Introduction
Burns, a series of complex pathophysiological
changes caused by heat such as high-temperature gas, liquid and
solid, can cause skin tissue damage, pain, loss of tissue fluid and
wound necrosis, resulting in systemic infection and even death
(1). Burn wounds, often have deeper
burn phenomenon, so avoiding burns is very important (2). After severe burns, the increase in
capillary permeability leads to severe inflammatory response. The
formation and release of a large number of inflammatory mediators
plays an important role in burned rats (3). The sustained or excessive release of
inflammatory mediators can lead to a variety of inflammatory
effects, cells activating the coagulation system and complement
activation, which causes damage to endothelial cells and distant
organs (4). As a power organ of
circulation, the structural and functional damage to the heart will
inevitably promote the occurrence and development of burn shock,
having become one of the important factors of early ischemic
hypoxia (5). The prevention and
treatment of myocardial damage after burns is very important during
burn treatment. Studies have shown that the application of stem
cells in local hypoxic myocardial tissues can effectively improve
the viability of damaged myocardial cells, repair damaged
myocardial tissues, and partially improve myocardial function
(6). With low immunogenicity,
multi-directional differentiation, easy separation and
proliferation, mesenchymal stem cells (MSCs) provide a new
treatment method for severe burns (7). Expressing in the tissues of burned
rats, miR-184 and miR-126 are also abnormally expressed in the
process of neovascularization, suggesting that they may be involved
in the formation of neovascularization (8). It is suggested that they may protect
the regulation of early burned myocardial injury. Studies have
shown that involved in key gene regulation, miR-184 and miR-126 may
be highly expressed in angiogenesis and endothelial cells,
regulating angiogenesis via cell signaling pathways such as VEGF
(9). The abnormal expression is
expected to become a new target for neovascularization therapy
(10).
In this study, miR-184 and miR-126 were studied to
investigate their expression changes in burned rats and their
correlation analysis.
Materials and methods
Experimental objects
A total of 40 healthy SD rats (provided by the
Experimental Animal Center of Guangxi Medical University, Guangxi,
China) were divided into 4 groups, with a body mass of 200±20 g,
including male and female, 10 for each group. The groups were:
normal control, model I, model II and model III groups. The
difference was not statistically significant concerning sex, age
and body weight in the four groups of rats (P>0.05) (data not
shown).
This study was approved by the Ethics Committee of
Ningbo No. 2 Hospital (Ningbo, China).
Reagents and instruments
PCR kit was purchased from Thermo Fisher Scientific
(China) Co., Ltd., Shanghai, China; RPMI-l640 medium from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA; 10% fetal bovine
serum from Shanghai Harling Biotechnology Co., Ltd., Shanghai,
China; Thiazolyl Blue (MTT) and dimethyl sulfoxide (DMSO) from
Suzhou Lianxiong Chemical Technology Co., Ltd., Suzhou, China; 3.5%
chloral hydrate from Qingdao Yulong Seaweed Co., Ltd., Qingdao,
China; Olympus IX71 inverted fluorescence microscope from Beijing
Presisi Instrument Co., Ltd., Beijing, China; YLS-5Q desktop super
temperature control scald instrument from Beijing Ruiyisi
Technology Co., Ltd., Beijing, China; super clean bench from
Chongqing Pharmaceutical Co., Ltd. Keyihuabo Branch Company,
Chongqing, China; ultra-low temperature refrigerator from Thermo
Fisher Scientific, Inc. Operations were performed strictly in
accordance with the respective instructions.
Specimen collection and
processing
Two rats were anaesthetised with pentobarbital
sodium (30 mg/kg) then sacrificed by cervical dislocation at 3, 6,
12, 24 and 48 h before and after burn, respectively (11). Abdominal aortic blood was collected
from the animals, and frozen at −20°C for testing after serum
separation.
Construction of burn rat model
In the construction of the model, the back hair of
rats was shaved, in an area of approximately 25 cm2.
Anesthesia was intraperitoneally injected with 3.5% chloral hydrate
(10 ml/kg), and then the YLS-5Q desktop super temperature control
scald instrument was used to corporate burns. The wounds in 4
groups were the same, and the burned area was 20 cm2.
The normal control group had no anesthesia and no burns. The hot
conditions of model I group were: 90°C for temperature, 15 sec for
time and 1000 g for pressure. Those of model II group were: 80°C
for temperature, 15 sec for time and 1000 g for pressure. Those of
model III group were: 80°C for temperature, 10 sec for time and 500
g for pressure.
RT-PCR detection of expression of
miR-184 and miR-126 in burned rats
After the above extraction of total mRNA in
accordance with the real-time PCR kit instructions, the reverse
transcription synthesis of cDNA was performed. Reaction was at 37°C
for 45 min and at 95°C for 5 min. The cDNA amplification reaction
system was 20 µl in total: pre-denaturation at 95°C for 10 min,
denaturation at 95°C for 10 sec, annealing at 60°C for 20 sec and
extension at 72°C for 10 sec, for a total of 40 cycles, and then
extension at 72°C for 5 min after the completion of the cycle. U6
was used as a reaction internal reference. All the samples were
repeated for 3 wells. The results were analyzed by 2−∆Cq
method (12). The primers for
RT-qPCR were synthesized by Suzhou Hongxun Biotechnology Co., Ltd.,
and the primer sequences are shown in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
|
| Upstream primer | Downstream
primer |
|---|
| miR-184 |
5′-ATCCAGAGACAAGACATGTAC-3′ |
5′-TTCAGATGTTCTAAGCCTACGG-3′ |
| miR-126 |
5′-TGGCGGTTTGCGGTGGAC-3′ |
5′-CCAGTGCAGGGTCCGAGGT-3′ |
| U6 |
5′-CGCTTCGGCAGCACATATAC-3′ |
5′-TTCACGAATTTGCGTGTCAT-3′ |
ELISA
ELISA was used to detect the expression levels of
miR-184 and miR-126 in peripheral blood. The detection method
referred to the kit instructions. The miR-184 and miR-126 detection
kits were purchased from Shanghai Jingkang Bioengineering Co.,
Ltd., Shanghai, China.
Observation indicators
The changes of miR-184 and miR-126 levels were
detected after operation, before modeling, at 3, 6, 12, 24 and 48 h
after burn. The relationship between miR-184 and miR-126 and the
burn degree of rats was observed, and the correlation between
miR-184 and miR-126 analyzed.
Statistical analysis
SPSS21.0 statistical software package (Shanghai
Kabei Information Technology Co., Ltd., Shanghai, China) was used
for the statistical analysis of data. Measurement data were
expressed as (mean ± SD). The t-test was used for comparison
between two groups, and F test for comparison among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
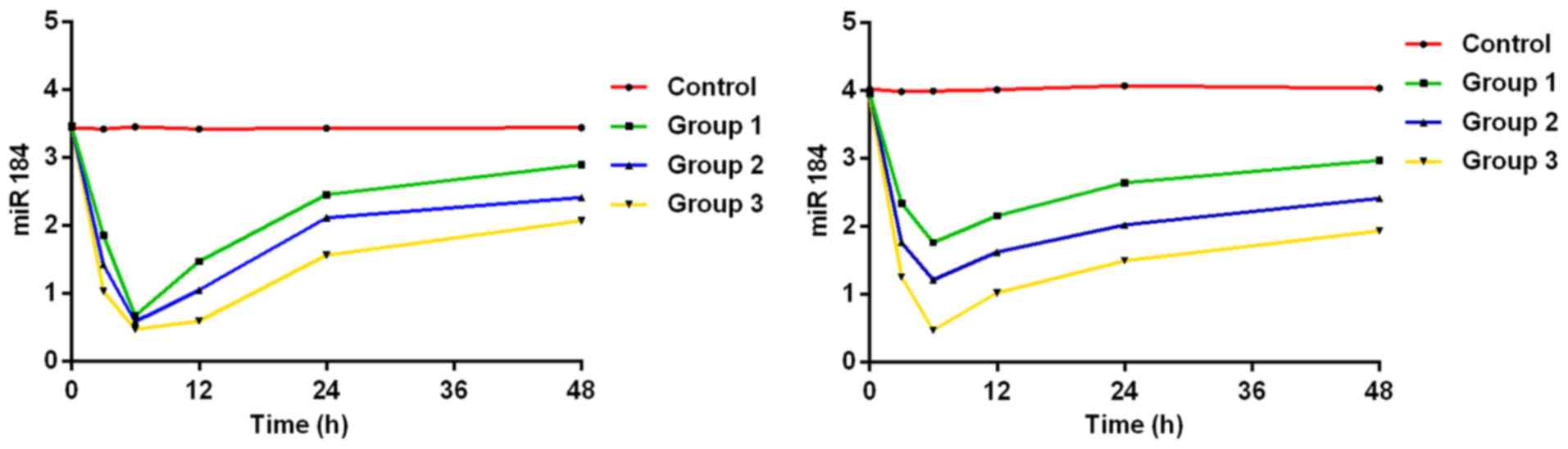
Expression level of miR-184
The results of analysis of variance showed that
there was a difference in the 4 groups of rats in the expression
level of miR-184 at 3, 6, 12, 24 and 48 h after burn (P<0.05),
but no difference before modeling (P>0.05). Those of miR-184 at
3, 6, 12, 24 and 48 h after burn decreased compared with those
before modeling in groups I, II and III (P<0.05). Those of
miR-184 at 24 h and 48 h after burn were higher than those at 6 h
after burn in groups I, II and III (P<0.05), and those of
miR-184 at 48 h after burn were higher than those at 12 h after
burn in groups I, II and III (P<0.05). There was no significant
difference in the expression level of miR-184 among 4 time points
in the control group (P>0.05). At the same time point, that of
miR-184 decreased with the increase of burn degree. The expression
levels of miR-184 were lower in groups I, II and III than those in
control group at each time point (P<0.05), those of miR-184 were
lower in groups II and III than those in group I (P<0.05), and
that of miR-184 was lower in group III than that in group II
(P<0.05). (Tables II and
III, Fig. 1).
 | Table II.RT-qPCR detection of expression level
of miR-184. |
Table II.
RT-qPCR detection of expression level
of miR-184.
| Groups | Control group | Group I | Group II | Group III | F value | P-value |
|---|
| Before modeling | 3.41±0.95 | 3.45±0.94 | 3.48±0.83 | 3.45±0.94 |
0.013 | 0.998 |
| 3 h | 3.44±1.10 | 1.85±0.82 | 1.76±0.63 | 1.25±0.51 |
8.143 | 0.001 |
| 6 h | 3.25±0.71 | 0.67±0.54 | 1.01±0.51 | 1.22±0.25 |
7.791 | 0.002 |
| 12 h | 3.01±0.72 | 0.73±0.28 | 1.01±0.38 | 1.05±0.24 |
8.180 | 0.001 |
| 24 h | 3.47±0.58 | 0.63±0.31 | 0.82±0.21 | 0.86±0.21 | 10.351 | 0.004 |
| 48 h | 3.51±0.57 | 0.52±0.23 | 0.63±0.39 | 0.76±0.32 |
8.242 | 0.001 |
| F value | 0.342 | 10.362 | 11.3523 | 11.595 |
|
|
| P-value | 0.885 |
0.001 |
0.001 |
0.001 |
|
|
 | Table III.ELISA detection of expression level of
miR-184 (ng/ml). |
Table III.
ELISA detection of expression level of
miR-184 (ng/ml).
| Groups | Control group | Group I | Group II | Group III | F value | P-value |
|---|
| Before modeling | 3.81±0.55 | 3.65±0.74 | 3.68±0.63 | 3.52±0.49 |
0.161 | 0.922 |
| 3 h | 3.74±1.12 | 0.85±0.22 | 1.76±0.84 | 2.25±0.41 | 14.201 | 0.001 |
| 6 h | 3.85±0.61 | 1.07±0.54 | 1.41±0.61 | 2.22±0.35 | 13.248 | 0.001 |
| 12 h | 4.01±0.42 | 0.63±0.26 | 1.11±0.33 | 2.06±0.34 | 14.120 | 0.001 |
| 24 h | 4.07±0.28 | 0.67±0.26 | 0.92±0.51 | 1.56±0.71 | 14.365 | 0.001 |
| 48 h | 4.01±0.47 | 0.57±0.23 | 0.73±0.44 | 1.36±0.62 | 12.364 | 0.001 |
| F value | 0.524 | 17.052 | 14.682 | 15.524 |
|
|
| P-value | 0.781 |
0.001 |
0.001 |
0.0001 |
|
|
Expression level of miR-126
The results of analysis of variance showed that
there was a difference among the four groups of rats in the
expression level of miR-126 at 3, 6, 12, 24 and 48 h after
operation (P<0.05), but no difference before modeling
(P>0.05). Those of miR-126 at 3, 6, 12, 24 and 48 h after burn
decreased compared with those before modeling in groups I, II and
III (P<0.05). Those of miR-126 at 24 and 48 h after burn
increased compared with those at 6 h after burn in groups I, II and
III (P<0.05), and those of miR-126 at 12 h after burn decreased
compared with those at 24 h after burn (P<0.05). There was no
significant difference in the expression level of miR-126 among the
four time points in control group (P>0.05). At the same time
point, the expression level of miR-126 decreased with the increase
of burn degree. The expression levels of miR-126 were lower in
groups I, II and III than those in control group at each time point
(P<0.05), those of miR-126 were lower in groups II and III than
those in group I (P<0.05), and that of miR-126 was lower in
group III than that in group II (P<0.05) (Tables IV and V, Fig.
2).
 | Table IV.RT-qPCR detection of expression level
of miR-126. |
Table IV.
RT-qPCR detection of expression level
of miR-126.
| Groups | Control group | Group I | Group II | Group III | t value | P-value |
|---|
| Before modeling | 3.48±0.21 | 3.59±0.32 | 3.64±0.41 | 3.38±0.35 |
0.264 | 0.752 |
| 3 h | 3.58±0.31 | 0.59±0.08 | 0.63±0.03 | 0.75±0.07 | 13.332 | 0.001 |
| 6 h | 3.23±0.21 | 0.56±0.04 | 0.56±0.05 | 0.60±0.05 | 14.253 | 0.001 |
| 12 h | 3.35±0.14 | 0.50±0.06 | 0.51±0.04 | 0.54±0.06 | 12.041 | 0.001 |
| 24 h | 3.49±0.31 | 0.62±0.07 | 0.65±0.07 | 0.68±0.08 | 13.542 | 0.001 |
| 48 h | 3.63±0.44 | 0.64±0.06 | 0.74±0.04 | 0.81±0.08 | 10.367 | 0.001 |
| F value | 0.347 | 14.352 | 12.321 | 15.213 |
|
|
| P-value | 0.782 |
0.001 |
0.001 |
0.001 |
|
|
 | Table V.ELISA detection of expression level
of miR-126 (ng/ml). |
Table V.
ELISA detection of expression level
of miR-126 (ng/ml).
| Groups | Control group | Group I | Group II | Group III | F value | P-value |
|---|
| Before
modeling | 3.81±0.55 | 3.65±0.74 | 3.68±0.63 | 3.52±0.49 |
0.256 | 0.785 |
| 3 h | 3.74±1.12 | 2.05±0.52 | 2.66±0.54 | 2.86±0.51 | 12.325 | 0.001 |
| 6 h | 3.85±0.61 | 1.27±0.74 | 1.81±0.71 | 2.12±0.55 | 15.238 | 0.001 |
| 12 h | 4.01±0.42 | 0.93±0.56 | 1.62±0.43 | 1.82±0.54 | 15.131 | 0.001 |
| 24 h | 4.07±0.28 | 0.57±0.36 | 0.72±0.41 | 1.06±0.41 | 13.383 | 0.001 |
| 48 h | 4.01±0.47 | 0.47±0.25 | 0.53±0.24 | 0.47±0.25 | 14.353 | 0.001 |
| F | 0.893 | 14.262 | 14.203 | 10.251 |
|
|
| P | 0.881 |
0.001 |
0.001 |
0.0001 |
|
|
Correlation analysis of miR-184 and
miR-126
The results of Pearson's correlation analysis showed
that the expression level of miR-184 was positively correlated with
that of miR-126 (r=0.832, P=0.002). miR-184 and miR-126 were
positively correlated with burn degree (r=0.901, P=0.001, r=0.775,
P=0.001) and time after burn (r=0.732, P=0.004, r=0.753,
P=0.002).
Discussion
Large-area deep burns have serious effects on the
body, causing varying degrees of damage to systems that maintain
the balance and stability of the internal environment (13). The immune defense system is also
affected, leading to serious disorders, which is mainly manifested
as severe damage to cellular immune function (14). The immune system is the first
activated body defense response. When immune cells release large
amounts of pro-inflammatory factors, it releases anti-inflammatory
factors that inhibit the excessive inflammation (15). However, lymphocyte injury has a great
impact on the body's immune response (16). The correlation between the expression
levels of miR-184 and miR-126 and burn degree in rats and time
after burn was analyzed, to help clinically prevent burn wounds
from deepening.
In this study, 3 rat models with different burn
degrees were constructed. The changes of miR-184 and miR-126 levels
in burned rats were detected before modeling, at 3, 6, 12, 24 and
48 h after burn. The results showed that the expression of miR-184
and miR-126 in burned rats significantly decreased, compared with
normal rats, with the increase of burn degree (P<0.05).
Therefore, it is speculated that with the increase of burn degree,
a large number of inflammatory factors are secreted, downregulating
the levels of miR-184 and miR-126 that have become markers for
evaluating burn severity and apoptosis, preventing burn wounds from
deepening. When the body is stimulated by burns, inflammatory
factors are rapidly activated, reaching a peak in a relatively fast
time. If the degree of inflammatory response in the body is
moderate and the changes of inflammatory factor levels are limited,
it is beneficial to protect the body and relieve body damage and
infection. Conversely, it may cause secondary damage to the tissue,
promoting disease development (17,18). In
this study, the expression levels of miR-184 and miR-126
continuously decreased with the prolongation of time after burn,
and the apoptosis degree in rats was getting higher and higher,
indicating that the inflammatory response of rats continues to
increase, and the secondary injury of burn wounds deepens. The
results of Pearson's correlation analysis showed that the
expression level of miR-184 was positively correlated with that of
miR-126 (r=0.832, P=0.002). miR-184 and miR-126 were positively
correlated with burn degree (r=0.901, P=0.001, r=0.775, P=0.001)
and time after burn (r=0.732, P=0.004, r=0.753, P=0.002). It was
reported that reducing the expression levels of miR-184 and miR-126
can effectively improve retinal and corneal damage caused by alkali
burn (19,20). Qiu et al (21) have also reported that the elevated
level of miR-126 increases the risk of sepsis in burn patients.
Huang et al (22) have also
reported that improving the levels of miR-184 and miR-126 can
improve the cardiac systolic function in burn patients. Their
conclusions are consistent with our hypothesis that improving the
levels of miR-184 and miR-126 can protect burn patients. In this
study, real-time PCR was used to detect the expressions of miR-184
and miR-126 in burned rats. It is speculated that they are not only
present in blood. It was found that the expression of miR-184 is
upregulated in other tissues, such as the brain (23). Therefore, miR-184 and miR-126 may
participate in the process of neovascularization, but its specific
mechanism of action needs further study and confirmation. We
studied a burn rat model. The burn condition is different from that
of the actual patient. Although SD rats have many similarities with
humans, the conclusion cannot represent the results of clinical
experiments. Therefore, our conclusions still require more clinical
experimental data for confirmation.
In summary, the expression levels of miR-184 and
miR-126 decrease in burned rats. The changes of their levels, as a
reference standard for the clinical efficacy evaluation, may be
used to evaluate burn degree, preventing burn wounds from
deepening.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX constructed burn rat model. YF performed PCR. TW
and PX helped with ELISA. SX and YF were involved in the writing of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Ningbo No. 2 Hospital (Ningbo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Earley ZM, Akhtar S, Green SJ, Naqib A,
Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM, et al:
Burn injury alters the intestinal microbiome and increases gut
permeability and bacterial translocation. PLoS One.
10:e01299962015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peterson JR, De La Rosa S, Sun H, Eboda O,
Cilwa KE, Donneys A, Morris M, Buchman SR, Cederna PS, Krebsbach
PH, et al: Burn injury enhances bone formation in heterotopic
ossification model. Ann Surg. 259:993–998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu L, Li X, Yang J, Chai J, Yu Y, Duan H,
Song H, Feng R, Wang T, Yin H, et al: Comparison of systemic
inflammation response and vital organ damage induced by severe
burns in different area. Int J Clin Exp Pathol. 8:6367–6376.
2015.PubMed/NCBI
|
|
4
|
Yu S, Shi M, Liu C, Liu Q, Guo J, Yu S and
Jiang T: Time course changes of oxidative stress and inflammation
in hyperoxia-induced acute lung injury in rats. Iran J Basic Med
Sci. 18:98–103. 2015.PubMed/NCBI
|
|
5
|
Ning J and Chang TM: Effects of homologous
and heterologous stroma-free hemoglobin and polyhemoglobin on
complement activation, leucocytes and platelets. Biomater Artif
Cells Artif Organs. 18:219–232. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, Xu J, Cai X, Ji L, Li J, Cao B,
Li J, Hu D, Li Y, Wang H, et al: Acute insulin resistance mediated
by advanced glycation endproducts in severely burned rats. Crit
Care Med. 42:e472–e480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu S, Xing C, Han J, Tso MO and Hong J:
Differentiation of rabbit bone marrow mesenchymal stem cells into
corneal epithelial cells in vivo and ex vivo. Mol Vis. 15:99–107.
2009.PubMed/NCBI
|
|
8
|
Wu Q, Wang JH, Li ZQ, Ren JL and Wu YH:
Expression of vascular endothelial growth factor in deep
second-degree scald wounds in rats. Zhongguo Yi Xue Ke Xue Yuan Xue
Bao. 36:650–653. 2014.(In Chinese). PubMed/NCBI
|
|
9
|
Yao QJ, Jia CY, Chen B, Tang CW, Xu MD,
Ding GB and Wang HT: Establishment of rat model of scalding with
high pressure steam. Zhonghua Shao Shang Za Zhi. 20:168–170.
2004.(In Chinese). PubMed/NCBI
|
|
10
|
Tian L, Gao J, Weng G, Yi H, Tian B,
O'Brien TD and Guo Z: Comparison of exendin-4 on beta-cell
replication in mouse and human islet grafts. Transpl Int.
24:856–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang X, Gao L, Zhang Y, Wang G, Liu Y,
Yan C and Sun H: A comparison of the effects of ketamine, chloral
hydrate and pentobarbital sodium anesthesia on isolated rat hearts
and cardiomyocytes. J Cardiovasc Med (Hagerstown). 12:732–735.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Yang W, Zhang X, Yang S, Peng G,
Wu T, Zhou Y, Huang C, Reinach PS, Li W, et al: MK2 inhibitor
reduces alkali burn-induced inflammation in rat cornea. Sci Rep.
6:281452016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quintana HT, Bortolin JA, da Silva NT,
Ribeiro FA, Liberti EA, Ribeiro DA and de Oliveira F: Temporal
study following burn injury in young rats is associated with
skeletal muscle atrophy, inflammation and altered myogenic
regulatory factors. Inflamm Res. 64:53–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge HW, Hu WW, Ma LL and Kong FJ:
Endoplasmic reticulum stress pathway mediates isoflurane-induced
neuroapoptosis and cognitive impairments in aged rats. Physiol
Behav. 151:16–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng SH, Liu Q, Zhao YJ, Shi Y and Wang Y:
Repair of finger deep burn with the island skin flap nourished by
the cutaneous nerve nutrient vessel of the dorsum of hand. Zhonghua
Zheng Xing Wai Ke Za Zhi. 21:98–100. 2005.(In Chinese). PubMed/NCBI
|
|
17
|
Cardoso AL, Bachion MM, Morais JM,
Fantinati MS, Almeida VL and Lino RSJ: Adipose tissue stromal
vascular fraction in the treatment of full thickness burns in rats.
Acta Cir Bras. 31:578–585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan S, Liang D, Lin M, Li Y and Wang Z:
Study on the rat models of corneal neovascularization induced by
alkali burn. Yan Ke Xue Bao. 21:165–172. 2005.(In Chinese).
PubMed/NCBI
|
|
19
|
Bai Y, Bai X, Wang Z, Zhang X, Ruan C and
Miao J: MicroRNA-126 inhibits ischemia-induced retinal
neovascularization via regulating angiogenic growth factors. Exp
Mol Pathol. 91:471–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang CH, Li BG, Fischer DR, Wang JJ,
Runnels HA, Monaco JJ and Hasselgren PO: Burn injury upregulates
the activity and gene expression of the 20 S proteasome in rat
skeletal muscle. Clin Sci (Lond). 99:181–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu P, Yao K, Zhu L, Zhou C and Cheng J:
Expression and significance of vascular endothelial growth factor
in rat cornea after cautery with alkali. Zhonghua Yan Ke Za Zhi.
38:311–314. 2002.(In Chinese). PubMed/NCBI
|
|
22
|
Huang T, Zhang K, Sun L, Xue X, Zhang C,
Shu Z, Mu N, Gu J, Zhang W, Wang Y, et al: Body protective
compound-157 enhances alkali-burn wound healing in vivo and
promotes proliferation, migration, and angiogenesis in vitro. Drug
Des Devel Ther. 9:2485–2499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murad N, Kokkinaki M, Gunawardena N,
Gunawan MS, Hathout Y, Janczura KJ, Theos AC and Golestaneh N:
miR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in
human retinal pigment epithelium and is downregulated in
age-related macular degeneration. FEBS J. 281:5251–5264. 2014.
View Article : Google Scholar : PubMed/NCBI
|