, maintained under
a 12-h light/dark cycle, and were allowed free access to water and
food. Rats were sacrificed by decapitation at 0, 1, 4, 7 and 14
days after nerve transection, and 5 mm of proximal sciatic nerve
segments were collected for subsequent reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
experiments. A total of 60 neonatal male SD rats were used for
Schwann cell isolation as previously described (25). Briefly, after anesthesia, sciatic
nerve was exposed, and an 8 mm of nerve segment was collected for
subsequent cell isolation.
Schwann cell culture and
transfection
Primary Schwann cells were isolated from sciatic
nerve segments using trypsin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Isolated cells were purified by the treatment of
anti-Thy1.1 antibody (1:1,000; cat. no. M7898; Sigma-Aldrich; Merck
KGaA) and rabbit complement (Sigma-Aldrich; Merck KGaA), as
previously described (25). Purified
Schwann cells were grown in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher
Scientific Inc.), 1% penicillin and streptomycin (Invitrogen;
Thermo Fisher Scientific Inc.), 2 µM forskolin (Sigma-Aldrich;
Merck KGaA), and 10 ng/ml human heregulin-β1 (HRG; Sigma; Merck
KGaA). Schwann cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2, and were passaged for no
more than three times prior to use. Cultured Schwann cells had a
purity >98% confirmed via immunostaining with S100β (1:200; cat.
no. ab52642; Abcam, Cambridge, MA, USA), a marker of Schwann cells.
For cell transfection, Schwann cells were transfected with a
miR-sc6 mimic (sense, 5′-UGGGGGACAGGGCAAGGUGG-3′ and antisense,
5′-CCACCUUGCCCUGUCCCCCA-3′), mimic (sense,
5′-UUCUCCGAACGUGUCACGU-3′ and antisense,
5′-ACGUGACACGUUCGGAGAA-3′), miR-sc6 inhibitor (sequence,
5′-CCACCUUGCCCUGUCCCCCA-3′) or inhibitor control (sequence,
5′-ACGUGACACGUUCGGAGAA-3′) (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) using Lipofectamine® RNAiMAX transfection reagent
(Invitrogen; Thermo Fisher Scientific Inc.), according to the
manufacturer's protocols. Briefly, the miR-sc6 mimic, mimic
control, miR-sc6 inhibitor or inhibitor control was diluted in
Opti-MEM I Medium (Gibco; Thermo Fisher Scientific Inc.).
Lipofectamine® RNAiMAX was then added into the
corresponding diluted complexes and incubated for 10–20 min at room
temperature. The transfection mixture containing the reagent and
miRNA diluted in Opti-MEM was then added onto the Schwann cells at
30–50% confluence, which were then incubated at 37°C under 5%
CO2 for 24 h. The experiment was repeated three
times.
5-ethynyl-2′-deoxyuridine (EdU)
proliferation assay
Schwann cells were suspended and seeded at a density
of 2×105 cells/ml onto 96-well plates precoated with
0.01% poly-L-lysine (Sigma; Merck KGaA), and transfected with
miR-sc6 mimic, mimic control, miR-sc6 inhibitor or inhibitor
control for 36 h. EdU (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) was then added into culture media and cells were cultured
for another 24 h. The cells were then fixed with 4%
paraformaldehyde (PFA; Xilong Scientific, Guangzhou, China) for 30
min at room temperature, stained with Hoechst 33342 (Beyotime
Institute of Biotechnology, Haimen, China) for 10 min at room
temperature, and observed with a DMR fluorescence microscope
(magnification, ×200; Leica Microsystems GmbH, Wetzlar, Germany).
The proliferation rate was calculated by dividing the number of
EdU-positive cells to the number of total cells. The experiment was
repeated three times.
Transwell assay
Schwann cells were transfected with miR-sc6 mimic,
mimic control, miR-sc6 inhibitor or inhibitor control. Following 36
h of transfection, the cells were suspended in DMEM, and seeded at
a density of 3×105 cells/ml onto the top chamber of a
6.5 mm transwell insert with 8 µm pores (Costar; Corning
Incorporated; Corning, NY, USA). A total of 500 µl DMEM
supplemented with 10% FBS were added to the bottom chamber of
transwell pre-coated with 10 µg/ml fibronectin. Schwann cells left
on the top chamber were wiped off by a cotton swab after a 24 h
incubation at 37°C with 5% CO2. The Schwann cells that
migrated to the lower chamber were fixed with 4% paraformaldehyde
for 30 min at room temperature, stained with 0.1% crystal violet
for 10 min at room temperature and then observed with a DMR
inverted microscope (magnification, ×200; Leica Microsystems GmbH).
Crystal violet staining the Schwann cells was dissolved by acetic
acid. The migratory ability of Schwann cells was calculated by the
optical density value of the dissolved crystal violet dye. The
experiment was repeated three times.
Renilla luciferase assay
The 3′-UTR of netrin-1 (Ntn1), erbB2 receptor
tyrosine-protein kinase 4 (ErbB4) or protein kinase C α (Prkcα) was
amplified and subcloned into the downstream region of the
luciferase gene stop codon in the luciferase reporter vector to
generate the p-Luc-UTR reporter plasmid (Promega Corporation,
Madison, WI, USA). A total of 120 ng constructed p-Luc-UTR reporter
plasmid was combined with 20 pmol miR-sc6 mimic and 20 ng Renilla
luciferase vector pRL-CMV (Promega Corporation) to generate a
mixture. On the day of the assay 293 cells were seeded onto 24-well
plates and then transfected with the mixture using the
Lipofectamine 2000 transfection system (Invitrogen; Thermo Fisher
Scientific, Inc.), and subsequently cultured for an additional 24
h. The firefly and Renilla luciferase activities were measured
using the dual-luciferase reporter assay system (Promega
Corporation).
RT-qPCR
RNA samples were isolated from proximal sciatic
nerve segments using TRIzol (Life technologies; Thermo Fisher
Scientific, Inc). Total RNA was reverse transcribed into cDNA using
Omniscript® Reverse Transcription Kit (Qiagen GmbH,
Hilden, Germany). RT-qPCR experiments were conducted using an
Applied Biosystems StepOne real-time PCR System and QuantiNova™
SYBR® Green PCR Kit (Qiagen GmbH). The following primer
pairs were used for qPCR: ErbB4 forward, 5′-GCATGTGATGGAATCGGCAC-3′
and reverse, 5′-TCCCCATGAATGCCAGTGAC-3′. The thermocycling
conditions were as follows: Initial denaturation for 5 min at 95°C;
40 cycles of denaturation for 30 sec at 95°C, annealing for 45 sec
at 60°C, and extension for 30 sec at 72°C; and final extension for
5 min at 72°C. The expression levels of ErbB4 were determined using
the 2−ΔΔCq method (26),
and were normalized to the internal reference gene GAPDH. The
relative expression levels of ErbB4 at 1, 4, 7 and 14 days after
sciatic nerve transection were compared with its expression levels
at 0 day.
Bioinformatics analysis
The 3′-UTRs of all genes in the rat genome were
downloaded, and sequence alignment was conducted using Basic Local
Alignment Search Tool (BLAST) (24).
The candidate target genes of miR-sc6 were predicted using
TargetScan. Functional genes associated with cell proliferation and
migration were examined using Database for Annotation,
Visualization, and Integrated Discovery (DAVID) (27) bioinformatic resources. The minimum
free energy of hybridization between miR-sc6 and the candidate
target genes was determined using the RNAhybrid software
(bibiserv.cebitec.uni-bielefeld.de/rnahybrid)
(28).
Statistical analysis
Statistical analyses were conducted using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). Three
independent experiments were performed, and the results were
presented as mean ± standard error of the mean. Student's t-test or
one-way analysis of variance followed by Dunnett's multiple
comparisons test was used for statistical comparison. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-sc6 promotes Schwann cell
proliferation
Schwann cells are the main cell types in the sciatic
nerve stumps and play important roles during peripheral nerve
regeneration (29), therefore, the
effect of miR-sc6 expression on Schwann cell proliferation was
investigated. Transfection using a miR-sc6 mimic or miR-sc6
inhibitor significantly increased or decreased the expression of
miR-sc6, respectively (Fig. 1A). EdU
assay was used to determine the effect of miR-sc6
upregulation/downregulation on the proliferative ability of Schwann
cells. Schwann cells transfected with miR-sc6 mimic after 36 h
exhibited a higher ratio of the number of EdU-positive cells to the
number of total cells, compared with the negative control group
(Fig. 1B). The results revealed that
transfection using miR-sc6 mimics led to a significantly elevated
proliferation rate (Fig. 1B).
Schwann cells were also transfected with a miR-sc6 inhibitor or its
corresponding negative control. Transfection with the miR-sc6
inhibitor led to a significantly reduced proliferation rate
compared with control (Fig. 1C).
miR-sc6 promotes Schwann cell
migration
To examine the effect of miR-sc6 on Schwann cell
migration, Transwell migration assay was performed. Schwann cells
transfected with miR-sc6 mimic showed an increased number of
migrated cells compared with those transfected with mimic control,
suggesting that an increased expression of miR-sc6 promoted the
migration of Schwann cells (Fig.
2A). However, transfection with the miR-sc6 inhibitor
significantly decreased the migration of Schwann cells (Fig. 2B).
Identification of potential candidate
target genes of miR-sc6
Considering that miRNAs perform their biological
functions through the direct regulation of their target genes
(30), the candidate target genes of
miR-sc6 were predicted. Bioinformatic analysis suggested that
ErbB4, Ntn1 and Prkcα were potential target genes of miR-sc6. The
sequences of ErbB4, Ntn1 and Prkcα in the seed region were also
completely complementary to that of miR-sc6 (Fig. 3A). All three candidate target genes
displayed negative free energy as determined by using the RNAhybrid
software (Fig. 3A). The results
revealed that ErbB4 has a free energy between −20 and −30 kcal/mol,
indicating that ErbB4 mRNA may bind with miR-sc6.
The expression association between miR-sc6 and its
potential target genes ErbB4, Ntn1 and Prkcα were determined using
previously obtained Solexa sequencing and microarray data (24,31). The
heatmap of the temporal expression patterns of miR-sc6 revealed
upregulation, while, the expression of ErbB4, Ntn1 and Prkcα were
downregulated after sciatic nerve transection. The results obtained
on day 7 revealed that the expression pattern of miR-sc6 may be
negatively associated with that of ErbB4, Ntn1 and Prkcα (Fig. 3B).
ErbB4 is a candidate target gene of
miR-sc6
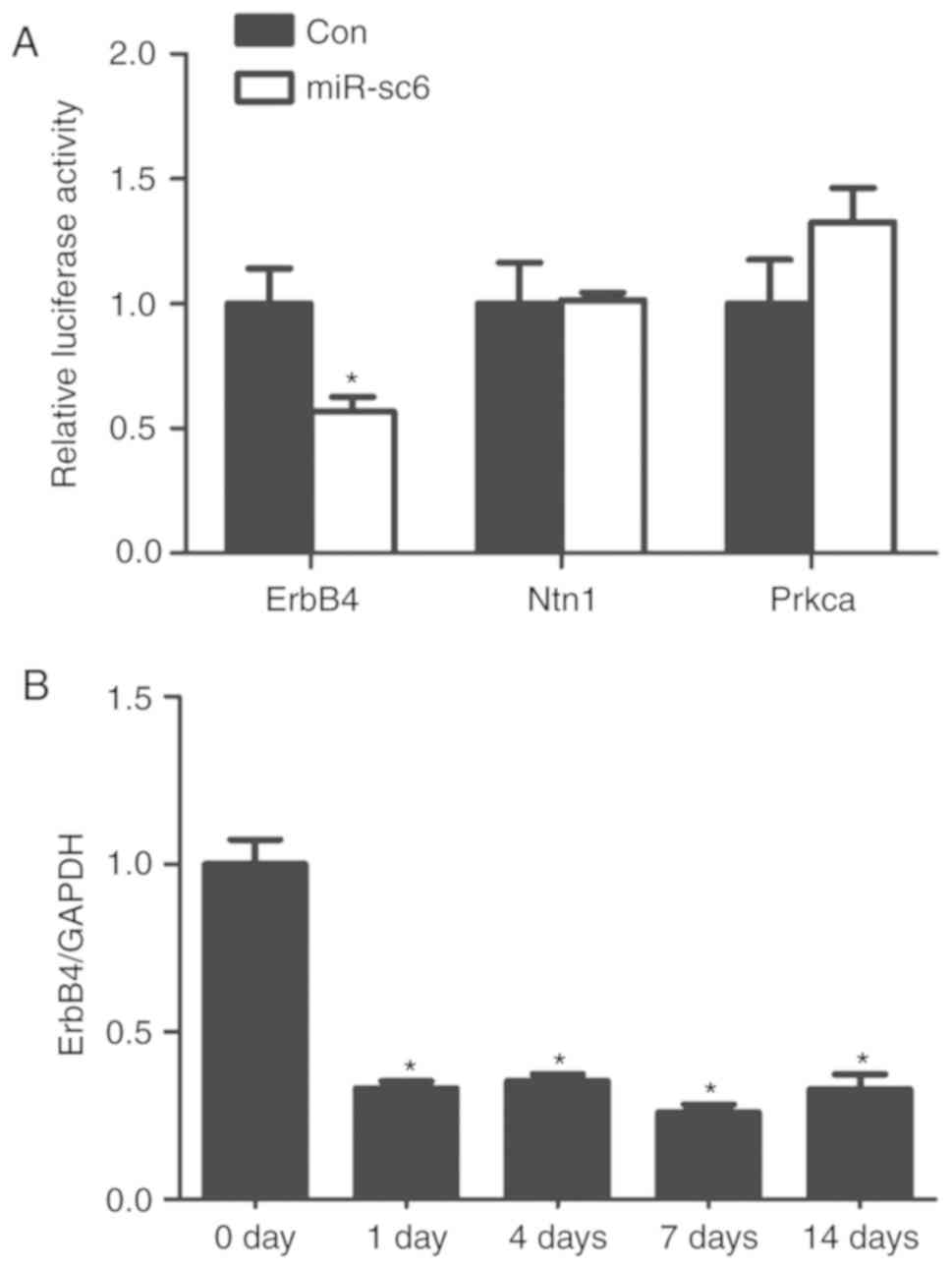
Renilla luciferase assay was performed to
investigate further whether miR-sc6 directly targets the 3′-UTR of
ErbB4, Ntn1 and Prkcα. Transfection using miR-sc6 mimic and
p-Luc-UTR reporter plasmid containing the 3′-UTR of ErbB4 showed a
significantly reduced relative luciferase activity compared with
cells transfected with its corresponding non-targeting negative
control (Fig. 4A). In contrast, 293
cells transfected with miR-sc6 mimic and p-Luc-UTR reporter plasmid
containing the 3′-UTR of Ntn1 or Prkcα did not show an altered
relative luciferase activity (Fig.
4A).
The expression levels of ErbB4 in the proximal nerve
segments at 0, 1, 4, 7 and 14 days after sciatic nerve transection
were measured to investigate further the association between
miR-sc6 and its candidate target gene ErbB4. Previous results from
Solexa sequencing showed that miR-sc6 was upregulated after sciatic
nerve transection, reaching a peak value at 7 days after nerve
injury (24). The temporal
expression patterns of ErbB4 at different timepoints after sciatic
nerve transection displayed opposite trends. The expression levels
of ErbB4 were decreased at 1, 4, 7 and 14 days after nerve injury,
reaching the lowest value at 7 days (Fig. 4B). These results collectively suggest
that ErbB4 might be the direct target of miR-sc6.
Discussion
The peripheral nerve system contains an internal
regenerative capacity after traumatic injury. However, the
functional recovery of peripheral nerve injury is generally not
desirable (32). Many strategies,
including the applications of neurotrophic factors (nerve growth
factor, brain-derived neurotrophic factor and glial-derived
neurotrophic factor), Schwann cells and stem cells, have been used
to facilitate peripheral nerve repair and regeneration (33–36).
Almost immediately after nerve injury, Schwann cells in the distal
region begin to dedifferentiate, engulf axon and myelin debris, and
clear a regenerative path. At the same time, Schwann cells in the
proximal region proliferate, migrate and form bands of Bungner to
guide axonal regrowth (37,38). Therefore, phenotypic modulation of
Schwann cells largely contributes to nerve regeneration. Recently,
it has been demonstrated that miRNAs are able to regulate Schwann
cell phenotype and may be of potential therapeutic interest for the
treatment of peripheral nerve injury in the future (21,39,40).
The advance of high-throughput analysis, especially
of gene sequencing analysis, is of benefit to the identification of
differentially expressed genes following peripheral nerve injury
(41). Previously, in our
laboratory, sequencing was performed to discover regular
alterations in the expression levels of miRNAs in proximal nerve
segments after rat sciatic nerve transection (19). In addition, by using Solexa
sequencing, a group of novel miRNAs were identified after rat
sciatic nerve transection and, were named as miR-scs since they
were discovered in rat sciatic nerve segments (24). The biological functions of certain of
these novel miRNAs were further investigated, and the results
showed that these miRNAs could affect the proliferation and
migration of Schwann cells. For example, miR-sc3 was found to
promote Schwann cell proliferation and migration by targeting
astrotactin 1 (Astn1), while miR-sc4 and miR-sc8 played an
inhibitory role on Schwann cell proliferation and migration by
targeting cyclin-dependent kinase 5 activator 1 (Cdk5r1) and the
epidermal growth factor receptor (Egfr), respectively (40,42,43).
The present study focused on miR-sc6, a miRNA
verified as a novel miRNA in rat whose expression levels were
upregulated after rat sciatic nerve transection. By using EdU
proliferation assay and Transwell migration assay, it was showed
that increased expression of miR-sc6 promoted Schwann cell
proliferation and migration, while decreased expression of miR-sc6
suppressed Schwann cell proliferation and migration. These results
suggested that peripheral nerve injury-induced upregulation of
miR-sc6 may support the proliferation and migration of Schwann
cells, and thus contribute to subsequent axon regrowth and nerve
regeneration. Bioinformatic analysis and Renilla luciferase assay
identified ErbB4 as the target gene of miR-sc6. The temporal
expression patterns of ErbB4 in proximal sciatic nerve segments at
0, 1, 4, 7 and 14 days after sciatic nerve transection were also
negatively associated with that of miR-sc6, implying that miR-sc6
may perform its biological functions by negatively regulating ErbB4
expression.
ErbB4 is a transmembrane receptor-tyrosine kinase
that belongs to the ErbB receptor family. The activation of the
neuregulin-1/ErbB signaling pathway can promote the proliferation
of neoplastic Schwann cells and the mitogenesis in malignant
peripheral nerve sheath tumors (44,45). A
previous study examined changes in ErbBs mRNA expression during
peripheral nerve regeneration and found that ErbB4 was
downregulated at 7, 14 and 28 days post-injury (46). This was consistent with our
observations. According to a previous study, other members of the
ErbB family exhibited different expression trends [for example,
ErbB1 (Egfr) was downregulated, ErbB3 was upregulated, and ErbB2
was not differentially expressed in the rat median nerve] (46). ErbB2 and ErbB3 were reported to be
essential for the migration and myelination of Schwann cells in
zebrafish (47). However, another
study showed that ErbB2 was dispensable for the maintenance of
established myelinated peripheral nerves in mice (48). Despite the complex and paradoxical
roles of ErbB2, the direct biological effect of ErbB4 on Schwann
cells remains unclear. Transgenic mice expressing a
dominant-negative ErbB4 receptor in non-myelinating Schwann cells
expressing a dominant negative ErbB4 exhibited elevated cell
apoptosis ad cell proliferation, which presented with a progressive
peripheral neuropathy (49). The
results of the present study revealed that ErbB4 (a target gene of
miR-sc6) may modulate Schwann cell physiology. Further
investigations will be performed to determine whether ErbB4
regulated Schwann cell proliferation and migration.
Taken together, the present study demonstrated the
possible biological functions of miR-sc6, a novel miRNA that was
dysregulated following peripheral nerve injury. The data may deepen
our understanding of the cellular and molecular mechanisms involved
in peripheral nerve injury and regeneration.
Acknowledgements
Not applicable.
Funding
The present study was supported by Postgraduate
Research & Practice Innovation Program of Jiangsu (grant no.
KYCX17-1910) and a Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
ZC and TQ conceived and designed the experiments.
CC, QL, HH, XW, PW and TQ performed the experiments. CC and TQ
analyzed the data. QL and TQ contributed reagents, materials and
analysis tools. TQ wrote the manuscript.
Ethical approval and consent to
participate
The present study was approved by the Administration
Committee of Experimental Animals (Jiangsu, China; no.
20170302-016). All applicable international, national and
institutional guidelines for the care and use of animals were
followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
O'Driscoll L: The emerging world of
microRNAs. Anticancer Res. 26:4271–4278. 2006.PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Didiano D and Hobert O: Molecular
architecture of a miRNA- regulated 3′UTR. RNA. 14:1297–1317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams AE: Functional aspects of animal
microRNAs. Cell Mol Life Sci. 65:545–562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shivdasani RA: MicroRNAs: Regulators of
gene expression and cell differentiation. Blood. 108:3646–3653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erson AE and Petty EM: MicroRNAs in
development and disease. Clin Genet. 74:296–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C: MicroRNAs: Role in cardiovascular
biology and disease. Clin Sci (Lond). 114:699–706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erson AE and Petty EM: miRNAs and cancer:
New research developments and potential clinical applications.
Cancer Biol Ther. 8:2317–2322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lynam-Lennon N, Maher SG and Reynolds JV:
The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos
Soc. 84:55–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pauley KM and Chan EK: MicroRNAs and their
emerging roles in immunology. Ann N Y Acad Sci. 1143:226–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu B, Zhou S, Yi S and Gu X: The
regulatory roles of non-coding RNAs in nerve injury and
regeneration. Prog Neurobiol. 134:122–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu D and Murashov AK: Molecular mechanisms
of peripheral nerve regeneration: Emerging roles of microRNAs.
Front Physiol. 4:552013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu X, Ding F, Yang Y and Liu J:
Construction of tissue engineered nerve grafts and their
application in peripheral nerve regeneration. Prog Neurobiol.
93:204–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bosse F: Extrinsic cellular and molecular
mediators of peripheral axonal regeneration. Cell Tissue Res.
349:5–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhatheja K and Field J: Schwann cells:
Origins and role in axonal maintenance and regeneration. Int J
Biochem Cell Biol. 38:1995–1999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bunge RP: Expanding roles for the Schwann
cell: Ensheathment, myelination, trophism and regeneration. Curr
Opin Neurobiol. 3:805–809. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campbell WW: Evaluation and management of
peripheral nerve injury. Clin Neurophysiol. 119:1951–1965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu B, Zhou S, Wang Y, Ding G, Ding F and
Gu X: Profile of microRNAs following rat sciatic nerve injury by
deep sequencing: Implication for mechanisms of nerve regeneration.
PLoS One. 6:e246122011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Phay M, Kim HH and Yoo S: Dynamic change
and target prediction of axon-specific microRNAs in regenerating
sciatic nerve. PLoS One. 10:e01374612015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi S, Wang QH, Zhao LL, Qin J, Wang YX, Yu
B and Zhou SL: miR-30c promotes Schwann cell remyelination
following peripheral nerve injury. Neural Regen Res. 12:1708–1715.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yi S, Yuan Y, Chen Q, Wang X, Gong L, Liu
J, Gu X and Li S: Regulation of Schwann cell proliferation and
migration by miR-1 targeting brain-derived neurotrophic factor
after peripheral nerve injury. Sci Rep. 6:291212016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li S, Zhang R, Yuan Y, Yi S, Chen Q, Gong
L, Liu J, Ding F, Cao Z and Gu X: MiR-340 regulates fibrinolysis
and axon regrowth following sciatic nerve injury. Mol Neurobiol.
54:4379–4389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Yu B, Wang Y, Yao D, Zhang Z and Gu
X: Identification and functional annotation of novel microRNAs in
the proximal sciatic nerve after sciatic nerve transection. Sci
China Life Sci. 54:806–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu B, Qian T, Wang Y, Zhou S, Ding G, Ding
F and Gu X: miR-182 inhibits Schwann cell proliferation and
migration by targeting FGF9 and NTM, respectively at an early stage
following sciatic nerve injury. Nucleic Acids Res. 40:10356–10365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID bioinformatics resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35:(Web Server Issue). W169–W175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palomo Irigoyen M, Tamayo Caro M, Pérez
Andrés E, Barreira Manrique A, Varela Rey M and Woodhoo A:
Isolation and purification of primary rodent schwann cells. Methods
Mol Biol. 1791:81–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Liu Q, Wang Y, Gu Y, Liu D, Wang C,
Ding G, Chen J, Liu J and Gu X: Differential gene expression
profiling and biological process analysis in proximal nerve
segments after sciatic nerve transection. PLoS One. 8:e570002013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gordon T: Nerve regeneration:
Understanding biology and its influence on return of function after
nerve transfers. Hand Clin. 32:103–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gordon T, Sulaiman O and Boyd JG:
Experimental strategies to promote functional recovery after
peripheral nerve injuries. J Peripher Nerv Syst. 8:236–250. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boyd JG and Gordon T: Glial cell
line-derived neurotrophic factor and brain-derived neurotrophic
factor sustain the axonal regeneration of chronically axotomized
motoneurons in vivo. Exp Neurol. 183:610–619. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oh SH, Kang JG, Kim TH, Namgung U, Song
KS, Jeon BH and Lee JH: Enhanced peripheral nerve regeneration
through asymmetrically porous nerve guide conduit with nerve growth
factor gradient. J Biomed Mater Res A. 106:52–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gonzalez-Perez F, Hernandez J, Heimann C,
Phillips JB, Udina E and Navarro X: Schwann cells and mesenchymal
stem cells in laminin- or fibronectin-aligned matrices and
regeneration across a critical size defect of 15 mm in the rat
sciatic nerve. J Neurosurg Spine. 28:109–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yi S, Tang X, Yu J, Liu J, Ding F and Gu
X: Microarray and qPCR analyses of wallerian degeneration in rat
sciatic nerves. Front Cell Neurosci. 11:222017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gaudet AD, Popovich PG and Ramer MS:
Wallerian degeneration: Gaining perspective on inflammatory events
after peripheral nerve injury. J Neuroinflammation. 8:1102011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang P, He J, Wang S, Wang X, Liu Q, Peng
W and Qian T: miR-3075 inhibited the migration of Schwann cells by
targeting Cntn2. Neurochem Res. 43:1879–1886. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian T, Wang X, Wang Y, Wang P, Liu Q, Liu
J and Yi S: Novel miR-sc4 regulates the proliferation and migration
of Schwann cells by targeting Cdk5r1. Mol Cell Biochem.
447:209–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yi S, Zhang H, Gong L, Wu J, Zha G, Zhou
S, Gu X and Yu B: Deep sequencing and bioinformatic analysis of
lesioned sciatic nerves after crush injury. PLoS One.
10:e01434912015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gu Y, Chen C, Yi S, Wang S, Gong L, Liu J,
Gu X, Zhao Q and Li S: miR-sc8 inhibits Schwann cell proliferation
and migration by targeting Egfr. PLoS One. 10:e01451852015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yi S, Wang S, Zhao Q, Yao C, Gu Y, Liu J,
Gu X and Li S: miR-sc3, a novel MicroRNA, promotes Schwann cell
proliferation and migration by targeting Astn1. Cell Transplant.
25:973–982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stonecypher MS, Byer SJ, Grizzle WE and
Carroll SL: Activation of the neuregulin-1/ErbB signaling pathway
promotes the proliferation of neoplastic Schwann cells in human
malignant peripheral nerve sheath tumors. Oncogene. 24:5589–5605.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stonecypher MS, Chaudhury AR, Byer SJ and
Carroll SL: Neuregulin growth factors and their ErbB receptors form
a potential signaling network for schwannoma tumorigenesis. J
Neuropathol Exp Neurol. 65:162–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Audisio C, Nicolino S, Scevola A, Tos P,
Geuna S, Battiston B and Perroteau I: ErbB receptors modulation in
different types of peripheral nerve regeneration. Neuroreport.
19:1605–1609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lyons DA, Pogoda HM, Voas MG, Woods IG,
Diamond B, Nix R, Arana N, Jacobs J and Talbot WS: erbb3 and erbb2
are essential for schwann cell migration and myelination in
zebrafish. Curr Biol. 15:513–524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Atanasoski S, Scherer SS, Sirkowski E,
Leone D, Garratt AN, Birchmeier C and Suter U: ErbB2 signaling in
Schwann cells is mostly dispensable for maintenance of myelinated
peripheral nerves and proliferation of adult Schwann cells after
injury. J Neurosci. 26:2124–2131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen S, Rio C, Ji RR, Dikkes P, Coggeshall
RE, Woolf CJ and Corfas G: Disruption of ErbB receptor signaling in
adult non-myelinating Schwann cells causes progressive sensory
loss. Nat Neurosci. 6:1186–1193. 2003. View
Article : Google Scholar : PubMed/NCBI
|