Introduction
Peripheral arterial disease (PAD) is caused by limb
artery atherosclerosis (1), which is
most common in the lower extremities (2). PAD affects ~20% of the population aged
>55 years, worldwide (3). The
Inter-Society Consensus for the Management of Peripheral Arterial
Disease (TASC II) determined that the prevalence of asymptomatic
PAD is ~3–10% and is increasing to 15–20% in people aged >70
years worldwide (4). Critical limb
ischemia (CLI) is the most severe clinical manifestation of PAD and
may cause intermittent claudication, gangrene and foot ulcers
(5). Patients with CLI therefore
have a risk of destructive complications, including amputation and
mortality (6,7). Smoking and diabetes are major risk
factors for PAD, in addition to arterial hypertension,
hypercholesterolemia, familial susceptibility and the male sex
(8). Prevalence is also particularly
age-associated, as 20% of individuals over the age of 70 are
affected (9). The pathophysiological
mechanism of PAD is complex and has not yet been fully elucidated
(1). Despite major improvements in
surgical endovascular techniques (1), there is currently no therapy that can
effectively treat and improve the prognosis of patients with severe
PAD (10). Thus, PAD still has a
high mortality and morbidity (9).
Therefore, identifying the molecular mechanism underlying PAD
development is critical for determining novel treatments. In recent
years, the cellular and molecular mechanisms of PAD (10) and the role of certain microRNAs
(miRNAs) in PAD have received increasing attention (11).
miRNAs are small non-coding RNAs that are ~22
nucleotide in length, which post-transcriptionally regulate gene
expression by degrading mRNAs or inhibiting protein translation
(4). As key regulators of certain
events, miRNAs serve important roles in the regulation of the
balance between cell proliferation and differentiation during
tumorigenesis and organ development (12). Previous studies have demonstrated
that miRNAs serve critical roles in the regulation of vascular cell
proliferation, differentiation and apoptosis (13–15).
However, the biological function of miRNAs in PAD has only recently
been elucidated. miRNAs have been reported to serve critical roles
in PAD and PAD-associated complications (13–15).
However, the role of miR-15b in PAD is still unclear.
The present study aimed to assess the role of
miR-15b in the development of PAD and its associated
mechanisms.
Materials and methods
Cell culture
Human vascular smooth muscle cells (hVSMCs) were
obtained from the Cell Bank of the Shanghai Institute of Cell
Biology (Shanghai, China). Cells were grown in 75 cm2
flasks with Dulbecco's Modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin (Beyotime Institute of
Biotechnology, Shanghai, China). Cells were then incubated at 37°C
with 5% CO2.
Cell transfection
100 nM Mimic controls (sense:
5′-UUCUCCGAACGUGUCACGUTT-3′; anti-sense:
5-′ACGUGACACGUUCGGAGAATT-3′), 100 nM miR-15b mimics (sense:
5′-UAGCAGCACAUCAUGGUUUACA-3′; anti-sense:
5′-UAAACCAUGAUGUGCUGCUAUU-3′), 100 nM miR-15b inhibitors
(5′-UGUAAACCAUGAUGUGCUGCUA-3′), 100 nM inhibitor controls
(5′-CAGUACUUUUGUGUAGUACAA-3′; all Shanghai GenePharma Co., Ltd.,
Shanghai, China), 2 µl control-plasmids (cat. no. sc-108083), 2 µl
insulin growth factor 1 receptor (IGF1R)-plasmids (cat. no.
sc-421057-ACT; both Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) and 100 nM miR-15b mimics+2 µl IGF1R-plasmids were transfected
into hVSMCs using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
Cells without any treatment were used as the control group.
Following 48 h, reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed to assess transfection
efficiency.
MTT assay
A MTT assay was performed to detect cell viability.
48 h following transfection, cells were seeded in 96-well plates
(2×104 cells/ml). Subsequently, 10 µl MTT reagent
(Beyotime Institute of Biotechnology, Haimen, China) was added to
each well and incubated for 4 h at 37°C. DMSO (100 µl; Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) was added to dissolve the
formazan crystals. Thermo Scientific™ Multiskan™ FC (Thermo Fisher
Scientific, Inc.) was then used to measure absorbance at a
wavelength of 490 nm. Each experiment was performed in
triplicate.
Cell apoptosis assay
Following transfection for 48 h, hVSMCs were
digested using 0.2% trypsin. Following a wash with PBS, cells were
fixed with 70% ethanol overnight at 4°C. The apoptosis of cells was
then assessed using the Annexin V-fluorescein
isothiocyanate/propidium iodide apoptosis detection kit [cat. no.
70-AP101-100; Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.,
Hangzhou, China] following the manufacturer's protocol. A FACS
Calibur flow cytometer with Cell Quest software (version 5.1; BD
Biosciences, San Jose, CA, USA) was utilized for the detection of
cell apoptosis rate. Each experiment was performed in
triplicate.
Transwell assay
To assess cell migration, un-coated transwell
chambers (pore size, 8 µm; Costar; Corning Inc., Corning, NY, USA)
were utilized in the present study. Cells (2×104) were
seeded into the upper chamber with serum-free DMEM and 600 µl DMEM
containing 30% FBS was added to the lower chamber. Following 48 h
of incubation at 37°C, the migratory cells on the lower chamber
were fixed with 4% paraformaldehyde at room temperature for 30 min
and then stained with 0.5 ml 0.1% crystal violet at room
temperature for 15 min. At the end of the experiment, migrated
cells were counted under a light microscope at a magnification of
×200 using five random fields of view. Each experiment was
performed in triplicate.
Dual luciferase reporter assay
TargetScan bioinformatics software (www.targetscan.org/vert_71) was utilized to
predict the targets of miR-15b and the binding sites between IGF1R
and miR-15b. To confirm the binding sites between miR-15b and the
3′-untranslated region (3′-UTR) of IGF1R, a dual luciferase
reporter assay was performed. The wild type (WT-IGF1R) and mutant
(MUT-IGF1R) 3′-UTRs of IGF1R were cloned into a pmiR-RB-ReportTM
dual luciferase reporter gene plasmid vector (Guangzhou RiboBio
Co., Ltd., Guangzhou, China). hVSMCs were then co-transfected with
WT-IGF1R or MUT-IGF1R with miR-15b mimics or mimic controls using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. After cell
transfection for 48 h, the dual-luciferase assay system (Promega
Corporation, Madison, WI, USA) was utilized to detect luciferase
activity. Luciferase activity was normalized to that of
renilla luciferase in the current study.
RT-qPCR
The TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for hVSMC RNA extraction and the
miScript Reverse Transcription kit (Qiagen GmbH, Hilden, Germany)
was used for reverse transcription. The QuantiFast SYBR Green PCR
kit (Qiagen GmbH) was used to perform RT-qPCR analysis under a CFX
Connect Real-Time System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). GAPDH and U6 were utilized as mRNA and miRNA controls,
respectively. Primer sequences as shown in Table I. The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
10 min, 35 cycles of 95°C for 15 sec and 55°C for 40 sec. Relative
gene expression was calculated using the 2−ΔΔCq method
(16).
 | Table I.Primer sequences for PCR. |
Table I.
Primer sequences for PCR.
| Gene | Direction | Sequences
(5′-3′) |
|---|
| miR-15b | F |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGTAAA |
|
| R |
ACGTAGCAGCACATCATGGTTT |
| IGF1R | F |
CCCCCTCGAGGATCCTGAATCTGTGCAAAC |
|
| R |
AAAAGCGGCCGCCTTCCCAGCGAAATCATC |
| AKT | F |
TAAAGAAGGAGGTCATCGTGG |
|
| R |
CGGGACAGGTGGAAGAAAA |
| mTOR | F |
ATGCTGTCCCTGGTCCTTATG |
|
| R |
GGGTCAGAGAGTGGCCTTCAA |
| P70S6K | F |
AGTAAAGCATCCCTTCATCGTGG |
|
| R |
TGATGTAAATGCCCCAAAGCC |
| GAPDH | F |
CTTTGGTATCGTGGAAGGACTC |
|
| R |
GTAGAGGCAGGGATGATGTTCT |
| U6 | F |
GCTTCGGCAGCACATATACTAAAAT |
|
| R |
CGCTTCACGAATTTGCGTGTCAT |
Western blotting
Protein was extracted from cells using
radioimmunoprecipitate lysate buffer containing PMSF (Beyotime
Institute of Biotechnology). Total protein was quantified using a
bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Protein samples (70 mg of each extract) were separated using
12% SDS-PAGE, electrotansfered to polyvinylidene difluoride
membranes and then blocked with 5% skimmed milk at room temperature
for 1.5 h. Subsequently, membranes were incubated with the
following primary antibodies overnight at 4°C: anti-IGF1R (1:1,000;
cat. no. sc-81464; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-protein kinase B (AKT; 1:1,000; cat. no. 4691; Cell
Signaling Technology Inc., Danvers, MA, USA), anti-phosphorylated
(p)-AKT (1:1,000; cat. no. 4060; Cell Signaling Technology Inc.),
anti-mechanistic target of rapamycin (mTOR; 1:1,000; cat. no. 2983;
Cell Signaling Technology Inc.), anti-p-mTOR (1:1,000; cat. no.
5536; Cell Signaling Technology Inc.), anti-ribosomal protein S6
kinase beta-1 (p70S6K; 1:1,000; cat. no. 2708; Cell Signaling
Technology Inc.), anti-p-P70S6K (1:1,000; cat. no. 9234; Cell
Signaling Technology Inc.) and anti-β-actin (1:1,000; cat. no.
4970; Cell Signaling Technology Inc.). Samples were then incubated
with horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibodies (1:1,000; cat. no. 7074; Cell Signaling Technology Inc.)
at room temperature for 2 h. To visualize immunoreactive proteins,
the enhanced chemiluminescence detection system (Thermo Fisher
Scientific, Inc.) was utilized. Gel-Pro Analyzer densitometry
software (Version 6.3, Media Cybernetics, Inc., Rockville, MD, USA)
was used for band density quantification.
Statistical analysis
Statistical analyses were performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ±
standard deviation. Comparisons between two groups were made using
Student's t-test and comparisons between multiple groups were
analyzed by one-way analysis of variance with a Tukey's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-15b affects the viability and
migration of hVSMCs
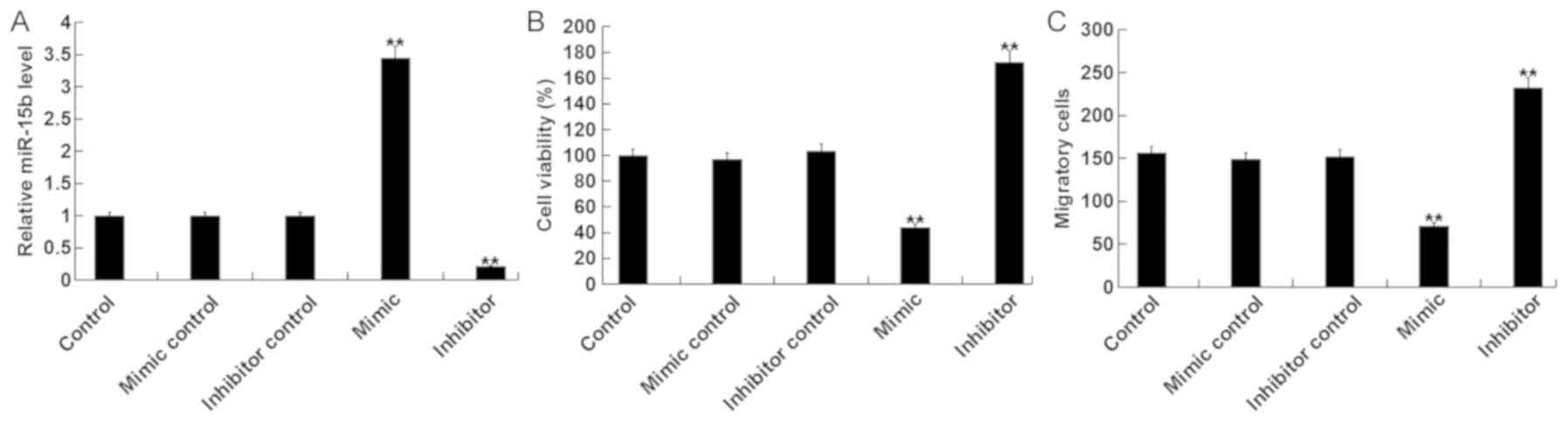
To assess the role of miR-15b in the viability and
migration of hVSMCs, miR-15b mimics, mimic controls, miR-15b
inhibitors or inhibitor controls were transfected into hVSMCs. A
total of 48 h following cell transfection, RT-qPCR was performed to
detect transfection efficiency. The results revealed that, compared
with the control group, the miR-15b mimic significantly increased
the level of miR-15b, while the miR-15b inhibitor significantly
decreased miR-15b (Fig. 1A).
Subsequently, MTT and transwell assays were performed to determine
whether miR-15b effected viability and migration of hVSMC cells. It
was demonstrated that, compared with the control group, the miR-15b
mimic significantly reduced the viability and migration of hVSMCs,
while the miR-15b inhibitor exhibited the opposite effect (Fig. 1B and C).
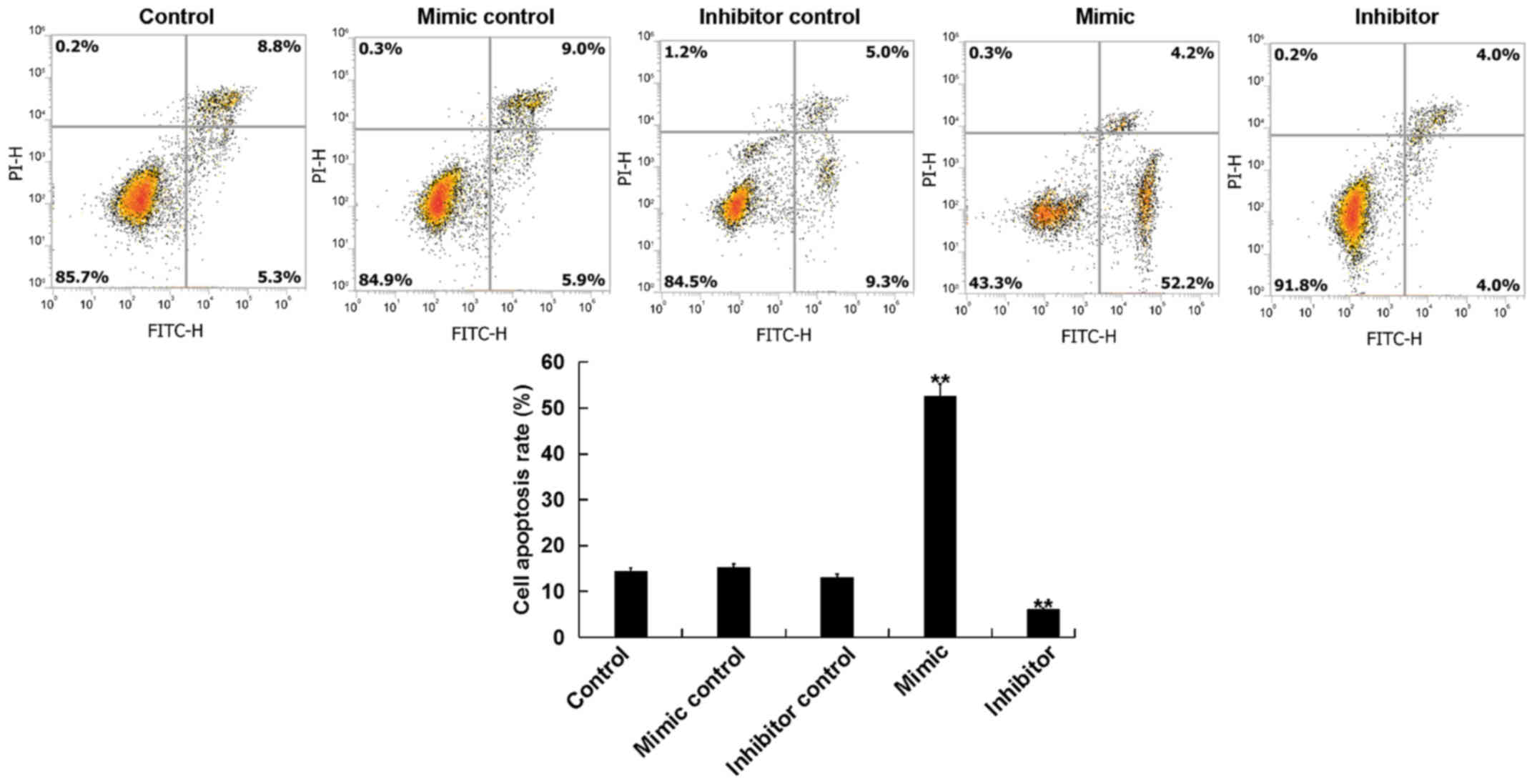
miR-15b affects the apoptosis of
hVSMCs
The current study further assessed whether miR-15b
affected the proliferation of hVSMCs by inducing cell apoptosis. A
total of 48 h following transfection, the apoptosis of hVSMCs was
analyzed via flow cytometry. The results revealed that, compared
with the control group, the miR-15b mimic significantly promoted
the apoptosis of hVSMCs, while the miR-15b inhibitor reduced hVSMCs
apoptosis (Fig. 2).
miR-15b directly targets IGF1R in
hVSMCs
To assess the molecular mechanism of miR-15b on
hVSMCs, bioinformatics software (TargetScan) was used to predict
the potential targets of miR-15b (Fig.
3A). A dual luciferase reporter assay was then performed to
confirm predictions. The results indicated that, compared with
cells co-transfected with WT-IGF1R and mimic control, the
luciferase activity in cells co-transfected with WT-IGF1R and
miR-15b mimic were significantly reduced (Fig. 3B). The results demonstrated that
IGF1R is a direct target of miR-15b.
Furthermore, the current study revealed that,
compared with the control group, the miR-15b mimic significantly
inhibited the expression of IGF1R in hVSMCs at the mRNA and protein
level, while the miR-15b inhibitor exhibited the opposite effects
(Fig. 3C and D).
miR-15b affects the phosphoinositide
3-kinase (PI3K)/AKT signaling pathway in hVSMCs
The PI3K/AKT signaling pathway, an IGF1R-mediated
downstream signaling pathway, was assessed in the current study.
The results indicated that, compared with the control group, the
mRNA levels of key components of the PI3K/AKT pathway, including
AKT, mTOR and P70S6K, were significantly decreased in hVSMCs
transfected with miR-15b mimics Additionally, treatment with the
miR-15b inhibitor exhibited the opposite effect (Fig. 4A-C). Compared with the control group,
the protein levels of AKT/p-AKT, mTOR/p-mTOR and P70S6K/p-P70S6K in
hVSMCs were significantly inhibited by miR-15b mimics and
significantly enhanced by miR-15b inhibitors (Fig. 4D-G).
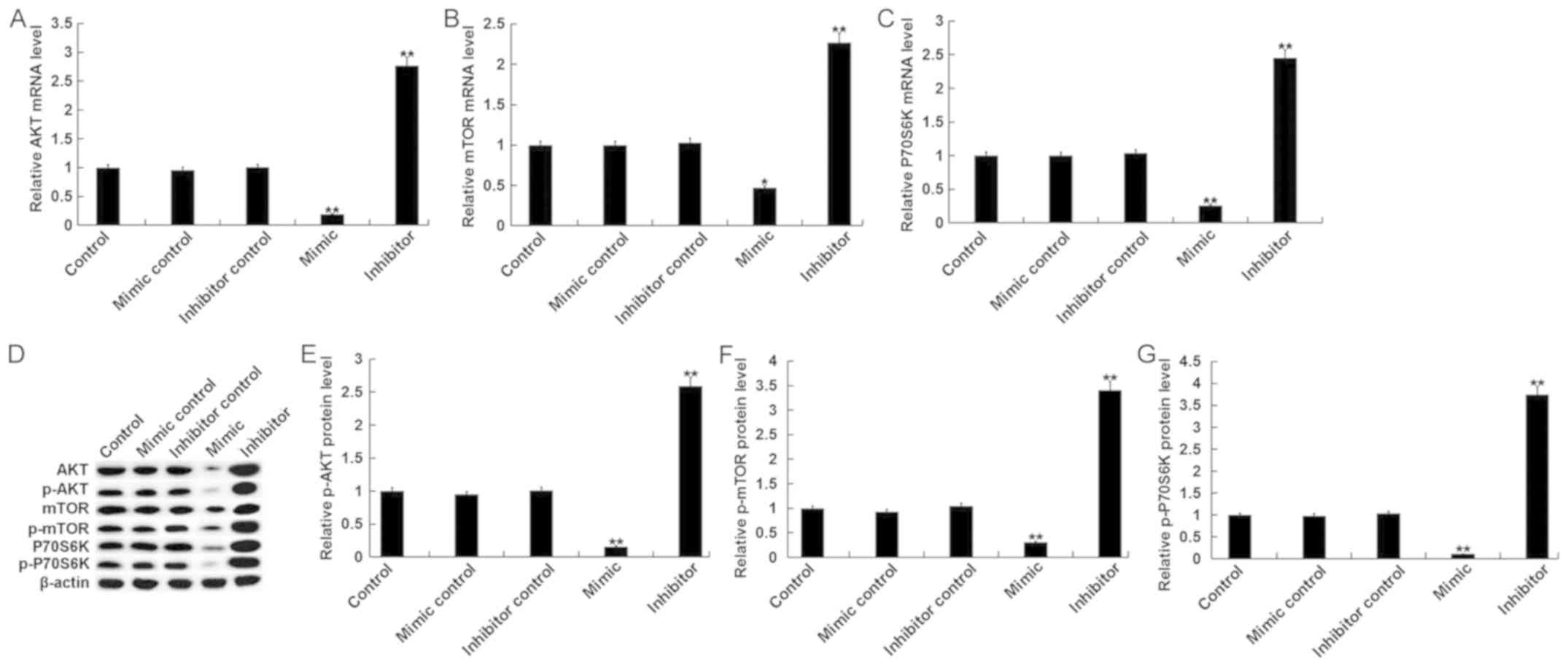 | Figure 4.Effect of miR-15b on the PI3K/AKT
signaling pathway in hVSMCs. Mimic controls, miR-15b mimics,
miR-15b inhibitors or inhibitor controls were transfected into
hVSMCs for 48 h. Subsequently, mRNA levels of (A) AKT, (B) mTOR and
(C) P70S6K, were assessed via reverse transcription-quantitative
polymerase chain reaction. (D) Western blotting was also performed
to assess the protein levels of AKT, p-AKT, mTOR, p-mTOR, P70S6K
and p-P70S6K. (E) p-AKT, (F) p-mTOR and (G) p-P70S6K were
quantified and presented as the fold of control. All data are
presented as the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. the control. miR, microRNA; PI3K, phosphoinositide
3-kinase; AKT, protein kinase B; hVSMCs, human vascular smooth
muscle cells; p, phosphorylated; mTOR, mechanistic target of
rapamycin; P70S6K, ribosomal protein S6 kinase beta-1. |
miR-15b mimics inhibit cell viability
and, migration, and induce apoptosis in hVSMCs by targeting
IGF1R
To assess whether miR-15b affects hVSMCs by directly
acting on IGF1R, a rescue experiment was performed. Mimic controls,
miR-15b mimics, control-plasmids, IGF1R-plasmids or miR-15b
mimic+IGF1R-plasmids were transfected into hVSMCs. A total of 48 h
following, transfection efficiency was detected via RT-qPCR and
western blotting. As presented in Fig.
5A and B, compared with the control group, the IGF1R-plasmid
significantly promoted the protein and mRNA expression of IGF1R in
hVSMCs. Furthermore, compared with the mimic control group, miR-15b
mimics markedly reduced the protein and mRNA expression of IGF1R in
hVSMCs, while the IGF1R-plasmid reversed this effect (Fig. 5C and D).
hVSMC viability, migration and apoptosis were
measured using MTT, transwell and flow cytometry assays,
respectively. The results revealed that, compared with the mimic
group, the reduced viability and migration, and the increased
apoptosis of hVSMCs induced by the miR-15b mimic was markedly
reversed following transfection with the IGF1R-plasmid (Fig. 6A-D).
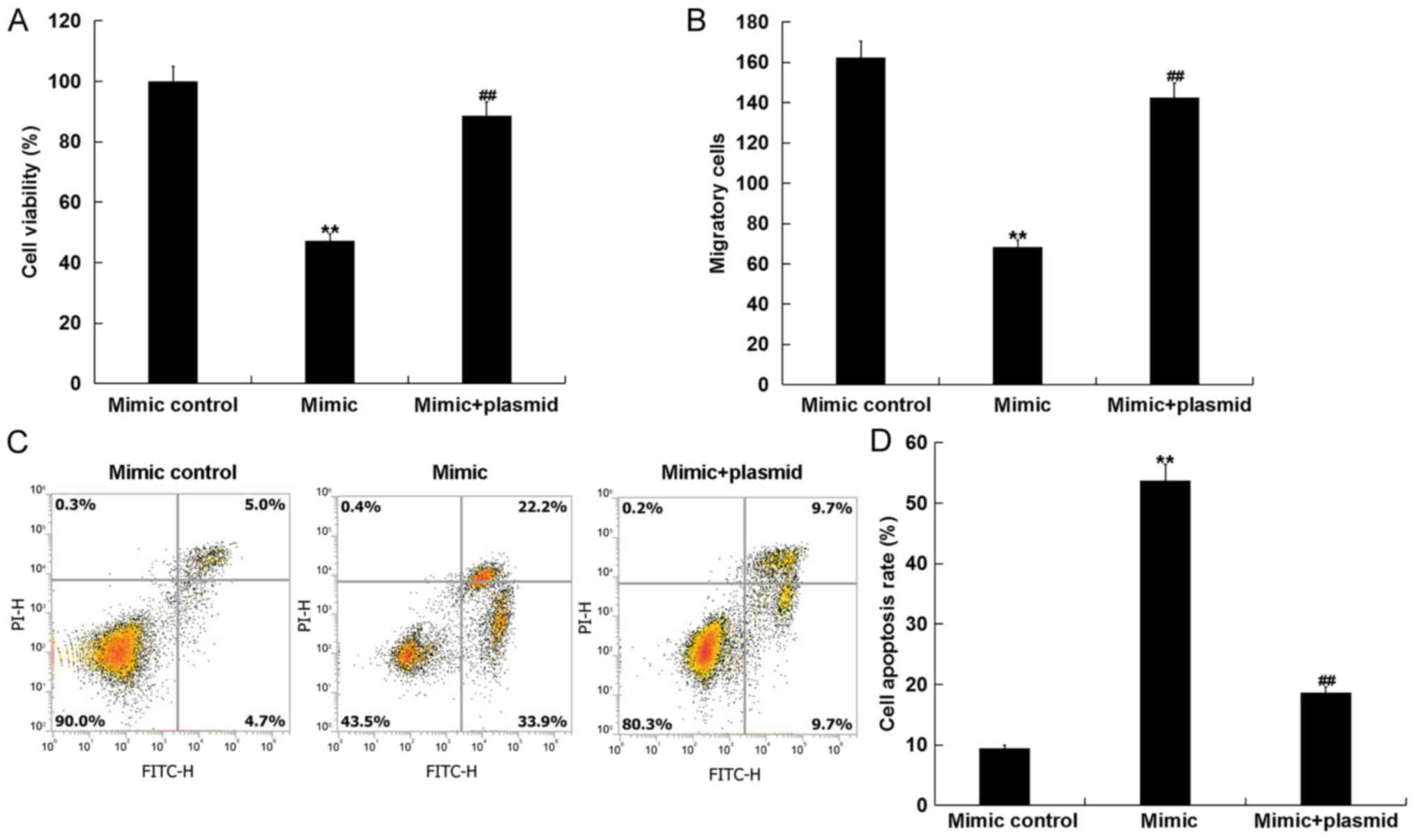 | Figure 6.miR-15b inhibits hVSMC cell viability
and migration, and induces cell apoptosis by directly targeting
IGF1R. hVSMCs were transfected with mimic controls, miR-15b mimics
or miR-15b mimic+IGF1R-plasmids for 48 h. (A) Cell viability, (B)
migration and (C) apoptosis with (D) quantification were assessed
via MTT, transwell and flow cytometry assays, respectively. All
data are presented as the mean ± standard deviation. **P<0.01
vs. the mimic control; ##P<0.01 vs. the mimic. miR,
microRNA; hVSMCs, human vascular smooth muscle cells; IGF1R,
insulin growth factor 1 receptor; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Discussion
The results of the present study indicated that
miR-15b overexpression significantly inhibited cell viability and
cell migration, induced apoptosis and repressed the PI3K/AKT
signaling pathway in hVSMCs. Furthermore, miR-15b downregulation
exhibited the opposite effect. IGF1R was also identified as a
direct target of miR-15b, which was negatively regulated by
miR-15b. In addition, the effect of miR-15b upregulation on hVSMCs
was eliminated by IGF1R upregulation. Therefore, these data
indicated that miR-15b inhibited the growth of hVSMCs by directly
targeting IGF1R. miR-15b may therefore be a novel therapeutic
target for future PAD treatment.
PAD is a leading cause of mortality worldwide that
is associated with widespread vascular atherosclerosis, affecting
vessels including the coronary arteries (9). It is considered to be a clinical
manifestation of systemic atherosclerosis (17). Although a large number of studies
have assessed the pathogenesis of atherosclerosis, its underlying
pathophysiology remains unclear (1,18,19).
Previous studies have also identified the differential expression
of certain genes in the peripheral monocytes of patients with a
variety of atherosclerotic conditions (20–22).
However, the association between PAD and certain miRNAs remains
largely unclear (1).
miRNAs are small non-coding RNAs, which are involved
in the regulation of multiple biological processes (1). They are considered to be part of a
network in which a modest change in the expression of one miRNA may
trigger a chain reaction involving multiple genes in the same or
different pathways (23). It has
been demonstrated that miRNAs can be used to diagnose PAD and to
further understand the molecular mechanism of disease development,
which illustrates its potential to be a therapeutic target for PAD
in the future (1).
miR-15b has been studied in a variety of diseases,
particularly in cancer. Li and Wang (24) suggested that miR-15b may prevent
amyloid-β accumulation by targeting nuclear factor-κB signaling and
beta-secretase 1, thus exhibiting a protective effect in
Alzheimer's disease. Furthermore, Sun et al (25) demonstrated that miR-15b levels were
decreased in human gliomas, which was associated with a poor
prognosis. Additionally, Wang et al (26) revealed that miR-15b facilitates
non-small cell lung carcinoma cell proliferation and invasion by
regulating the expression of metallopeptidase inhibitor 2. miR-15b
has also been revealed to contribute to extracellular matrix
degradation in intervertebral disc degeneration by regulating the
expression of SMAD family member 3 (27). However, the role of miR-15b in PAD
remains unclear. Therefore, the current study was performed to
assess the role of miR-15b in the growth of hVSMCs.
To determine the role of miR-15b in the growth of
hVSMCs, miR-15b was up- or downregulated in hVSMCs. Further
analysis demonstrated that miR-15b overexpression significantly
inhibited cell viability and migration, and induced apoptosis in
hVSMCs, while miR-15b downregulation exhibited the opposite
results. To further assess the molecular mechanism of miR-15b on
hVSMCs, the targets of miR-15b were predicted using TargetScan
software. The results revealed that IGF1R was a target of miR-15b
that was negatively regulated by miR-15b in hVSMCs. IGF1R, as a
transmembrane receptor tyrosine kinase, is an anti-apoptotic
oncogene that is closely associated with the insulin receptor
(InsR), which forms homodimers or heterodimerizes with InsR to
discriminate its ligands (IGF-1 and IGF-2) (28). Upon binding to its ligand, IGF1R
activates the PI3K/AKT and the mitogen activated protein kinase
pathway (29). The current study
also indicated that miR-15b overexpression repressed the PI3K/AKT
pathway in hVSMCs, while miR-15b downregulation promoted PI3K/AKT
pathway. Finally, to assess whether miR-15b affected the growth of
hVSMCs by directly targeting IGF1R, a rescue experiment was
performed. The inhibitory effect of miR-15b on the growth of hVSMCs
was eliminated by IGF1R overexpression, indicating that miR-15b
prevented the proliferation and migration, and induced the
apoptosis of hVSMCs by directly targeting IGF1R.
In summary, the results of the current study
indicated that miR-15b regulates cell proliferation, migration and
apoptosis by regulating the PI3K/AKT signaling pathway via IGF1R
targeting. Therefore, the exogenous overexpression of miR-15b may
serve as a promising method for the treatment of PAD.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data sets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YS collaborated to design the study. YS, YG, TS and
CY were responsible for data collection and analysis. ZN and XW
collaborated to data analysis. All authors collaborated to
interpret results and develop the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stather PW, Sylvius N, Wild JB, Choke E,
Sayers RD and Bown MJ: Differential microRNA expression profiles in
peripheral arterial disease. Circ Cardiovasc Genet. 6:490–497.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Togliatto G, Trombetta A, Dentelli P,
Gallo S, Rosso A, Cotogni P, Granata R, Falcioni R, Delale T, Ghigo
E and Brizzi MF: Unacylated ghrelin induces oxidative stress
resistance in a glucose intolerance and peripheral artery disease
mouse model by restoring endothelial cell miR-126 expression.
Diabetes. 64:1370–1382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen L, Liu C, Sun D, Wang T, Zhao L, Chen
W, Yuan M, Wang J and Lu W: MicroRNA-133a impairs perfusion
recovery after hindlimb ischemia in diabetic mice. Biosci Rep.
38:BSR201803462018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu PY, Hsi E, Wang TM, Lin RT, Liao YC
and Juo SH: MicroRNA let-7g possesses a therapeutic potential for
peripheral artery disease. J Cell Mol Me. 21:519–529. 2017.
View Article : Google Scholar
|
|
5
|
Dua A and Lee CJ: Epidemiology of
peripheral arterial disease and critical limb ischemia. Tech Vasc
Interv Radiol. 19:91–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abu Dabrh AM, Steffen MW, Undavalli C, Asi
N, Wang Z, Elamin MB, Conte MS and Murad MH: The natural history of
untreated severe or critical limb ischemia. J Vasc Surg.
62:1642–1651.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rollins KE, Jackson D and Coughlin PA:
Meta-analysis of contemporary short- and long-term mortality rates
in patients diagnosed with critical leg ischaemia. Br J Surg.
100:1002–1008. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mejias SG and Ramphul K: Prevalence of
peripheral arterial disease among diabetic patients in Santo
Domingo, Dominican Republic and associated risk factors. Arch Med
Sci Atheroscler Dis. 3:e35–e40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kloos W, Vogel B and Blessing E: MiRNAs in
peripheral artery disease-something gripping this way comes. Vasa.
43:163–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganta VC, Choi MH, Kutateladze A, Fox TE,
Farber CR and Annex BH: A MicroRNA93-interferon regulatory
factor-9-immunoresponsive gene-1-itaconic acid pathway modulates
M2-like macrophage polarization to revascularize ischemic muscle.
Circulation. 135:2403–2425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang J, Song XW, Tian J, Chen HY, Li DF,
Wang JF, Ren AJ, Yuan WJ and Lin L: Overexpression of microRNA-378
attenuates ischemia-induced apoptosis by inhibiting caspase-3
expression in cardiac myocytes. Apoptosis. 17:410–423. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Yuan P and He Y: Role of microRNAs
in peripheral artery disease (review). Mol Med Rep. 6:695–700.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zampetaki A and Mayr M: MicroRNAs in
vascular and metabolic disease. Circ Res. 110:508–522. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shantikumar S, Caporali A and Emanueli C:
Role of microRNAs in diabetes and its cardiovascular complications.
Cardiovasc Res. 93:583–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katare R, Riu F, Mitchell K, Gubernator M,
Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami
AP, et al: Transplantation of human pericyte progenitor cells
improves the repair of infarcted heart through activation of an
angiogenic program involving micro-RNA-132. Circ Res. 109:894–906.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paraskevas KI, Kotsikoris I, Koupidis SA,
Giannoukas AD and Mikhailidis DP: Ankle-brachial index: A marker of
both peripheral arterial disease and systemic atherosclerosis as
well as a predictor of vascular events. Angiology. 61:521–523.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu MY, Li CJ, Hou MF and Chu PY: New
insights into the role of inflammation in the pathogenesis of
atherosclerosis. Int J Mol Sci. 18:E20342017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Pietro N, Formoso G and Pandolfi A:
Physiology and pathophysiology of oxLDL uptake by vascular wall
cells in atherosclerosis. Vascul Pharmacol. 84:1–7. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu S, Zhao H, Shi J, Abzhanov A, Crawford
K, Ohno-Machado L, Zhou J, Du Y, Kuo WP, Zhang J, et al: Peripheral
arterial occlusive disease: Global gene expression analyses suggest
a major role for immune and inflammatory responses. BMC Genomics.
9:3692008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wingrove JA, Daniels SE, Sehnert AJ,
Tingley W, Elashoff MR, Rosenberg S, Buellesfeld L, Grube E, Newby
LK, Ginsburg GS and Kraus WE: Correlation of peripheral-blood gene
expression with the extent of coronary artery stenosis. Circ
Cardiovasc Genet. 1:31–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masud R, Shameer K, Dhar A, Ding K and
Kullo IJ: Gene expression profling of peripheral blood mononuclear
cells in the setting of peripheral arterial disease. J Clin
Bioinforma. 2:62012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Hong W and Yang Z: MiR-122
inhibits cell proliferation and tumorigenesis of breast cancer by
targeting IGF1R. PLoS One. 7:e470532012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J and Wang H: miR-15b reduces amyloid-β
accumulation in SH-SY5Y cell line through targeting NF-κB signaling
and BACE1. Biosci Rep. 38:BSR201800512018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun G, Yan S, Shi L, Wan Z, Jiang N, Li M
and Guo J: Decreased expression of miR-15b in human gliomas is
associated with poor prognosis. Cancer Biother Radiopharm.
30:169–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Zhan Y, Jin J, Zhang C and Li W:
MicroRNA-15b promotes proliferation and invasion of non-small cell
lung carcinoma cells by directly targeting TIMP2. Oncol Rep.
37:3305–3312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang L, Yang C, Yin H, Zhao K, Liu W, Hua
W, Wang K, Song Y, Tu J, Li S, et al: MicroRNA-15b silencing
inhibits IL-1β-induced extracellular matrix degradation by
targeting SMAD3 in human nucleus pulposus cells. Biotechnol Lett.
39:623–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Solarek W, Czarnecka AM, Escudier B,
Bielecka ZF, Lian F and Szczylik C: Insulin and IGFs in renal
cancer risk and progression. Endocr Relat Cancer. 22:R253–R264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: An update. Nat Rev
Cancer. 12:159–169. 2012. View
Article : Google Scholar : PubMed/NCBI
|