Introduction
Sepsis is extremely common in intensive care units
(ICUs) and emergency departments, with rising morbidity and
mortality annually. Since sepsis is now recognized as one of the
most challenging problems in critical care medicine, its rapid and
early diagnosis and treatment have become particularly important
(1,2). Sepsis is mainly characterized by
microcirculatory dysfunction, and its pathophysiology can be
divided into two parts: Vascular endothelial cell dysfunction, and
clot formation caused by a hypercoagulative state. Vascular
endothelial cells play key roles in sepsis and sepsis-induced
multiple organ dysfunction syndrome (MODS) because the vascular
endothelial system, especially microvascular endothelium is mainly
attacked during sepsis. The progression of sepsis can be indirectly
reflected by the extent of endothelial cell injury and the degree
of microthrombosis. Thus for the risk stratification and prognosis
prediction, the investigation of markers for vascular endothelial
cell damage is highly required (3).
Endothelial cell-specific molecule1 (endocan), the
von Willebrand factor (vWF), and a disintegrin-like and
metalloprotease with thrombospondin type 1 motif (ADAMTS-13) are
three vascular endothelial cell markers to indicate endothelial
damage and dysfunction. In this study, we measured the circulating
levels of endocan, vWF, ADAMTS-13, and vWF/ADAMTS-l3 in septic
patients and explored the relationships of these indicators with
sepsis severity and prognosis. Furthermore, the application of
these markers during sepsis was elucidated.
Subjects and methods
Patients
The clinical data of 301 patients with systemic
inflammatory reaction syndrome (SIRS) or sepsis, according to
ACCP/SCCM criteria (4), who were
treated in the Emergency Department in Beijing Chao-Yang Hospital
(Beijing, China) from October 2014 to October 2015, were analyzed.
According to the diagnostic criteria, these patients were divided
into SIRS, sepsis, severe sepsis, and septic shock groups.
Additionally, 40 healthy individuals in our center during the same
period, were selected as the healthy control group.
Exclusion criteria were: i) Patients younger than 18
years, ii) patients or family members who refused to participate in
the study, iii) patients with psychiatric disorders, iv) patients
with tumors, v) patients who had undergone organ transplantation or
who exhibited long-term use of immunosuppressive agents, vi)
patients with allergic reactions, and vii) patients with
dysfunction of two or more organs before the onset of the
disease.
This study was approved by the Ethics Comittee of
Institutional Review Board of Beijing Chao-Yang Hospital, Capital
Medical University (Beijing, China). Patients who participated in
this research had complete clinical data. The signed informed
consents were obtained from the patients or the guardians.
Methods
Blood samples were collected from the elbow vein at
baseline and 5 days later. The samples were placed in non-additive
tubes, and centrifuged at 4,000 × g for 5 min at 4°C. The serum was
collected in 1.5 ml centrifugal tubes, and then stored at −80°C for
further analysis. Endocan, vWF and ADAMTS-13 (SEC463Hu, CEA833Hu
and SEA950Hu; Cloud-Clone Corp., Katy, TX, USA) concentrations were
measured by the double-antibody sandwich enzyme-linked
immunosorbent assay (DAS-ELISA). Procalcitonin (PCT) was measured
by the MINI VIDAS® (Block Scientific Inc., New York,
USA) fully automated analyzer. The patients' past histories, vital
signs, routine test results, and radiographic examination findings
were recorded. Baseline data were used for scoring on the following
assessments: Acute Physiology and Chronic Health Evaluation (APACHE
II) (5), Mortality in Emergency
Department Sepsis (MEDS) (6), and
Sequential Organ Failure Assessment (SOFA) (7). The outcome on the 28 th day after
enrollment was used as the endpoint. During the follow-up,
non-survivors were defined as patients who died of various reasons
and survivors were defined as those who remained alive.
Statistical analysis
All statistical analysis was performed using SPSS
19.0 software (SPSS, Inc., Chicago, IL, USA). Normally distributed
data were expressed as mean ± standard deviations, and non-normally
distributed data were expressed as medians (25–75% interquartile
ranges). Comparisons of measurement data between two or more groups
were performed using one-way analysis of variance, whereas paired
comparisons were performed using Least Significant Difference-t
test. For non-normally distributed data, comparisons of the medians
between two groups were performed using non-parametric tests,
Kruskal-Wallis method. The test level α′ (α0.05 =
0.05/number of paired comparisons; α0.01 = 0.01/number
of paired comparisons) was adjusted according to the number of
paired comparisons; a P-value of <α′ was regarded as
statistically significant. The count data were compared using the
Chi-square test. The independent predictive factors of the
prognostic indicators were determined by binary logical regression
analysis. The diagnostic and prognostic values of each indicator
were evaluated using the receiver operating characteristic (ROC)
curves. The area under the curve (AUC) was compared by:
Z=(A1-A2)/SE12+SE22,
test criterion: Z0.05=1.96,
Z0.01=2.58, Z>Z0.05, then P<0.05.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General characteristics of
patients
Patients in all groups were comparable in terms of
age, sex, previous diseases, and infection sites. The 28-day
mortality, PCT level, MEDS score, APACHE II score, and SOFA score
increased progressively in patients with sepsis (lowest), severe
sepsis, or septic shock (highest) with significant difference
(P<0.001; Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | Control | SIRS | Sepsis | Severe sepsis | Septic shock | P-value |
|---|
| Number | 40 | 40 | 77 | 132 | 52 |
|
| Age, years | 66.5 (26 to
82) | 65 (14
to 85) | 65 (20 to 93) | 69 (26
to 90) | 67 (23
to 94) | 0.602 |
| Male n (%) | 55.0 | 63.6 | 55.8 | 61.4 | 65.4 | 0.758 |
| Infection site
(n) |
|
Lung |
|
| 54 (70.1%) | 88 (66.7%) | 36 (69.2%) | 0.860 |
|
Abdominal cavity |
|
| 3 (3.9%) | 6 (4.5%) | 4 (7.7%) | 0.650 |
| Urinary
system |
|
| 1 (1.3%) | 7 (5.3%) | 2 (3.8%) | 0.430 |
|
Gastrointestinal tract |
|
| 7 (9.1%) | 7 (5.3%) | 2 (3.8%) | 0.486 |
| Skin
and soft tissue |
|
| 2 (2.6%) | 5 (3.8%) | 3 (5.8%) | 0.635 |
| Central
system |
|
| 3 (3.9%) | 5 (3.8%) | 1 (1.9%) | 0.911 |
|
Hepatobiliary system |
|
| 7 (9.1%) | 14 (10.6%) | 4 (7.7%) | 0.821 |
| Previous disease
history n (%) |
|
COPD |
| 12 (30.0%) | 27 (35.1%) | 51 (38.6%) | 16 (30.8%) | 0.658 |
|
Cardiovascular diseases |
| 13 (32.5%) | 29 (37.7%) | 53 (40.2%) | 17 (32.7%) | 0.659 |
|
Cerebrovascular diseases |
| 9
(22.5%) | 19 (24.7%) | 39 (29.6%) | 20 (38.4%) | 0.283 |
|
Diabetes |
| 11 (27.5%) | 27 (35.1%) | 49 (37.1%) | 21 (40.4%) | 0.617 |
|
Others |
| 4
(10.0%) | 12 (15.6%) | 25 (18.9%) | 10 (19.2%) | 0.564 |
| 28-day mortality n
(%) |
| 1 (2.5%) | 16 (20.8%) | 43 (32.6%) | 36 (69.2%) | <0.001 |
| PCT (ng/l) |
| 0.05
(0.05–0.43) | 2.59
(0.41–5.09) | 3.97
(1.04–8.06) | 9.27
(6.84–13.30) | <0.001 |
| MEDS score |
| 8.0
(5.5–10.5) | 9.0
(7.0–11.0) | 11.0
(8.0–14.0) | 14.5
(12.0–16.0) | <0.001 |
| APACHE II
score |
| 9.1±3.6 | 12.0±5.2 | 16.2±6.6 | 22.9±6.7 | <0.001 |
| SOFA score |
| 2.0 (1.0–2.5) | 3.0 (2.0–6.0) | 7.5 (5.0–9.0) | 11.0
(10.0–14.0) | <0.001 |
Levels of all biomarkers in each
group
The levels of endocan and vWF, as well as
vWF/ADAMTS-13 ratio progressively increased in the control
(lowest), SIRS, sepsis, severe sepsis, and septic shock (highest)
groups. However, the ADAMTS-13 level gradually decreased. The
differences among these groups were statistically significant
(P<0.05; Table II).
 | Table II.Levels of all biomarkers in each
group. |
Table II.
Levels of all biomarkers in each
group.
| Items | Control | SIRS | Sepsis | Severe sepsis | Septic shock | P-value |
|---|
| Number | 40 | 40 | 77 | 132 | 52 |
|
| Endocan |
| (ng/ml) | 32.53±9.13 | 47.31±6.11 | 52.86±5.17 |
54.99±5.28a | 58.25±4.08 | <0.001 |
| vWF (ng/l) | 1729.49±565.60 | 2736.22±436.98 | 3199.17±425.61 | 3909.27±334.02 | 4161.97±275.65 | <0.001 |
| ADAMTS-13 |
| (pg/ml) | 328.06±49.22 | 268.33±24.64 | 238.38±25.96 | 215.44±21.89 | 199.10±14.66 | <0.001 |
| vWF/ADAMTS1 | 5.43±2.06 | 10.32±2.14 | 13.59±2.40 | 18.34±2.54 | 21.01±2.01 | <0.001 |
Levels of all biomarkers in survivors
and non-survivors
The levels of endocan and vWF, as well as
vWF/ADAMTS-13 ratio were significantly higher in non-survivors than
survivors on days 1 and 5 (P<0.01). However, ADAMTS-13 levels
significantly decreased in non-survivors (P<0.01). Furthermore,
levels of endocan and vWF, as well asvWF/ADAMTS-13 ratio were
significantly lower on day 5 than day 1 in survivors (all
P<0.01), whereas ADAMTS-13 levels significantly increased
(P<0.01). In addition, levels of endocan and vWF, as well as
vWF/ADAMTS-13 ratio significantly increased on day 5 in
non-survivors (P<0.05), whereas ADAMTS-13 significantly
decreased over the same period (P<0.01; Table III).
 | Table III.Comparisons of biomarkers between
survivors and non-survivors on days 1 and 5 after admission. |
Table III.
Comparisons of biomarkers between
survivors and non-survivors on days 1 and 5 after admission.
| Variables | Survivors | Non-survivors | P-value |
|---|
| Number | 205 | 96 |
|
| Endocan
(ng/ml) |
| Day
1 | 53.14±6.55 | 56.22±3.71 | <0.01 |
| Day
5 | 46.29±6.84 | 57.73±8.16 | <0.01 |
|
P-value | <0.01 | 0.049 |
|
|
Difference (%) | −12.9 | +2.7 |
|
| vWF (ng/l) |
| Day
1 | 3476.83±586.27 | 3965.26±468.88 | <0.01 |
| Day
5 | 3296.10±557.98 | 4052.11±482.55 | <0.01 |
|
P-value | <0.01 | 0.002 |
|
|
Difference (%) | −5.2 | +2.2 |
|
| ADAMTS-13
(pg/ml) |
| Day
1 | 233.05±31.03 | 206.86±18.64 | <0.01 |
| Day
5 | 247.32±33.91 | 199.99±21.45 | <0.01 |
|
P-value | <0.01 | <0.01 |
|
|
Difference (%) | +6.1 | −3.3 |
|
| vWF/ADAMTS-13 |
| Day
1 | 15.35±3.90 | 19.39±3.22 | <0.01 |
| Day
5 | 13.73±3.60 | 20.61±3.93 | <0.01 |
|
P-value | <0.01 | <0.01 |
|
|
Difference (%) | −10.6 | +6.3 |
|
The disease condition was more critical in
non-survivors than survivors. In the survivors, the levels of
endocan and vWF, as well as vWF/ADAMTS-13 ratio decreased after 4
days of treatment, while the ADAMTS-13 levels increased, suggesting
an improvement in disease condition. The changes of biomarker
levels were reversed in the non-survivors.
Independent predictors of 28-day
mortality in septic patients
On day 1, ADAMTS-13 levels, vWF/ADAMTS-13 ratio, and
MEDS score were independent predictors of 28-day mortality for
sepsis.
Logistic regression analysis was performed using the
levels of endocan, vWF, ADAMTS-13, and vWF/ADAMTS-13 ratio, as well
as the MEDS scores, on day 1 of admission. Since vWF/ADAMTS-13 is
the ratio of VWF to ADAMTS-13, the input of all three indicators
into the equation might influence each other, thus, vWF, ADAMTS-13,
and vWF/ADAMTS-13 values were separately input into the equation,
and the other indicators were kept unchanged. This produced two
probability equations that enabled identification of ADAMTS-13
level, vWF/ADAMTS-13 ratio, and MEDS score as independent
predictors of the 28-day mortality from sepsis on day 1 of
admission. The equation (i):
(P=1/[1+e-(−9.875 + 0.290 × MEDS + 0.239 ×
vWF/ADAMTS - 13)])
was obtained by logistic regression analyses
involving endocan, vWF/ADAMTS-13 ratio, and MEDS score. The
equation (ii) P=1/[1+e-(0.296 × MEDS - 0.029 × ADAMTS - 13)] was
obtained by logistic regression analyses involving endocan, vWF,
and ADAMTS-13 levels, as well as MEDS score (Tables IV and V).
 | Table IV.Independent predictors of 28-day
mortality in septic patients on day 1 of admission-equation
(i). |
Table IV.
Independent predictors of 28-day
mortality in septic patients on day 1 of admission-equation
(i).
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Independent
predictors | B | SE | Wald | P-value | Exp (B) | 5% | 95% |
|---|
| MEDS | 0.290 | 0.049 | 35.325 | <0.001 | 1.337 | 0.199 | 0.412 |
| vWF/ADAMTS-13 | 0.239 | 0.050 | 23.179 | <0.001 | 1.269 | 0.147 | 0.362 |
| Constant | −9.875 | 1.775 | 30.952 | <0.001 | 0.000 |
|
|
 | Table V.Independent predictors of 28-day
mortality in septic patients on day 1 of admission-equation
(ii). |
Table V.
Independent predictors of 28-day
mortality in septic patients on day 1 of admission-equation
(ii).
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Independent
predictors | B | SE | Wald | P-value | Exp (B) | 5% | 95% |
|---|
| MEDS |
0.296 | 0.050 | 35.032 | <0.001 | 1.345 |
0.206 |
0.432 |
| ADAMTS-13 | −0.029 | 0.008 | 13.053 | <0.001 | 0.972 | −0.047 | −0.014 |
On the fifth day of admission, levels of endocan,
vWF and ADAMTS-13, as well as vWF/ADAMTS-13 ratio were independent
predictors of 28-day mortality for sepsis.
Logistic regression analysis was performed on the
endocan, vWF and ADAMTS-13 levels, as well as the vWF/ADAMTS-13
ratio, on day 5 of admission. The vWF, ADAMTS-13, and vWF/ADAMTS-13
values were separately input into the equation, with other
indicators unchanged. Therefore, two probability equations were
obtained: equation (iii) P=1/[1+e-(−12.622 + 0.106 × endocan +
0.380 × vWF/ADAMTS - 13)] was obtained by logistic
regression analyses involving endocan level and vWF/ADAMTS-13
ratio. Equation (iv):
P =1/[1+e-(0.106 × endocan + 0.001 × vWF - 0.042 ×
ADAMTS - 13)]
was obtained by logistic regression analyses
involving endocan, vWF and ADAMTS-13 levels. On the fifth day of
admission, endocan, vWF and ADAMTS-13 levels, as well as
vWF/ADAMTS-13 ratio, were independent predictors of the 28-day
mortality (Tables VI and VII).
 | Table VI.Independent predictors of 28-day
mortality in septic patients on day 5 of admission-equation
(iii). |
Table VI.
Independent predictors of 28-day
mortality in septic patients on day 5 of admission-equation
(iii).
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Independent
predictors | B | SE | Wald | P-value | Exp (B) | 5% | 95% |
|---|
| Endocan | 0.106 | 0.028 | 14.353 | <0.001 | 1.112 | 0.056 | 0.174 |
| vWF/ADAMTS13 | 0.380 | 0.063 | 36.031 | <0.001 | 1.463 | 0.265 | 0.549 |
| Constant | −12.622 | 1.538 | 67.343 | <0.001 | 0.000 |
|
|
 | Table VII.Independent predictors of 28-day
mortality in septic patients on day 5 of admission-equation
(iv). |
Table VII.
Independent predictors of 28-day
mortality in septic patients on day 5 of admission-equation
(iv).
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Independent
predictors | B | SE | Wald | P-value | Exp (B) | 5% | 95% |
|---|
| Endocan |
0.106 | 0.028 | 14.499 | <0.001 | 1.111 |
0.058 |
0.168 |
| vWF |
0.001 | 0.000 |
7.563 |
0.006 | 1.001 |
0.000 |
0.002 |
| ADAMTS-13 | −0.042 | 0.009 | 23.682 | <0.001 | 0.958 | −0.065 | −0.028 |
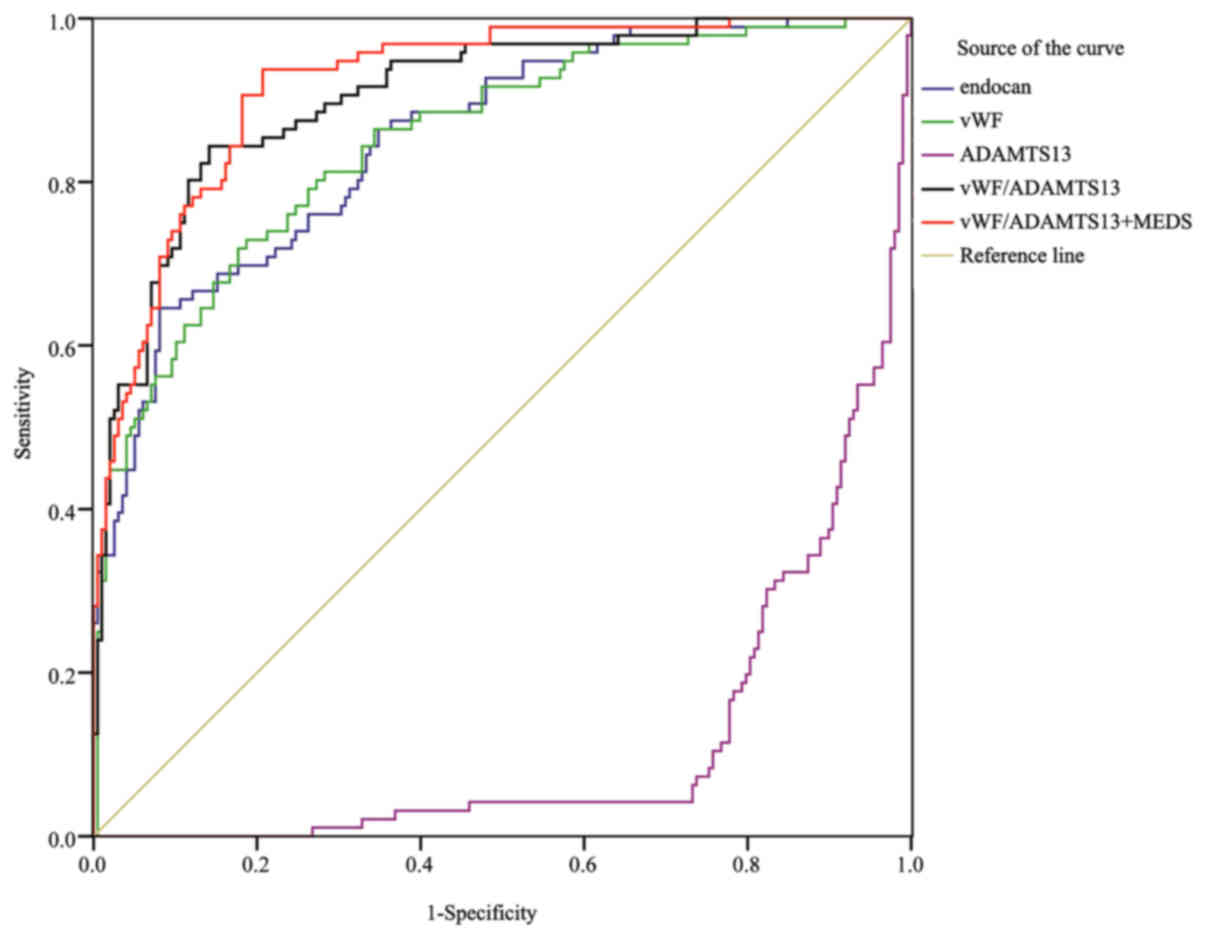
ROC curves of the independent
predictors of 28-day mortality in septic patients
Fig. 1 shows the ROC
curves of endocan, vWF, ADAMTS-13, vWF/ADAMTS-13 ratio, and MEDS
score in predicting the 28-day mortality rate of sepsis on day 1 of
admission. Table VIII shows the
area under the ROC curve (AUC) and 95% confidence interval (95% CI)
for each indicator. The MEDS score exhibited the highest AUC
(0.809) among all the indicators, which was significantly higher
than the AUC (0.656) of endocan (P<0.01), although it showed no
significant difference when comparing with the AUCs of other
indicators. The combination of vWF/ADAMTS-13 ratio with MEDS score
(AUC 0.856) remarkably increased the prognostic value, especially
when comparing with endocan (0.656, P<0.01), vWF (0.751,
P<0.01), or ADAMTS-13 (0.761, P<0.05) alone.
 | Table VIII.Area under ROC curve (AUC) of all
indicators in predicting the 28-day mortality from sepsis on day 1
of admission. |
Table VIII.
Area under ROC curve (AUC) of all
indicators in predicting the 28-day mortality from sepsis on day 1
of admission.
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|---|
| Predictors | AUC | SE | P | 5% | 95% |
|---|
| Endocan | 0.656a | 0.031 | <0.001 | 0.595 | 0.717 |
| vWF | 0.751a | 0.030 | <0.001 | 0.693 | 0.810 |
| ADAMTS-13 | 0.761b | 0.028 | <0.001 | 0.706 | 0.815 |
| vWF/ADAMTS-13 | 0.790 | 0.027 | <0.001 | 0.737 | 0.844 |
| MEDS | 0.809 | 0.028 | <0.001 | 0.755 | 0.863 |
| vWF/ADAMTS-13 +
MEDS | 0.856 | 0.024 | <0.001 | 0.808 | 0.903 |
In Table IX, the
results are shown of the assessment of the prognostic capabilities
of all indicators in septic patients. The combination of
vWF/ADAMTS-13 ratio with MEDS score exhibited superior sensitivity
(70.8), specificity (89.4), positive predictive value (PPV) (76.4),
and negative predictive value (NPV) (86.3), compared with
individual indicators.
 | Table IX.The cut-off value of each indicator
for the prognosis of sepsis and the relevant evaluation results on
day 1 of admission. |
Table IX.
The cut-off value of each indicator
for the prognosis of sepsis and the relevant evaluation results on
day 1 of admission.
| Prognostic
factors | Cut-off value | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) |
|---|
| Endocan
(ng/ml) | 51.84 | 92.7 | 40.9 | 43.2 | 92.0 |
| vWF (ng/l) | 3674.67 | 81.3 | 59.1 | 49.1 | 86.7 |
| ADAMTS-13
(pg/ml) | 221.58 | 62.6 | 81.3 | 61.9 | 81.7 |
| vWF/ADAMTS-13 | 17.51 | 76.0 | 71.2 | 56.1 | 86.0 |
| MEDS score | 12.5 | 69.8 | 86.4 | 71.3 | 85.5 |
| vWF/ADAMTS-13 +
MEDS | 0.47 | 70.8 | 89.4 | 76.4 | 86.3 |
Fig. 2 shows the ROC
curves of endocan, vWF, ADAMTS-13, and vWF/ADAMTS-13 ratio in
predicting the 28-day mortality rate of sepsis on the fifth day of
admission. The AUC (0.905) of the vWF/ADAMTS-13 ratio was the
highest among all indicators, but without significant difference
when comparing with the AUCs of other biomarkers. The combination
of vWF/ADAMTS-13 ratio with endocan (AUC 0.921) remarkably
increased the prognostic value, especially when comparing with
endocan (0.853, P<0.05) or vWF (0.850, P<0.05) alone. The
predictive value of vWF/ADAMTS-13 + endocan (AUC 0.921) on day 5
was significantly higher than that of vWF/ADAMTS-13 + MEDS score
(AUC 0.856, P<0.05) on day 1 (Table
X). The combination of endocan level+vWF/ADAMTS-13 ratio
exhibited superior sensitivity (93.8), specificity (79.3), PPV
(68.7), and NPV (96.3), compared with individual indicators
(Table XI).
 | Table X.Area under ROC curve (AUC) of all
indicators in predicting the 28-day mortality from sepsis on day
5. |
Table X.
Area under ROC curve (AUC) of all
indicators in predicting the 28-day mortality from sepsis on day
5.
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|---|
| Predictors | AUC | SE | P-value | 5% | 95% |
|---|
| vWF |
0.850a | 0.024 | <0.001 | 0.803 | 0.897 |
| Endocan |
0.853a | 0.023 | <0.001 | 0.807 | 0.898 |
| ADAMTS-13 | 0.886 | 0.020 | <0.001 | 0.847 | 0.924 |
| vWF/ADAMTS-13 | 0.905 | 0.019 | <0.001 | 0.868 | 0.941 |
| Endocan +
vWF/ADAMTS-13 | 0.921 | 0.016 | <0.001 | 0.889 | 0.952 |
 | Table XI.The cut-off value of each indicator
for the prognosis of sepsis and the relevant evaluation results on
day 5. |
Table XI.
The cut-off value of each indicator
for the prognosis of sepsis and the relevant evaluation results on
day 5.
| Prognostic
factors | Cut-off value | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) |
|---|
| Endocan
(ng/ml) | 55.89 | 64.6 | 91.9 | 79.5 | 84.3 |
| vWF (ng/l) | 3814.84 | 71.9 | 82.3 | 66.3 | 85.8 |
| ADAMTS-13
(pg/ml) | 229.19 | 73.2 | 95.8 | 89.7 | 88.0 |
| vWF/ADAMTS-13 | 16.93 | 84.4 | 85.9 | 74.3 | 91.9 |
| Endocan +
vWF/ADAMTS-13 | 0.22 | 93.8 | 79.3 | 68.7 | 96.3 |
Discussion
As one of the leading causes of death in critically
ill patients, studies have shown that the case-fatality rate in
patients with severe sepsis or septic shock reached 20–54%
(5,8–11).
Despite the application of more advanced life support and new
generations of antibiotics in recent years, the number of annual
deaths from sepsis continues to increase. Furthermore, the fatality
rate increases by 5–10% for each hour when the appropriate
antibiotic treatment is delayed (12,13).
Therefore, the early diagnosis and treatment of sepsis are both
particularly important. Biomarkers can increase the accuracy of
diagnosis and help to monitor the infection process. In the absence
of typical clinical symptoms, validated biomarkers can objectively
reflect the severity of the disease and furthermore monitor the
pathophysiological process of the disease, as well as the response
to treatment interventions (14).
Thereafter, it is essential to search for biomarkers that are
valuable for early diagnosis, accurate stratification, sensitive
therapeutic monitoring, and precise prediction of prognosis.
In this study, the levels of endocan and vWF, as
well as vWF/ADAMTS-13 ratio progressively increased in the healthy
control (lowest), SIRS, sepsis, severe sepsis, and septic shock
(highest) groups, whereas ADAMTS-13 progressively decreased, with
statistically significant difference, suggesting that these
biomarkers may play specific roles in risk stratification.
Importantly, patients with more severe disease conditions exhibited
higher endocan, vWF, and vWF/ADAMTS-13 ratio levels, and lower
ADAMTS-13 levels. Because vascular endothelial injury increases
with severity rate of sepsis, the endothelial cells become more
active, followed by the occurrence of MODS. Therefore, these
combined biomarkers not only indicate disease severity but may
serve as independent indicators of organ dysfunction and poor
prognosis (15,16).
Based on our results, MEDS scoring might be
preferentially on day 1 to predict the likelihood of death. If
patients survive to day 5, the vWF/ADAMTS-13 ratio would be a more
feasible biomarker to predict death risk. Furthermore, comparisons
of AUC have shown that the AUCs of the combined vWF/ADAMTS-13 ratio
were higher than those of vWF or ADAMTS-13 alone on both days 1 and
5, suggesting that vWF/ADAMTS-13 ratio is superior to vWF or
ADAMTS-13 alone in both determining disease severity and predicting
prognosis. Claus et al (17)
found that vWF/ADAMTS-13 ratio was associated with the severity and
prognosis of organ failure in patients with systemic inflammation,
and that this combined ratio was more valuable than vWF alone in
decision-making, which is consistent to our finding. Finally, we
also found that the combination of two indicators was superior to a
single indicator: for septic patients in the emergency department,
endocan + vWF/ADAMTS-13 was the most valuable indicator for
predicting the 28-day fatality rate on day 5.
Vascular endothelial cells play an important role in
sepsis and sepsis-induced MODS. The progression of sepsis can be
indirectly reflected by the extent of endothelial cell injury and
the degree of microthrombus formation. Specifically secreted by
endothelial cells, endocan is continuously released from cells when
endothelial cells are damaged in a septic patient, thus mediating
its physiological functions in regulating leukocyte adhesion and
migration, as well as in preventing leukocytes from entering tissue
and causing tissue damage. The serum level of endocan increased
significantly and was correlated with severity of sepsis (3,18).
In the pathogenesis of sepsis, vascular endothelial
cells and platelet-derived vWF molecules form multimers that are
much larger than those in normal plasma. These ‘ultralarge’ vWF
(UL-vWF) multimers are connected with the P-selectin on the
endothelial cell surface in a beaded chain fashion and bind to the
glycoprotein Ib (GpIb) on the surface of circulating platelets.
Other platelets are assembled around the UL-vWF via the activated
glycoprotein IIb-IIIa (GPIIB-IIIA) complexes, forming large
platelet thrombosis that may cause embolism. Once vascular
endothelial cells are injured, UL-vWF cleaves from the surface of
endothelial cells. These free UL-vWF-platelets can block downstream
small blood vessels, resulting in ischemia of tissues/organs,
ultimately causing MODS (19). The
damage of vascular endothelial cells will inevitably lead to the
massive release of vWF (20,21). High vWF level, as an important
biomarker of vascular endothelial damage, has been confirmed in
sepsis (15,17,22).
ADAMTS-13 is an enzyme that degrades vWF-platelet
complexes, reducing thrombus formation (23), avoiding microvenous thrombosis, and
regulating the thrombus reaction in the injured arteries (24). Increased vWF levels along with
decreased ADAMTS-13 levels have been detected in septic patients
(25). Martin et al (26) reported the formation of a large
number of vWF polymers during sepsis, which consumed ADAMTS-13
in vivo. Additionally, sepsis was associated with the
excessive production of interleukin-6, a proinflammatory cytokine,
which reduced the speed of ADAMTS-13 in degrading vWF polymers.
Abnormal secretion of ADAMTS-13 in septic patients may explain the
concomitant decrease in ADAMTS-13 activity (27,28). As
ADAMTS-13 activity dramatically decreases, plasma vWF
macromolecules cannot be degraded. Resultantly, more platelets bind
to vWF to form small thrombi, which block the microvessels and
aggravate microcirculation disorders (15,24).
A sepsis biomarker should be able to identify either
the onset of SIRS or compensated anti-inflammatory response
syndrome (CARS) before the onset of MODS and aid in the lowering of
mortality rates. However, because of the vague and broad definition
of sepsis along with its various manifestations and severity
levels, it is difficult to ascertain a definitive biomarker which
could aid in therapeutic strategies. Currently, no biological
molecular markers are used in the diagnosis or prognosis of sepsis
in the United States (29).
Studies have reported the high specificity and
sensitivity of C-reactive protein (CRP) in the sepsis diagnosis.
However, other reports showed that CRP levels are not indicative of
survival in sepsis patients (30).
PCT has mixed value as a biomarker in the diagnosis and prognosis
of sepsis. Elevations of PCT are not as specific for infection as
was once believed, which may be elevated in a number of disorders
in the absence of infection, especially following trauma (31–33). PCT
levels may vary early during the development of sepsis and the
test's predictive power is probably only significant later in the
patient's course (34–35).
In this study, several biomarkers were evaluated to
predict total in hospital mortality early in suspected sepsis, and
we found that endocan, vWF and ADAMTS-13 levels, were easily and
timely acquired for clinicians to rapidly diagnose and extend
treatment beyond the standard therapy. Moreover, a combination of
vWF/ADAMTS-13 ratio may be more effective. These biomarkers had the
best predictive performance, which outperformed clinical criteria
and other more extensively studied biomarkers (e.g. CRP and PCT)
used for diagnosis and mortality prediction in the setting of
sepsis. Our findings suggested that endocan, vWF and ADAMTS-13
levels, as well as vWF/ADAMTS-13 ratio, when combined with the
clinical SIRS criteria that defined eligibility for enrollment, may
provide valuable tool to predict mortality in sepsis.
In the present study there were also some
limitations. As a single-center study, it was limited by its small
sample size, and the findings need to be further validated in
large-scale multiple-center studies. Second, systematic scoring was
not performed on day 5, which might compromise the conclusions of
the analysis regarding the predictors of 28-day mortality from
sepsis. Finally, while patients who had received treatment in other
hospitals were excluded during enrollment, some subjects in our
study might still have been treated with oral drugs for several
days (on their own) before admission, which might have affected the
measurement results of all indicators.
In conclusion, Endocan, vWF and ADAMTS-13 levels, as
well as vWF/ADAMTS-13 ratio are valuable in the risk stratification
and prognostic evaluation of sepsis, providing novel sepsis
biomarkers in clinic.
Acknowledgements
The authors sincerely thank the ED staff, the
Physical examination center of Beijing Chao-Yang Hospital, and the
Biochemistry Laboratory staff for their helpful contributions to
this study.
Funding
The authors have not received a specific grant for
this research from any funding agency in the public, commercial or
not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CSL designed this study, QZ conducted the trial and
collected and analyzed data, performed statistical analysis,
drafted and wrote the manuscript. CSL takes responsibility for the
paper as a whole. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Comittee of
Institutional Review Board of Beijing Chao-Yang Hospital, Capital
Medical University (Beijing, China). Patients who participated in
this research had complete clinical data. The signed informed
consents were obtained from the patients or the guardians
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lever A and Mackenzie I: Sepsis:
Definition, epidemiology, and diagnosis. BMJ. 335:879–883. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zambon M, Ceola M, Almeida-de-Castro R,
Gullo A and Vincent JL: Implementation of the surviving sepsis
campaign guidelines for severe sepsis and septic shock: We could go
faster. J Crit Care. 23:455–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pierrakos C and Vincent JL: Sepsis
biomarkers: A review. Crit Care. 14:R152010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G;
SCCM/ESICM/ACCP/ATS/SIS, : 2001 SCCM/ESICM/ACCP/ATS/SIS
International Sepsis Definitions Conference. Crit Care Med.
31:1250–1256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knaus WA, Draper EA, Wagner DP and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shapiro NI, Wolfe RE, Moore RB, Smith E,
Burdick E and Bates DW: Mortality in Emergency Department Sepsis
(MEDS) score: a prospectively derived and validated clinical
prediction rule. Crit Care Med. 31:670–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vincent JL, Moreno R, Takala J, Willatts
S, De Mendonça A, Bruining H, Reinhart CK, Suter PM and Thijs LG:
The SOFA (Sepsis-related Organ Failure Assessment) score to
describe organ dysfunction/failure. On behalf of the working group
on sepsis-related problems of the European Society of Intensive
Care Medicine. Intensive Care Med. 22:707–710. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shirakawa K, Naitou K, Hirose J, Takahashi
T and Furusako S: Presepsin (sCD14-ST): Development and evaluation
of one-step ELISA with a new standard that is similar to the form
of presepsin in septic patients. Clin Chem Lab Med. 49:937–939.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alberti C, Brun-Buisson C, Burchardi H,
Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R,
Boulmé R, et al: Epidemiology of sepsis and infection in ICU
patients from an international multicentre cohort study. Intensive
Care Med. 28:108–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vincent JL, Sakr Y, Sprung CL, Ranieri VM,
Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR and Payen D;
Sepsis occurrence in acutely Ill patients investigators, : Sepsis
in European intensive care units: Results of the SOAP study. Crit
Care Med. 34:344–353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esteban A, Frutos-Vivar F, Ferguson ND,
Peñuelas O, Lorente JA, Gordo F, Honrubia T, Algora A, Bustos A,
García G, et al: Sepsis incidence and outcome: Contrasting the
intensive care unit with the hospital ward. Crit Care Med.
35:1284–1289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonsalves MD and Sakr Y: Early
identification of sepsis. Curr Infect Dis Rep. 12:329–335. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bauer M and Reinhart K: Molecular
diagnostics of sepsis - where are we today? Int J Med Microbiol.
300:411–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levy MM: Preface biomarkers in critical
illness. Crit Care Clin. 27:xiii–xv. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bongers TN, de Maat MP, van Goor ML,
Bhagwanbali V, van Vliet HH, Gómez García EB, Dippel DW and Leebeek
FW: High von Willebrand factor levels increase the risk of first
ischemic stroke: Influence of ADAMTS13, inflammation, and genetic
variability. Stroke. 37:2672–2677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nguyen TC, Liu A, Liu L, Ball C, Choi H,
May WS, Aboulfatova K, Bergeron AL and Dong JF: Acquired ADAMTS-13
deficiency in pediatric patients with severe sepsis. Haematologica.
92:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Claus RA, Bockmeyer CL, Budde U, Kentouche
K, Sossdorf M, Hilberg T, Schneppenheim R, Reinhart K, Bauer M,
Brunkhorst FM, et al: Variations in the ratio between von
Willebrand factor and its cleaving protease during systemic
inflammation and association with severity and prognosis of organ
failure. Thromb Haemost. 101:239–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scherpereel A, Depontieu F, Grigoriu B,
Cavestri B, Tsicopoulos A, Gentina T, Jourdain M, Pugin J, Tonnel
AB and Lassalle P: Endocan, a new endothelial marker in human
sepsis. Crit Care Med. 34:532–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chng WJ, Yip CY, Baliwag MB and Liu TC:
Differential effect of the ABO blood group on von Willebrand factor
collagen binding activity and ristocetin cofactor assay. Blood
Coagul Fibrinolysis. 16:75–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sadler JE: Biochemistry and genetics of
von Willebrand factor. Annu Rev Biochem. 67:395–424. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Mourik JA, Boertjes R, Huisveld IA,
Fijnvandraat K, Pajkrt D, van Genderen PJ and Fijnheer R: von
Willebrand factor propeptide in vascular disorders: a tool to
distinguish between acute and chronic endothelial cell
perturbation. Blood. 94:179–185. 1999.PubMed/NCBI
|
|
22
|
Lerolle N, Dunois-Lardé C, Badirou I,
Motto DG, Hill G, Bruneval P, Diehl JL, Denis CV and Baruch D: von
Willebrand factor is a major determinant of ADAMTS-13 decrease
during mouse sepsis induced by cecum ligation and puncture. J
Thromb Haemost. 7:843–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong JF, Moake JL, Nolasco L, Bernardo A,
Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K and
López JA: ADAMTS-13 rapidly cleaves newly secreted ultralarge von
Willebrand factor multimers on the endothelial surface under
flowing conditions. Blood. 100:4033–4039. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ley K, Laudanna C, Cybulsky MI and
Nourshargh S: Getting to the site of inflammation: The leukocyte
adhesion cascade updated. Nat Rev Immunol. 7:678–689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reiter RA, Varadi K, Turecek PL, Jilma B
and Knöbl P: Changes in ADAMTS13 (von-Willebrand-factor-cleaving
protease) activity after induced release of von Willebrand factor
during acute systemic inflammation. Thromb Haemost. 93:554–558.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin K, Borgel D, Lerolle N, Feys HB,
Trinquart L, Vanhoorelbeke K, Deckmyn H, Legendre P, Diehl JL and
Baruch D: Decreased ADAMTS-13 (A disintegrin-like and
metalloprotease with thrombospondin type 1 repeats) is associated
with a poor prognosis in sepsis-induced organ failure. Crit Care
Med. 35:2375–2382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao WJ, Niiya M, Zheng XW, Shang DZ and
Zheng XL: Inflammatory cytokines inhibit ADAMTS13 synthesis in
hepatic stellate cells and endothelial cells. J Thromb Haemost.
6:1233–1235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ono T, Mimuro J, Madoiwa S, Soejima K,
Kashiwakura Y, Ishiwata A, Takano K, Ohmori T and Sakata Y: Severe
secondary deficiency of von Willebrand factor-cleaving protease
(ADAMTS13) in patients with sepsis-induced disseminated
intravascular coagulation: Its correlation with development of
renal failure. Blood. 107:528–534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Biron BM, Ayala A and Lomas-Neira JL:
Biomarkers for sepsis: What is and what might be? Biomark Insights.
10 (Suppl 4):7–17. 2015.PubMed/NCBI
|
|
30
|
Pepys MB and Hirschfield GM: C-reactive
protein: A critical update. J Clin Invest. 111:1805–1812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maruna P, Nedelníková K and Gürlich R:
Physiology and genetics of procalcitonin. Physiol Res. 49 (Suppl
1):S57–S61. 2000.PubMed/NCBI
|
|
32
|
Becker KL, Snider R and Nylen ES:
Procalcitonin assay in systemic inflammation, infection, and
sepsis: Clinical utility and limitations. Crit Care Med.
36:941–952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Billeter A, Turina M, Seifert B, Mica L,
Stocker R and Keel M: Early serum procalcitonin, interleukin-6, and
24-hour lactate clearance: useful indicators of septic infections
in severely traumatized patients. World J Surg. 33:558–566. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brunkhorst FM, Al-Nawas B, Krummenauer F,
Forycki ZF and Shah PM: Procalcitonin, C-reactive protein and
APACHE II score for risk evaluation in patients with severe
pneumonia. Clin Microbiol Infect. 8:93–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bele N, Darmon M, Coquet I, Feugeas JP,
Legriel S, Adaoui N, Schlemmer B and Azoulay E: Diagnostic accuracy
of procalcitonin in critically ill immunocompromised patients. BMC
Infect Dis. 11:2242011. View Article : Google Scholar : PubMed/NCBI
|