Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
inflammatory disease mainly characterized by a systemic autoimmune
disorder, cartilage degradation and synovial inflammation (1). Several risk factors, including
heredity, infection and gonadal hormone contribute to RA (2). Synovial tissue in patients with RA
features the production of inflammatory cytokines, including tumor
necrosis factor α and interleukin-1β by macrophages, and T and B
cells, and inhibiting these cytokines may ameliorate the clinical
symptoms of RA (3). However, the
mechanisms underlying the genesis and progression of RA remain
elusive, and no appropriate therapeutic strategies have been
developed to cure this disease (4).
Aberrant gene expression in RA is associated with synovial
inflammation; however, the precise mechanisms that lead to altered
gene expression in RA remain poorly understood.
MicroRNAs (miRNAs/miRs) are small noncoding RNAs of
22–24 nucleotides in length that regulate target gene expression by
binding to the 3′-untranslated region, and miRNAs have roles in
biological processes, including cell proliferation, apoptosis and
differentiation (5). Hundreds of
miRNAs have been detected in various organisms and most of them
have an essential role in regulating gene expression through
targeting mRNA translation or inducing mRNA cleavage (6). A large number of studies have revealed
that miRNAs, including miR-155, miR-146, miR-132 and miR-16, are
involved in RA. miR-155 is upregulated in the synovial membrane and
synovial fluid macrophages from patients with RA (7). miR-146 appears to be associated with
the inflammatory response in RA (8).
miR-16 was reported to be overexpressed in the peripheral blood
mononuclear cells of RA patients (8). However, the effects and mechanisms of
miRNA gene expression in RA are not well understood.
The present study evaluated the expression levels of
miR-155, miR-203 and miR-146 in rat synovial fibroblasts of control
and RA groups. The aim of the present study was to evaluate the
effects of miRNA on synovial fibroblast viability, apoptosis and
the cell cycle, and the underlying mechanisms, including
inflammatory signaling pathways in RA, were also investigated.
Materials and methods
Animals
A total of 3 healthy male Sprague Dawley rats (age,
8 weeks; weight, 200±20 g) were purchased from the Laboratory
Animal Center of Huangzhong University of Science and Technology
(Wuhan, China). The experimental rats had been bred under constant
conditions, including 55–60% humidity at 23±2°C and were provided
with water and food ad libitum. All animal experimental
procedures were approved by the Institutional Animal Care and Use
Committee at Tongji Hospital, Tongji Medical College, Huazhong
University of Science and Technology.
Isolation and culture of synovial
fibroblasts
Rat knee joint synovial tissue was cut into small
fragments and digested in Dulbecco's modified Eagle's medium (DMEM)
containing 1 mg/ml collagenase II (both Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C for 2 h. The synovial fibroblasts
were collected after centrifugation and maintained in DMEM
containing 5 mmol/l glucose and 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc.) in a humidified atmosphere containing 5%
CO2 at 37°C. The cells were passaged when they reached
confluency. The fourth generation of cells was used for the
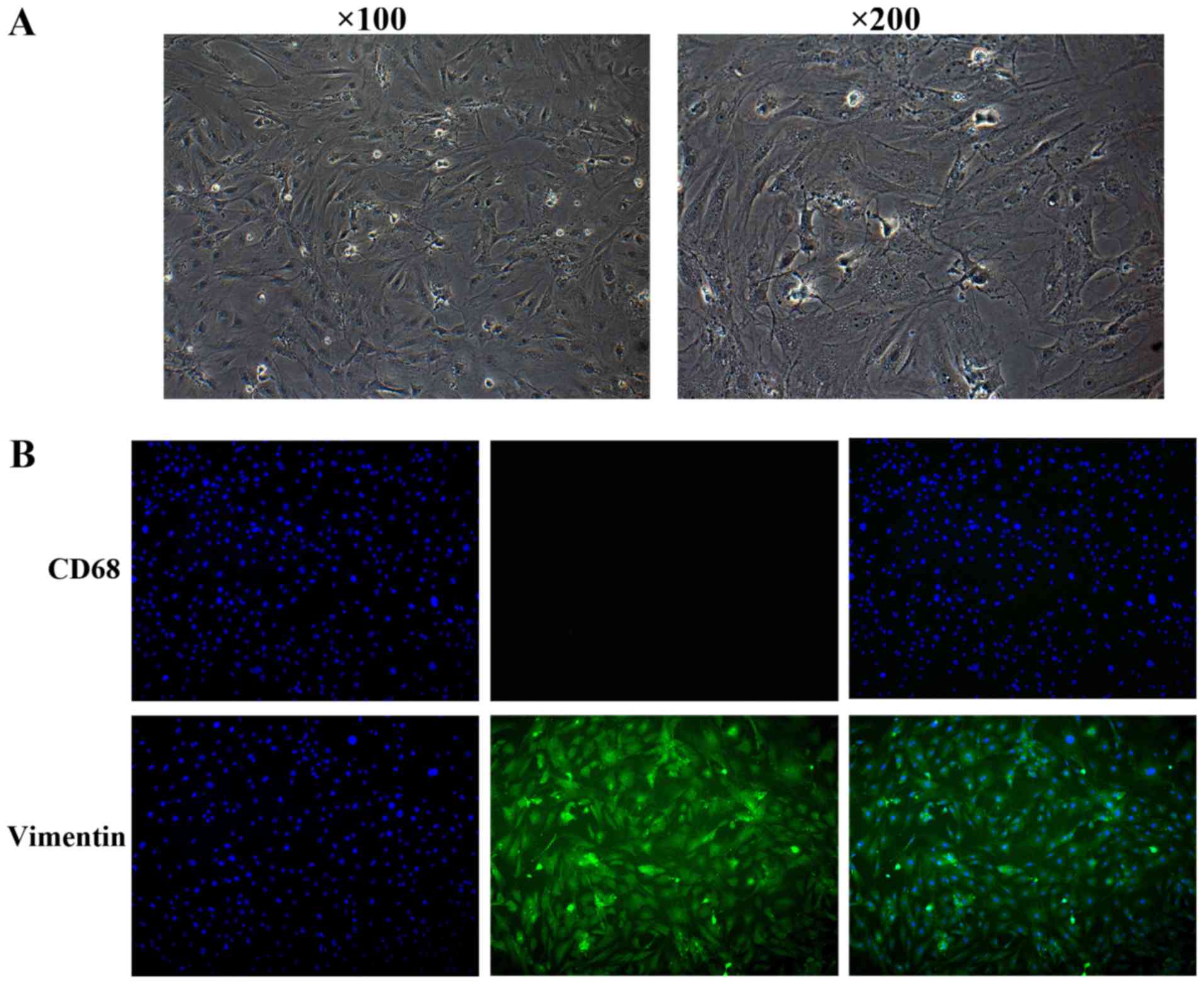
experiment. Microscopy images displaying the cell morphology are
presented in Fig. 1A. The purity of
the cells was tested using immunofluorescence with rabbit
anti-vimentin (cat. no. ab45939; 1:100 dilution; Abcam, Cambridge,
MA, USA) and mouse anti-CD68 (cat. no. ab125212; 1:50 dilution)
antibodies. Following blocking in 5% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at room
temperature, cells were incubated with anti-vimentin and -CD68
primary antibodies for 1 h at room temperature. Cells were washed
with PBS, incubated for 1 h at room temperature with
AlexaFluor® 488-conjugated secondary antibodies (cat.
no. ab150081; 1:500; Abcam) and incubated for 1 h at room
temperature with nuclear DNA was labeled in blue with DAPI.
Cell transfection and reagents
Synovial fibroblasts were divided into a negative
control + RA group, miR-155 mimics + RA group and a miR-155
inhibitor + RA group. Cells were cultured until 60–70% confluent
and then transfected with miR-155 mimics, miR-155 inhibitor or
negative control (NC; all GenePharm S.A. Pallini, Greece) by using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. After 48 h, the synovial fibroblasts were harvested for
further experiments. Lipopolysaccharide (LPS; 1 ng/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to
synovial fibroblasts for 24 h prior to transfection. The following
sequences were used: miRNA inhibitor NC,
5′-CAGUACUUUUGUGUAGUACAA-3′; miRNA-155-5P inhibitor,
5′-ACCCCUAUCACAAUUAGCAUUAA-3′; miRNA mimics NC sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense,
5′-ACGUGACACGUUCGGAGAATT-3′; miRNA-155-5P mimics sense,
5′-UUAAUGCUAAUUGUGAUAGGGGU-3′ and anti-sense,
5′-CCCUAUCACAAUUAGCAUUAAUU-3′.
MTT assay
Cell viability was measured using an MTT assay.
Cells were seeded into 96-well plates at a density of
1×105 cells/well and then stimulated with LPS. After
transfection with miRNA inhibitor, miRNA mimics or negative control
for 48 h, 20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added
to each well, followed by incubation for 4 h. At the end of the
incubation period, the medium was removed and 150 µl
dimethylsulfoxide was added to each well. After shaking at low
speed for 10 min, the absorbance of the dye that had formed was
measured at a wavelength of 490 nm.
Cell apoptosis assay
Apoptosis analysis was performed with the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) flow
cytometry kit (BD Biosciences, Franklin Lakes, NJ, USA) according
to the manufacturer's instructions. After the different treatments,
cells were washed three times with ice-cold PBS and re-suspended in
200 µl binding buffer at a concentration of 1×106
cells/ml. Subsequently, 10 µl Annexin V-FITC and 10 µl PI were
added and cells were incubated for 30 min at 4°C in the dark.
Finally, 300 µl binding buffer was added and cells were analyzed by
flow cytometry (Cytomics FC 500; Beckman Coulter, Brea, CA, USA)
within 1 h.
Cell cycle assay
Cells were washed thrice with ice-cold PBS and then
fixed with 70% (v/v) ethanol at −70°C for 1 h. Following washing
with PBS, a staining solution [10 mmol/l Tris (pH 7.0), 0.1%
Nonidet P-40, 1 mmol/l NaCl, 0.7 µg/ml ribonuclease A and 5 µg/ml
propidium iodide] was added to the cells. After incubation for 30
min in the dark, the cellular DNA content was determined using flow
cytometry.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells by using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and
complementary (c)DNA was generated using a PrimeScript II 1st
strand cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan).
Subsequently, cDNA sequences were amplified with SYBR Premix Ex
Taq™ (Takara Bio, Inc.) with specific primers listed in
Table I. The thermocycling
conditions consisted of an initial step of 3 min at 95°C, followed
by 35 cycles of 95°C for 5 sec, 56°C for 10 sec and 72°C for 25
sec. PCR products were analyzed using an optimized the
2−ΔΔCq method (9).
 | Table I.Primers used for polymerase chain
reaction. |
Table I.
Primers used for polymerase chain
reaction.
| Name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-155 | TTAATGCTAATCGTG |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACCCCTAT |
| miR-203 |
AGTGGTTCTTAACAGTT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAACTGTTG |
| miR-16 | TAGCAGCACGTAAA |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG
CGCCAATA |
| β-catenin |
AATGGCTTGGAATGAGA |
AGTGAAAAGAACGGTAG |
| GSK-3β |
CGGGATCCGCCACCATGTCGGGGCGACCGAGA |
CCCCCCAGGAATTCTCAGGTAGAGTTGGAGGCTGA |
| MMP-7 |
CCAAATAGCCCAAAATGGACTTC |
TGTAATATGCGGTAAGTCTCGAGTATATG |
| Cyclin D1 |
TGTTCGTGGCCTAAGATGAAG |
GGAAGTGTTCGATGAAATCGTG |
| GAPDH |
CCATCAATGACCCCTTCATTG |
CATGGGTGGAATCATATTGGAAC |
Statistical analysis
SPSS 18.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was applied to analyze the data. Values are expressed as
the mean ± standard deviation. Significant differences between
groups were assessed using Student's t-test or one-way analysis of
variance followed by Duncan's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Phenotypic characteristics of synovial
fibroblasts
Synovial fibroblasts were isolated from rats. After
reaching confluence, cells were passaged 4 times, and the
phenotypic characteristics of the cells were observed. The cells
had fusiform or cylindrical shapes and the nuclei were ovoid in the
cells (Fig. 1A). The synovial
fibroblast cultures were negative for CD68 and positive for
vimentin (Fig. 1B).
Effect of LPS treatment on cell
viability
A number of studies have suggested that LPS and its
pattern recognition receptor, Toll-like receptor 4, have a critical
role in the development of RA (10,11). In
the present study, cells were stimulated with LPS at a
concentration of 1, 10 or 100 mg/l for 24, 48 or 72 h. The results
indicated that the cell viability was reduced in a dose- and
time-dependent manner (Fig. 2). In
order to ensure that LPS induces an inflammatory response in
synovial fibroblasts, while minimizing the adverse impact of LPS in
the subsequent experiments, the conditions of 1 mg/l and 24 h were
selected to induce the RA model in the present study (12).
miR-155, miR-203 and miR-16 RNA
expression in synovial fibroblasts
The mRNA expression levels of miR-155, miR-203 and
miR-16 were analyzed by RT-qPCR in control and RA cells. As
presented in Fig. 3, miR-155 and
miR-16 expression levels were significantly higher in the RA group
than in the control group, and miR-155 levels were much higher than
miR-16 levels in the RA group. miR-155 was therefore selected for
further study.
miR-155 regulates the viability of
synovial fibroblasts with RA
The following five groups were set up: Control, RA +
mimics NC (LPS stimulation and transfection of mimics NC), RA +
inhibitor NC (LPS stimulation and transfection of inhibitor NC), RA
+ mimics 155 (LPS stimulation and transfection of miR-155 mimics)
and RA + inhibitor 155 (LPS stimulation and transfection of miR-155
inhibitor). The results indicated that the cell viability in the RA
+ mimics NC and RA + inhibitor NC groups was lower than that in the
control group (Fig. 4). Compared
with that in the RA + mimics NC and RA + inhibitor NC groups, the
RA + mimics 155 group had a reduced cell viability, but the RA +
inhibitor 155 group had a significantly elevated cell viability
(Fig. 4). Taken together, miR-155
inhibits the viability of synovial fibroblasts with RA.
miR-155 regulates apoptosis of
synovial fibroblasts with RA
Cell apoptosis was measured by Annexin V-FITC/PI
staining and flow cytometric analysis. As presented in Fig. 5, the percentages of apoptotic cells
in the RA + mimics NC and RA + inhibitor NC groups were
significantly higher than those in the control group. The RA +
mimics 155 group had a markedly elevated percentage of apoptotic
cells but the RA + inhibitor 155 group had a significantly
decreased apoptotic rate (Fig. 5).
These results suggest that miR-155 increased the apoptosis of
synovial fibroblasts with RA.
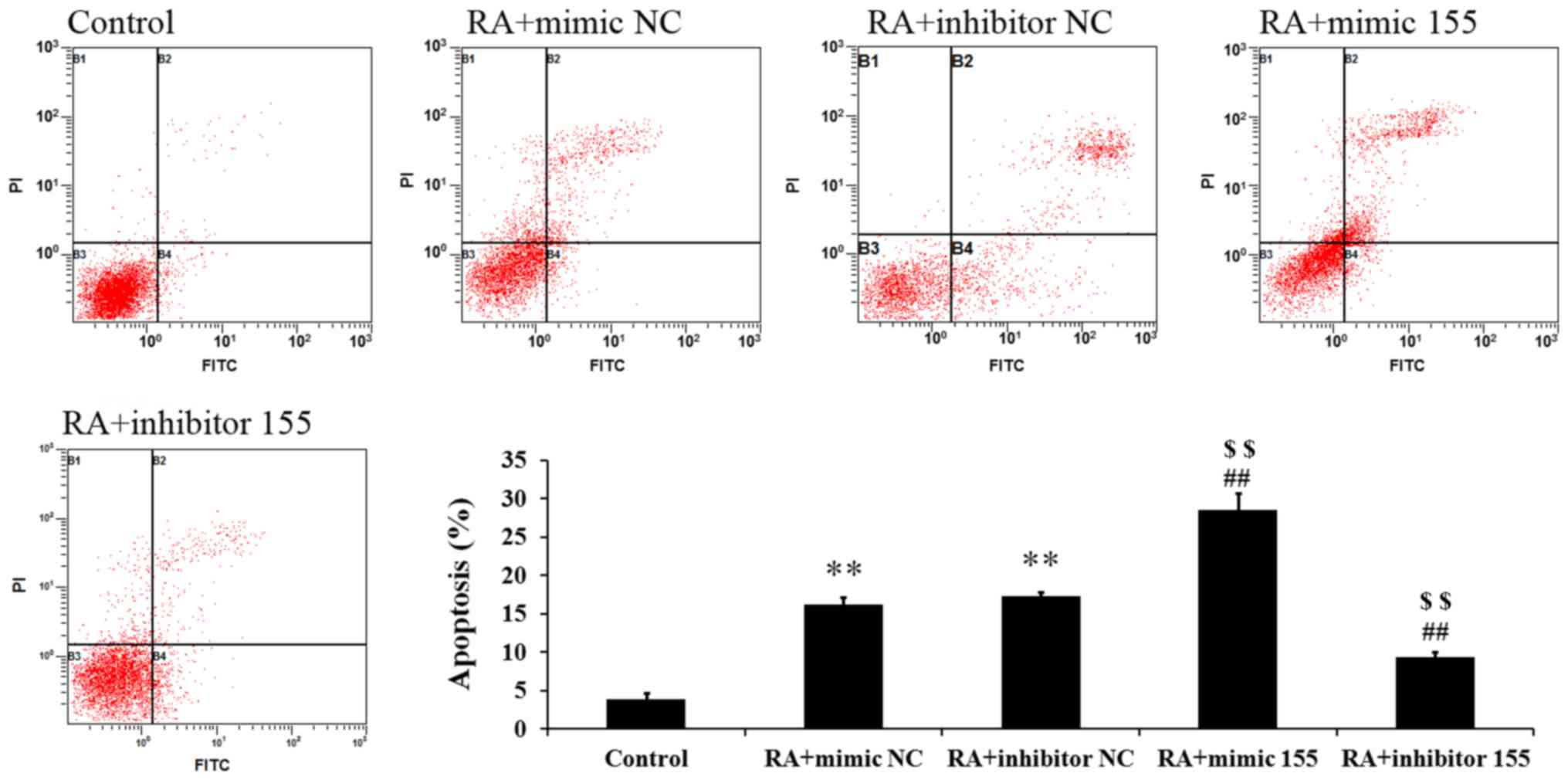 | Figure 5.Effect of miR-155 on the apoptosis of
LPS-induced synovial fibroblasts. The percentage of apoptotic cells
was detected by flow cytometry. Quadrants: B1, necrotic cells; B2,
apoptotic cells; B3, live cells; B4, early apoptotic cells.
**P<0.01 vs. control group; ##P<0.01 vs. RA +
mimic NC; $$P<0.01 vs. RA + inhibitor NC. miR,
microRNA; RA, rheumatoid arthritis; NC, negative control; FITC,
fluorescein isothiocyanate; PI, propidium iodide; mimic 155,
miR-155 mimics. |
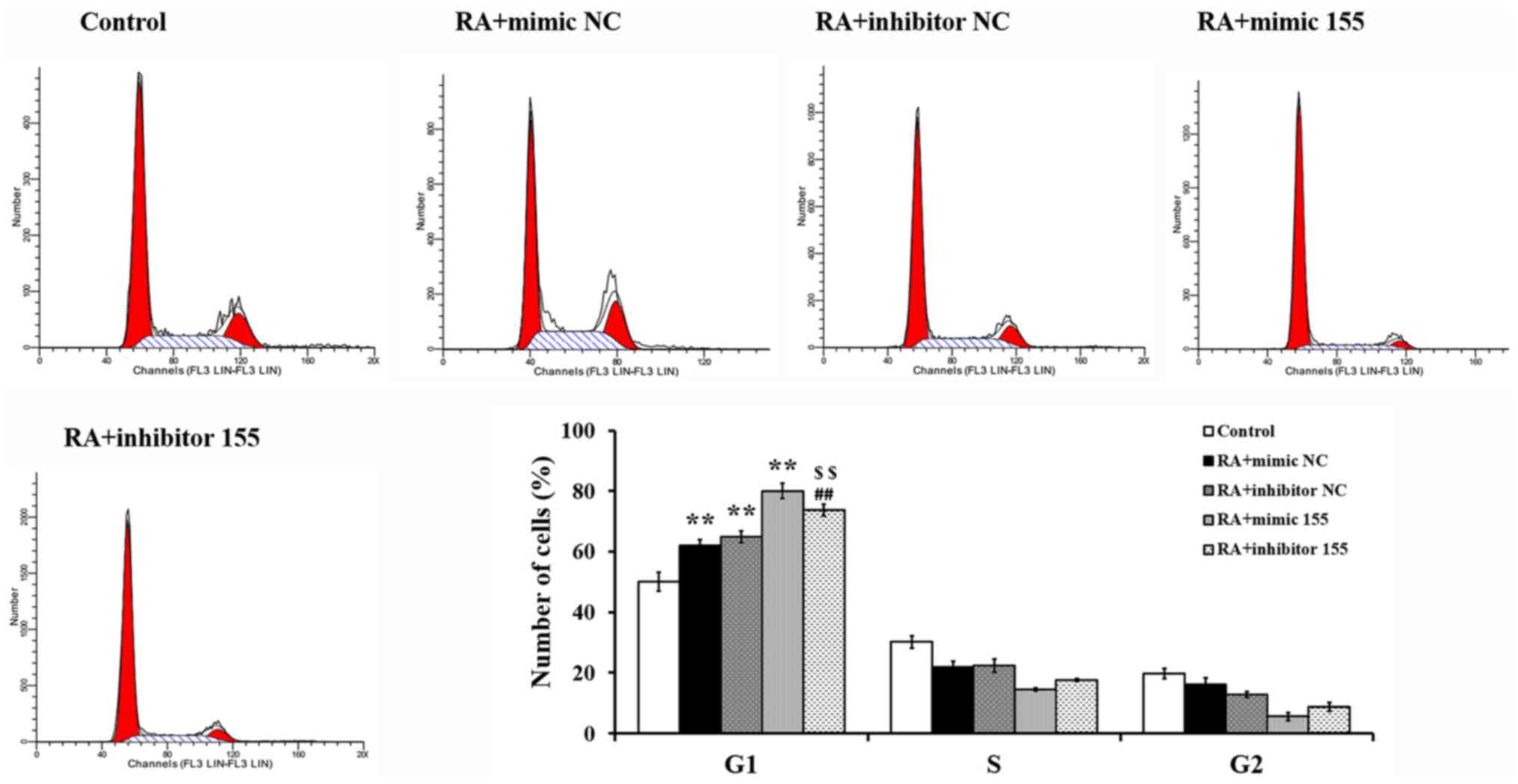
miR-155 regulates the cell cycle of
synovial fibroblasts with RA
The cell cycle was measured by flow cytometry
following PI staining. As presented in Fig. 6, a significant cell cycle arrest in
G1 phase was detected in the RA + mimics NC, RA +
inhibitor NC and RA + mimics 155 groups, while this arrest was
abrogated in the RA + inhibitor 155 group and the cell cycle
progression was restored partially compared to the RA + mimics 155
group. These results indicated that miR-155 induced cycle arrest in
G1 phase in synovial fibroblasts with RA.
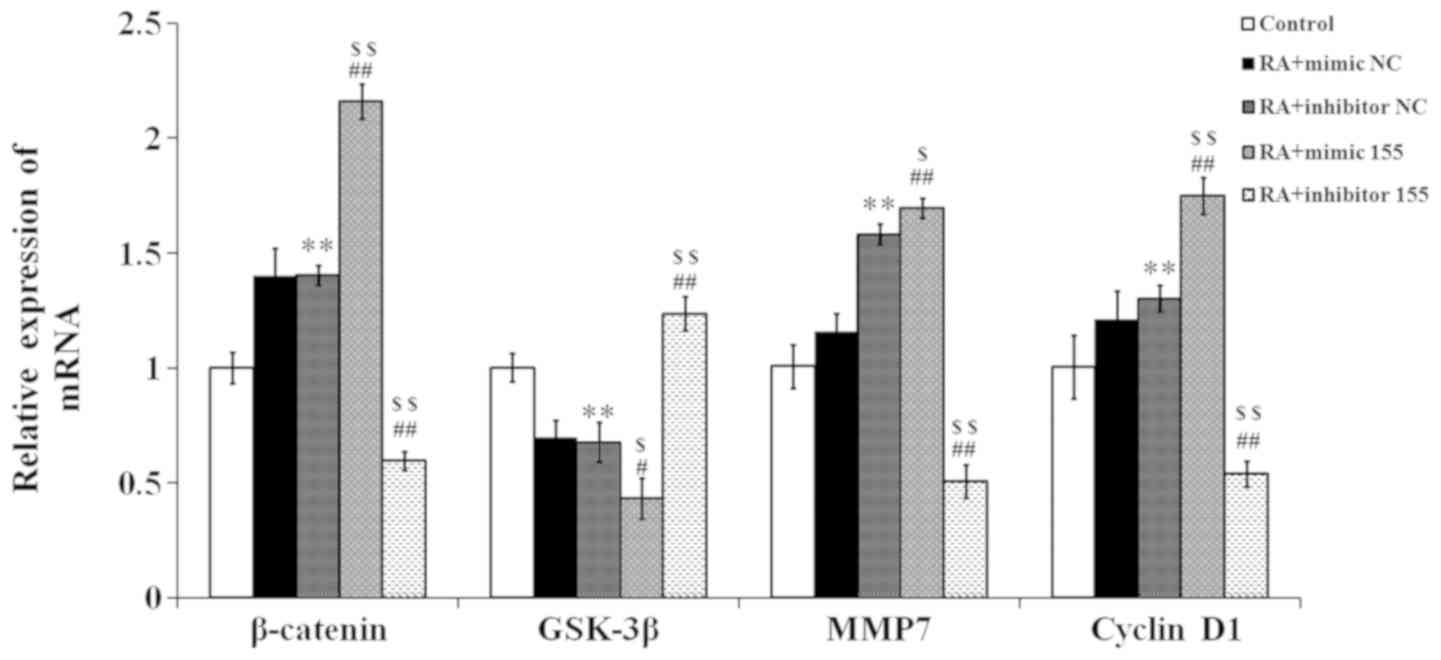
miR-155 regulates Wnt effector protein
expression in synovial fibroblasts with RA
The effector proteins of the Wnt signaling pathway
were quantified by RT-qPCR. As presented in Fig. 7, the expression levels of β-catenin,
matrix metalloproteinase (MMP)7 and cyclin D1 in the RA + mimics NC
and RA + inhibitor NC groups were higher than those in the control
group, while the glycogen synthase kinase (GSK)-3β expression in
the RA + mimics NC and RA + inhibitor NC groups was lower than that
in the control group. In the RA + mimics 155 group, the expression
levels of β-catenin, MMP7 and cyclin D1 were significantly elevated
compared with those in the NC + RA groups, but those in the RA +
inhibitor 155 group were reduced. Conversely, the expression of
GSK-3β was significantly decreased in the RA + mimics 155 group,
but increased in the RA + inhibitor 155 group compared with that in
the NC + RA groups.
Discussion
Accumulating evidence indicates that synovial
inflammation contributes to the progression of RA (13,14). The
presence of synovitis in a significant proportion of patients with
primary RA has been increasingly recognized. Based on this
observation, further studies have implicated joint inflammation and
synovitis in the pathogenesis of RA (15,16). By
regulating various cellular processes, including cell
proliferation, immune responses, inflammation, apoptosis and cell
signaling, miRNAs have an important role in the development and
progression of various diseases. Diverse miRNA expression profiles
(such as the deregulation of miRNA expression) were identified as
crucial small-molecular regulators in severe joint diseases,
including osteoarthritis and RA (7,14,17). The
present study mainly investigated the role of miR-155 in RA.
RA synoviocytes are considered as the effector cells
of cartilage and bone destruction (18). Joint synovial cells are divided into
two cell types: Macrophage-like synoviocytes (MLS) and
fibroblast-like synoviocytes (FLS). Among them, MLS are negative
for vimentin and positive for CD68, while FLS are negative for CD68
and positive for vimentin (19).
Synovial fibroblasts have been reported to have a crucial role in
RA pathogenesis (20). In the
present study, synovial fibroblasts were isolated and identified as
FLS, not MLS. LPS initiates the signaling cascades that cause
inflammation and finally result in RA (21). Therefore, the present study used
LPS-stimulated cells as the RA model to evaluate the effect of
miR-155 on RA development.
miR-155 and miR-16 have been reported to be
associated with RA (22,23). Jin et al (24) reported that overexpression of miR-155
in synovial fluid mononuclear cells leads enhanced proinflammatory
factors. The results of the present study indicated that miR-155
expression exhibited a marked difference between the control and RA
groups, indicating the crucial role of miR-155 in RA. In a previous
study, miR-155 was induced in response to inflammatory stimuli and
acted as a positive regulator of inflammation in RA in vivo,
indicating a role in clinical and experimental arthritis.
Furthermore, the present results suggested that miR-155 inhibits
the viability of synovial fibroblasts and induces cell apoptosis
and cell cycle arrest. In addition, miRNA elevated the expression
of β-catenin by lowering GSK-3β expression, which activated the Wnt
pathway, and enhanced the expression of the target genes cyclin D1
and MMP7. The Wnt signaling pathway regulates cell proliferation,
differentiation, adhesion, morphology and motility, as well as
inflammation (25). Thus, the
present results suggested that miR-155 contributes to inflammation
in RA and may be a promising therapeutic target. In order to
confirm the hypothesis that miR-155 is a target for RA treatment,
miR-155 was inhibited in synovial fibroblasts induced with LPS, and
the results indicated that the cell viability was significantly
increased, while the apoptotic rate was significantly decreased
compared with those in the RA + NC groups. Deregulation of miR-155
in RA monocytes may contribute to the production of
pro-inflammatory chemokines by these cells and to their
accumulation at sites of inflammation (26). Of note, the present study indicated
that inhibition of miR-155 blocks the Wnt signaling pathway in
synovial fibroblasts induced with LPS by reducing the expression of
β-catenin and increasing the expression of GSK-3β.
In conclusion, the present study demonstrated that
miR-155 inhibits RA synovial fibroblast viability and induces
apoptosis and cell cycle arrest by regulating the Wnt signaling
pathway. It was inferred that miR-155 may be a potential target for
RA treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the innovation fund of
Huazhong University of Science and Technology (grant no.
3202754).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FR designed the experiment and was responsible for
the acquisition of funding. HL cultured the cells, performed vector
transfection and was a major contributor in writing the manuscript.
PL assessed the expression of mRNA using reverse
transcription-quantitative polymerase chain reaction analyses. YG
analyzed cell apoptosis and cell cycle distribution using flow
cytometry. JL contributed to the design of the present study and
measured the cell viability using the MTT assay. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures on animals were approved
by the Institutional Animal Care and Use Committee at Tongji
Hospital, Tongji Medical College, Huazhong University of Science
and Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klareskog L, Catrina AI and Paget S:
Rheumatoid arthritis. Lancet. 373:659–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lipsky PE, van der Heijde DM, St Clair EW,
Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P,
Feldmann M, et al: Infliximab and methotrexate in the treatment of
rheumatoid arthritis. Anti-tumor necrosis factor trial in
rheumatoid arthritis with concomitant therapy study group. N Engl J
Med. 343:1594–1602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salemi S, Biondo MI, Fiorentino C, Argento
G, Paolantonio M, Di Murro C, Malagnino VA, Canzoni M, Diamanti AP
and D'Amelio R: Could early rheumatoid arthritis resolve after
periodontitis treatment only?: Case report and review of the
literature. Medicine (Baltimore). 93:e1952014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ham O, Lee CY, Kim R, Lee J, Oh S, Lee MY,
Kim J, Hwang KC, Maeng LS and Chang W: Therapeutic potential of
differentiated mesenchymal stem cells for treatment of
osteoarthritis. Int J Mol Sci. 16:14961–14978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurowska-Stolarska M, Alivernini S,
Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna
M, Fraser AR, Stolarski B, et al: MicroRNA-155 as a proinflammatory
regulator in clinical and experimental arthritis. Proc Natl Acad
Sci USA. 108:11193–11198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pauley KM, Satoh M, Chan AL, Bubb MR,
Reeves WH and Chan EK: Upregulated miR-146a expression in
peripheral blood mononuclear cells from rheumatoid arthritis
patients. Arthritis Res Ther. 10:R1012008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roelofs MF, Wenink MH, Brentano F,
Abdollahi-Roodsaz S, Oppers-Walgreen B, Barrera P, van Riel PL,
Joosten LA, Kyburz D, van den Berg WB and Radstake TR: Type I
interferons might form the link between Toll-like receptor (TLR)
3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis
(RA). Ann Rheum Dis. 68:1486–1493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gierut A, Perlman H and Pope RM: Innate
immunity and rheumatoid arthritis. Rheum Dis Clin North Am.
36:271–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe T, Takahashi N, Hirabara S,
Ishiguro N and Kojima T: Hyaluronan inhibits Tlr-4-dependent RANKL
expression in human rheumatoid arthritis synovial fibroblasts. PLoS
One. 11:e01531422016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andersson AK, Li C and Brennan FM: Recent
developments in the immunobiology of rheumatoid arthritis.
Arthritis Res Ther. 10:2042008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang ZC, Lu H, Zhou Q, Yu SM, Mao YL,
Zhang HJ, Zhang PC and Yan WJ: MiR-451 inhibits synovial
fibroblasts proliferation and inflammatory cytokines secretion in
rheumatoid arthritis through mediating p38MAPK signaling pathway.
Int J Clin Exp Pathol. 8:14562–14567. 2015.PubMed/NCBI
|
|
15
|
Miao CG, Yang YY, He X, Li XF, Huang C,
Huang Y, Zhang L, Lv XW, Jin Y and Li J: Wnt signaling pathway in
rheumatoid arthritis, with special emphasis on the different roles
in synovial inflammation and bone remodeling. Cell Signal.
25:2069–2078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Umar S, Hedaya O, Singh AK and Ahmed S:
Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion
in rheumatoid arthritis synovial fibroblasts by ASK1 regulation.
Toxicol Appl Pharmacol. 287:299–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karouzakis E, Gay RE, Gay S and Neidhart
M: Epigenetic control in rheumatoid arthritis synovial fibroblasts.
Nat Rev Rheumatol. 5:266–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ando A, Hagiwara Y, Onoda Y, Hatori K,
Suda H, Chimoto E and Itoi E: Distribution of type A and B
synoviocytes in the adhesive and shortened synovial membrane during
immobilization of the knee joint in rats. Tohoku J Exp Med.
221:161–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Philippe L, Alsaleh G, Suffert G, Meyer A,
Georgel P, Sibilia J, Wachsmann D and Pfeffer S: TLR2 expression is
regulated by microRNA miR-19 in rheumatoid fibroblast-like
synoviocytes. J Immunol. 188:454–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin Y, Chen Y, Wang W, Wang Z, Tang G,
Zhang P, He Z, Liu Y, Dai SM and Shen Q: HMGB1-LPS complex promotes
transformation of osteoarthritis synovial fibroblasts to a
rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death
Dis. 5:e10772014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mookherjee N and El-Gabalawy HS: High
degree of correlation between whole blood and PBMC expression
levels of miR-155 and miR-146a in healthy controls and rheumatoid
arthritis patients. J Immunol Methods 400–401. 106–110. 2013.
View Article : Google Scholar
|
|
23
|
Filkova M, Aradi B, Senolt L, Ospelt C,
Vettori S, Mann H, Filer A, Raza K, Buckley CD, Snow M, et al:
Association of circulating miR-223 and miR-16 with disease activity
in patients with early rheumatoid arthritis. Ann Rheum Dis.
73:1898–1904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin HM, Kim TJ, Choi JH, Kim MJ, Cho YN,
Nam KI, Kee SJ, Moon JB, Choi SY, Park DJ, et al: MicroRNA-155 as a
proinflammatory regulator via SHIP-1 down-regulation in acute gouty
arthritis. Arthritis Res Ther. 16:R882014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Lau W, Barker N, Low TY, Koo BK, Li VS,
Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M,
et al: Lgr5 homologues associate with Wnt receptors and mediate
R-spondin signalling. Nature. 476:293–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elmesmari A, Gilchrist D, Fraser A, Brewer
J, Mcinnes I and Kurowskastolarska M: The role of miR-155 in
monocyte migration in Rheumatoid arthritis. 2012.
|