Introduction
The majority of cell culture experiments are
conducted on two-dimensional surfaces, including micro-well plates,
tissue culture flasks and Petri dishes, due to the ease and
convenience of two-dimensional cultures (1). However, two-dimensional cultures may
have limitations for the evaluation of cell and tissue physiology,
including the communication between a cell and its matrix and
between adjacent cells (2). To
overcome these limitations, three-dimensional culture techniques
have been applied (3,4). Three-dimensional cultures have
advantages including the ability to represent in vivo
morphologies and the potential for use in drug discovery with
primary and stem cells (5). In a
previous study, three-dimensional culture platforms were made using
engineered microenvironments, such as highly porous biomimetic
scaffolds, which exhibited higher cell differentiation efficiency
compared with their two-dimensional counterparts (4). Furthermore, three-dimensional cultures
have been shown to support the long-term expansion of nephrogenic
progenitor cells (6).
Gingiva-derived stem cells (GDSCs) display multipotency with high
proliferation characteristics (7,8)
Lovastatin is a cholesterol-lowering agent (9); it is involved in regulation of the
mevalonate pathway and also affects Akt pathways that are involved
in cell proliferation and apoptosis, leading to antiproliferative
effect (10). However, the effects
of lovastatin on mesenchymal stem cells with three-dimensional
cultures have not been well elucidated. Therefore, the purpose of
the present study was to evaluate the effects of lovastatin on the
proliferation and osteogenic differentiation of human
gingiva-derived stem cells (GDSCs) using concave microwells. To the
best of the authors' knowledge, this investigation is the first to
elucidate the effects of lovastatin on three-dimensional spheroid
cultures using mesenchymal stem cells derived from gingiva.
Materials and methods
Isolation and culture of human
GDSCs
Gingival tissues were collected from 75-year-old
female undergoing periodontal surgery on August 2013 at Seoul St
Mary's Hospital, College of Medicine, The Catholic University of
Korea. The design of the study was reviewed and approved by the
Institutional Review Board of the Catholic University of Korea,
College of Medicine (no. KC11SISI0348). Informed consent was
obtained from all participants according to the Act on Legal Codes
for Biomedical Ethics and Safety and the Declaration of Helsinki.
Human GDSCs were isolated and cultivated following the protocol
published in the present authors' previous study (7). The gingival tissues were collected and
maintained in sterile phosphate-buffered saline (PBS; Welgene,
Inc.) containing 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA) at 4°C. The tissues were
de-epithelialized, separated into 1–2-mm2 fragments, 0.2
µm filtered, and digested in modified in α-minimum essential medium
(α-MEM; Gibco; Thermo Fisher Scientific, Inc.) containing dispase
(1 mg/ml; Sigma-Aldrich; Merck KGaA) and collagenase type IV (2
mg/ml; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. The cell
suspension was filtered with a 70-µm cell strainer (Falcon; BD
Biosciences) and the cells were then incubated at 37°C in a
humidified incubator with 5% CO2. After 24 h, the
non-adherent cells were washed with PBS.
Formation of spheres and evaluation of
cellular morphology
Fig. 1 demonstrates
the overview of the present study design. Cells were plated onto
silicon elastomer-based concave microwells (StemFIT 3D; MicroFIT)
of 600 µm diameter at a density of 4×105 cells/well and
cultured in osteogenic media (StemPro® Osteogenesis
Differentiation Kit; Gibco; Thermo Fisher Scientific, Inc.) at
37°C. The medium was refreshed at 3-day intervals. To examine the
effect of lovastatin, the cells were cultured in the presence of
lovastatin (Abcam) at final concentrations of 0 (untreated
control), 2 and 6 µM using dimethyl sulfoxide (DMSO) as the vehicle
at plating. The concentrations of lovastatin used in the present
study were based on those used in previously published studies
(11–14). Equal amounts of DMSO were added to
each culture sample to offset the influence of this dissolving
vehicle (15). The cells expressed
CD44 surface marker and the cell spheroids were positive for SSEA-4
(7,16). The morphology of the microspheres was
viewed under an inverted microscope (CKX41; Olympus Corporation) on
days 2, 7 and 14 following plating. The diameter of the cell
spheroids was measured at each time point.
Determination of cytotoxicity
The cytotoxicity of lovastatin was evaluated on days
2, 7 and 14 with a Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocol. The
absorbance at 450 nm was measured spectrophotometrically using a
microplate reader (BioTek Instruments, Inc.).
Alkaline phosphatase activity
assays
Stem cell spheroids grown with osteogenic media
(StemPro® Osteogenesis Differentiation kit; Gibco;
Thermo Fisher Scientific, Inc.) were obtained on day 2 and 8.
Alkaline phosphatase activity assays were tested using a
commercially available kit (K412-500, BioVision, Inc., Milpitas,
CA, USA) following the manufacturers protocol. The cells were
resuspended with an assay buffer, sonicated and then centrifuged at
15,000 × g for 10 min at 4°C to remove insoluble material. The
supernatant was mixed with p-nitrophenylphosphate substrate and
incubated at 25°C for 60 min. The optical density was determined
spectrophotometrically at 405 nm.
Alizarin red-S staining
To investigate mineralized nodule formation, cells
were grown with osteogenic media (StemPro® Osteogenesis
Differentiation kit; Gibco; Thermo Fisher Scientific, Inc.) for 14
days. The cell spheroids were fixed with 4% paraformaldehyde at
room temperature and stained with Alizarin red-S (ScienCell
Research Laboratories, Inc.) at room temperature for 30 min.
Inverted microscopy (CKX41) was used for evaluation of the stained
cells (magnification, ×100). The relative value of mineralization
was determined by measuring the relative intensity of staining
using image processing and analysis software (ImageJ version 1.8.0;
National Institutes of Health).
Statistical analysis
Data are presented as the mean ± standard deviation
with 95% confidence of intervals (95% CI). Experiments were
performed at least three times. A test of normality was performed
with a Shapiro-Wilk test. Two-way analysis of variance (ANOVA) was
performed for evaluation of the effects of concentration and time
and one-way ANOVA was used to determine the differences among
groups, followed by Tukey's post hoc test. The analysis was
conducted with SPSS 12 for Windows (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant result.
Results
Evaluation of cell morphology and
cellular viability
GDSCs formed spheroids in the concave microwells.
The morphology of the spheroids at day 2 is shown in Fig. 2A-C. The morphologies of the spheroids
at days 7 and 14 were similar to those at day 2 (Fig. 2D-I). No obvious changes in morphology
were observed as the incubation time increased. The diameters of
the spheroids were smallest in the 6 µM group at day 2 (P<0.001;
Fig. 3). The average diameters of
the stem cell spheroids at day 2 were 309.9±32.7 (95% CI:
280.5–339.2), 331.2±24.0 (95% CI: 301.9–360.6) and 231.4±24.4 (95%
CI: 202.0–260.7) µm for 0, 2 and 6 µM lovastatin, respectively
(P=0.001). The average diameters at day 7 were 322.2±27.1 (95% CI:
292.9–351.5), 376.4±27.9 (95% CI: 347.1–405.8) and 235.9±29.6 (95%
CI: 206.6–265.2) µm for 0, 2 and 6 µM lovastatin, respectively
(P<0.001). The average diameters at day 14 were 275.8±33.6 (95%
CI: 246.4–305.1), 346.4±11.8 (95% CI: 317.1–375.8) and 317.7±38.2
(95% CI: 288.3–347.0) µm for 0, 2 and 6 µM, respectively (P=0.027;
Table I). In general, the diameters
of the spheroids were maintained throughout the incubation
period.
 | Table I.Cell spheroid diameters (µm) for
various lovastatin concentrations at different time points. |
Table I.
Cell spheroid diameters (µm) for
various lovastatin concentrations at different time points.
|
| Lovastatin |
|---|
|
|
|
|---|
| Time point | 0 µM | 2 µM | 6 µM |
|---|
| Day 2 | 309.9±32.7
(280.5–339.2) | 331.2±24.0
(301.9–360.6) | 231.4±24.4
(202.0–260.7) |
| Day 7 | 322.2±27.1
(292.9–351.5) | 376.4±27.9
(347.1–405.8) | 235.9±29.6
(206.6–265.2) |
| Day 14 | 275.8±33.6
(246.4–305.1) | 346.4±11.8
(317.1–375.8) | 317.7±38.2
(288.3–347.0) |
Cell cytotoxicity
Cell cytotoxicity was measured for the spheroids
after culturing for 2, 7 and 14 days (P=0.035; Fig. 4). The CCK-8 assay results for the 0,
2 and 6 µM groups on day 2 were 0.088±0.004 (95% CI: 0.073, 0.104),
0.089±0.001 (95% CI: 0.073, 0.105) and 0.092±0.002 (95% CI: 0.076,
0.108), respectively (P=0.108). The CCK-8 assay results at day 7
were 0.104±0.016 (95% CI: 0.088, 0.120), 0.117±0.019 (95% CI:
0.101, 0.133) and 0.110±0.026 (95% CI: 0.094, 0.126) for the 0, 2
and 6 µM groups, respectively (P=0.634). No statistically
significant differences were detected between the groups at days 2
and 7 (P>0.05). The CCK-8 assay values at day 14 were
0.093±0.004 (95% CI: 0.077, 0.108), 0.123±0.026 (95% CI: 0.107,
0.138) and 0.142±0.027 (95% CI: 0.126, 0.158) for the 0, 2 and 6 µM
groups, respectively (P=0.012; Table
II).
 | Table II.Cytotoxicity of the cell spheroids for
various lovastatin concentrations at different time points. |
Table II.
Cytotoxicity of the cell spheroids for
various lovastatin concentrations at different time points.
|
| Lovastatin |
|---|
|
|
|
|---|
| Time point | 0 µM | 2 µM | 6 µM |
|---|
| Day 2 | 0.088±0.004 (0.073,
0.104) | 0.089±0.001 (0.073,
0.105) | 0.092±0.002 (0.076,
0.108) |
| Day 7 | 0.104±0.016 (0.088,
0.120) | 0.117±0.019 (0.101,
0.133) | 0.110±0.026 (0.094,
0.126) |
| Day 14 | 0.093±0.004 (0.077,
0.108) | 0.123±0.026 (0.107,
0.138) | 0.142±0.027 (0.126,
0.158) |
Alkaline phosphatase activity
assay
The results of the alkaline phosphatase activity
assay on days 2 and 8 are shown in Fig.
5. The absorbance values at 405 nm on day 2 for the 0, 2 and 6
µM groups were 0.437±0.017, 0.513±0.028 and 0.472±0.018,
respectively (P<0.05). The value for the 2 µM group was
significantly higher compared with that of the 0 µM group
(P<0.05). The absorbance values at 405 nm on day 8 for the 0, 2
and 6 µM groups were 0.553±0.022, 0.562±0.016 and 0.578±0.018,
respectively (P>0.05).
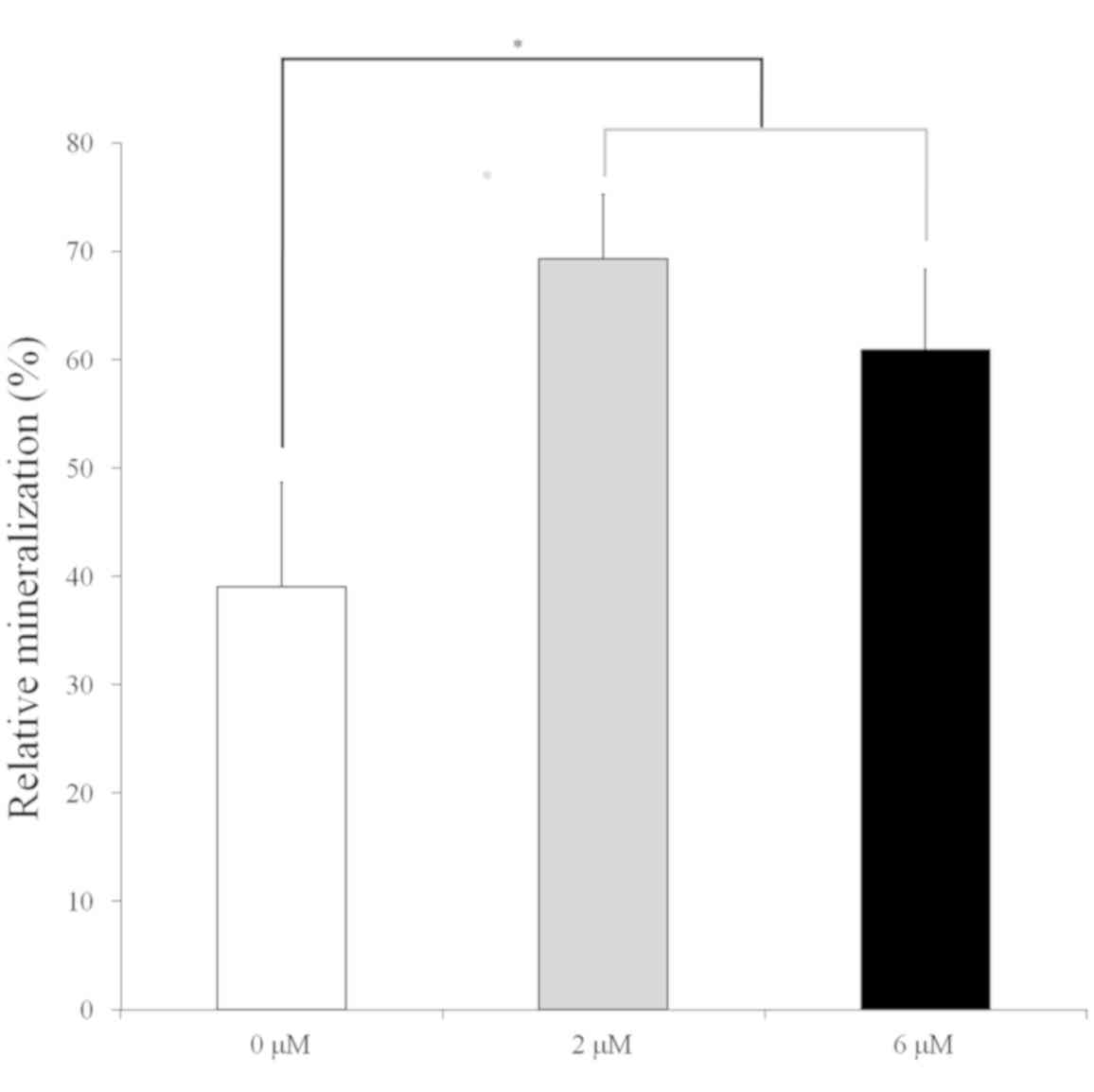
Mineralization assay
Mineralized extracellular deposits were observed
after Alizarin red-S staining on day 14 (Fig. 6). Higher mineralization was observed
in the 2 and 6 µM groups when compared with the 0 µM control
(Fig. 7; P<0.05). The relative
values of the 0, 2 and 6 µM groups on day 14 were 39.0±9.6 (95% CI:
23.6, 54.3), 69.3±6.0 (95% CI: 59.8, 78.8) and 60.9±7.5 (95% CI:
49.0, 72.8), respectively (P=0.001).
Discussion
The present study clearly demonstrates that
lovastatin at the tested concentrations did not adversely affect
the viability of the stem cell spheroids, and increased their
osteogenic differentiation.
Statins are drugs that are widely used for lowering
serum cholesterol, but have also been shown to enhance new bone
formation in vitro and in rodents in previous studies
(15,17–19). The
locally delivery of lovastatin using biodegradable polymer
nanobeads of poly(lactic-co-glycolide acid) has been found to
improve fracture healing in rats (20). In another study, lovastatin-loaded
biodegradable polyurethanes exhibited sustained release of
biologically active lovastatin, and the released lovastatin
significantly enhanced the osteogenic differentiation of
osteoblastic cells in vitro (21). Similarly, the present study using
cell spheroids without scaffold confirmed the increased osteogenic
differentiation of stem cells when cultured with 2–6 µM lovastatin,
suggesting a potential application in therapeutic agents for bone
formation.
In a previous study, statins impaired the survival
of primary human mesenchymal progenitor cells via mevalonate
depletion, nuclear factor κB signaling and B-cell lymphoma
2/adenovirus E1B 19 kDa protein-interacting protein 3 when 1 and 10
µM simvastatin or atorvastatin was used (22). In another study, exposure to
simvastatin (0–20 µM) induced a reduction in sphere-forming
capacity and cell viability, accompanied by a concentration- and
time-dependent increase in caspase-3/7 activity (23). Conversely, lovastatin (0.01–1 µM) was
found to prevent mesenchymal stem cells from undergoing
hypoxia/serum deprivation-induced apoptosis through inhibition of
the mitochondrial apoptotic pathway, leading to attenuation of
caspase-3 activation (24). In an
in vitro study, simvastatin inhibited mesenchymal stem cell
apoptosis and increased vascular endothelial growth factor, and
combined treatment with simvastatin and mesenchymal stem cells
induced a significant improvement in blood reperfusion and a
notable increase in capillary density (25).
Mesenchymal stem cells have been applied in tissue
engineering, for tissues including bone, cartilage, fat and other
connective tissue (26). Mesenchymal
stem cells have been characterized from a variety of dental-related
tissues, including periodontal ligaments, papilla, follicle, dental
pulp of exfoliated deciduous and adult teeth, and the maxillary
sinus membrane, which represent rich sources of mesenchymal stem
cells (27,28). These stem cells have the capacity for
self-renewal and multi-lineage differentiation, including
osteogenic, chondrogenic and adipogenic differentiation (29). In addition, dental stem cells display
several advantages, including a high proliferation rate, high
viability and easy induction to distinct cell lineages (27). Moreover, human GDSCs can be harvested
during routine practice under local anesthesia and may be
considered an excellent source for tissue-engineering purposes
(7). Further studies are warranted
to evaluate the effects of combination therapy using animal
models.
The present study demonstrated that cell spheroids
formed from stem cells combined with the application of lovastatin
at the tested concentrations had enhanced osteogenic
differentiation capability. Therefore, combinations of lovastatin
and stem cell spheroids may potentially be useful for tissue
engineering purposes.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
Catholic Institute of Cell Therapy in 2019, the Research Fund of
Seoul St. Mary's Hospital, The Catholic University of Korea and the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Science,
Information and Communication Technology & Future Planning
(grant no. NRF-2017R1A1A1A05001307).
Availability of data and materials
All data generated or analyzed during the present
study are included in the published article.
Authors' contributions
BK, JT, YK and JP designed the study, performed the
experiments, were responsible for data collection and analysis, and
participated in drafting the manuscript. All the authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures involving human participants were in
accordance with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards, and were approved by
the Institutional Review Board of the Catholic University of Korea,
College of Medicine (no. KC11SISI0348). Informed consent was
obtained from the participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee J, Cuddihy MJ and Kotov NA:
Three-dimensional cell culture matrices: State of the art. Tissue
Eng Part B Rev. 14:61–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haycock JW: 3D cell culture: A review of
current approaches and techniques. Methods Mol Biol. 695:1–15.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Itaka K, Ohba S, Nishiyama N,
Chung UI, Yamasaki Y and Kataoka K: 3D spheroid culture system on
micropatterned substrates for improved differentiation efficiency
of multipotent mesenchymal stem cells. Biomaterials. 30:2705–2715.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Justice BA, Badr NA and Felder RA: 3D cell
culture opens new dimensions in cell-based assays. Drug Discov
Today. 14:102–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Araoka T, Wu J, Liao HK, Li M, Lazo
M, Zhou B, Sui Y, Wu MZ, Tamura I, et al: 3D culture supports
long-term expansion of mouse and human nephrogenic progenitors.
Cell Stem Cell. 19:516–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tae JY, Lee H, Lee H, Ko Y and Park JB:
Osteogenic potential of cell spheroids composed of varying ratios
of gingiva-derived and bone marrow stem cells using concave
microwells. Exp Ther Med. 16:2287–2294. 2018.PubMed/NCBI
|
|
9
|
Wajid N, Anwar SS, Ali F, Zahoor M, Hamid
N, Aslam MM and Ali A: Medicinal significance of lovastatin. Int J
Pharm Sci Res. 6:971–977. 2015.
|
|
10
|
Thibault A, Samid D, Tompkins AC, Figg WD,
Cooper MR, Hohl RJ, Trepel J, Liang B, Patronas N, Venzon DJ, et
al: Phase I study of lovastatin, an inhibitor of the mevalonate
pathway, in patients with cancer. Clin Cancer Res. 2:483–491.
1996.PubMed/NCBI
|
|
11
|
Maeda T, Kawane T and Horiuchi N: Statins
augment vascular endothelial growth factor expression in
osteoblastic cells via inhibition of protein prenylation.
Endocrinology. 144:681–692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee H, Lee H, Na CB and Park JB: Effects
of simvastatin on the viability and secretion of vascular
endothelial growth factor of cell spheroids cultured in growth
media. Implant Dent. 27:480–487. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maeda T, Matsunuma A, Kurahashi I,
Yanagawa T, Yoshida H and Horiuchi N: Induction of osteoblast
differentiation indices by statins in MC3T3-E1 cells. J Cell
Biochem. 92:458–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang SH, Lin HY, Changou CA, Chen CH, Liu
YR, Wang J, Jiang X, Luh F and Yen Y: Integrin β3 and LKB1 are
independently involved in the inhibition of proliferation by
lovastatin in human intrahepatic cholangiocarcinoma. Oncotarget.
7:362–373. 2016.PubMed/NCBI
|
|
15
|
Park JB, Zhang H, Lin CY, Chung CP, Byun
Y, Park YS and Yang VC: Simvastatin maintains osteoblastic
viability while promoting differentiation by partially regulating
the expressions of estrogen receptors α. J Surg Res. 174:278–283.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SI, Ko Y and Park JB: Evaluation of
the maintenance of stemness, viability, and differentiation
potential of gingiva-derived stem-cell spheroids. Exp Ther Med.
13:1757–1764. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mundy G, Garrett R, Harris S, Chan J, Chen
D, Rossini G, Boyce B, Zhao M and Gutierrez G: Stimulation of bone
formation in vitro and in rodents by statins. Science.
286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JB: The use of simvastatin in bone
regeneration. Med Oral Patol Oral Cir Bucal. 14:e485–e488.
2009.PubMed/NCBI
|
|
19
|
Park JB: Combination of simvastatin and
bone morphogenetic protein-2 enhances the differentiation of
osteoblasts by regulating the expression of phospho-Smad1/5/8. Exp
Ther Med. 4:303–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garrett IR, Gutierrez GE, Rossini G, Nyman
J, McCluskey B, Flores A and Mundy GR: Locally delivered lovastatin
nanoparticles enhance fracture healing in rats. J Orthop Res.
25:1351–1357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshii T, Hafeman AE, Nyman JS, Esparza
JM, Shinomiya K, Spengler DM, Mundy GR, Gutierrez GE and Guelcher
SA: A sustained release of lovastatin from biodegradable,
elastomeric polyurethane scaffolds for enhanced bone regeneration.
Tissue Eng Part A. 16:2369–2379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Müller AL, Ngo MA, Sran K, Bellan D,
Arora RC, Kirshenbaum LA and Freed DH: Statins impair survival of
primary human mesenchymal progenitor cells via mevalonate
depletion, NF-κB signaling, and Bnip3. J Cardiovasc Transl Res.
8:96–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Torres CG, Olivares A and Stoore C:
Simvastatin exhibits antiproliferative effects on spheres derived
from canine mammary carcinoma cells. Oncol Rep. 33:2235–2244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu R, Chen J, Cong X, Hu S and Chen X:
Lovastatin protects mesenchymal stem cells against hypoxia- and
serum deprivation-induced apoptosis by activation of PI3K/Akt and
ERK1/2. J Cell Biochem. 103:256–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Zhang D, Zhang Y, He G and Zhang F:
Augmentation of neovascularization in murine hindlimb ischemia by
combined therapy with simvastatin and bone marrow-derived
mesenchymal stem cells transplantation. J Biomed Sci. 17:752010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong SH, Lee JE, Kim BB, Ko Y and Park
JB: Evaluation of the effects of cimicifugae rhizoma on the
morphology and viability of mesenchymal stem cells. Exp Ther Med.
10:629–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lei M, Li K, Li B, Gao LN, Chen FM and Jin
Y: Mesenchymal stem cell characteristics of dental pulp and
periodontal ligament stem cells after in vivo transplantation.
Biomaterials. 35:6332–6343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JH, Ko SY, Lee JH, Kim DH and Yun JH:
Evaluation of the periodontal regenerative properties of patterned
human periodontal ligament stem cell sheets. J Periodontal Implant
Sci. 47:402–415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|